Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11898
Peer-review started: June 17, 2022
First decision: August 21, 2022
Revised: September 3, 2022
Accepted: October 11, 2022
Article in press: October 11, 2022
Published online: November 16, 2022
Processing time: 143 Days and 16.1 Hours
Pulmonary hypertension (PH) is a severe complication of bronchopulmonary dysplasia (BPD) in premature neonates and is closely related to prognosis. However, there is no effective and safe treatment for PH due to BPD in infants. Successful treatment for cases of BPD-associated PH with Tadalafil combined with bosentan is rare. This case may make a significant contribution to the literature because PH is difficult to manage as a serious complication of BPD in preterm infants. Mortality is high, especially when it is complicated by heart failure.
An extremely premature neonate with a gestational age of 26+5 wk and birth weight of 0.83 kg was diagnosed with BPD associated with PH; oral sildenafil did not improve the PH. The infant experienced sudden cardiac arrest and serious heart failure with severe PH. After a series of treatments, including cardiopulmonary resuscitation, mechanical ventilation, and inhaled nitric oxide (iNO), the respiratory and circulatory status improved but the pulmonary artery pressure remained high. Then oral sildenafil was replaced with oral tadalafil and bosentan; pulmonary artery pressure improved, and the infant recovered at our hospital. After 2 years of follow-up, she is in good condition, without any cardiovascular complications.
INO can effectively improve the respiratory and circulatory status of infants with PH associated with premature BPD. B-type natriuretic peptide should be routinely measured during hospitalization to evaluate the risk and prognosis of BPD-associated PH in preterm infants. Tadalafil combined with bosentan for the treatment of PH associated with premature BPD was better than sildenafil in this case. Further studies are needed to explore the efficacy and safety of different vasodilators in the treatment of PH associated with premature BPD.
Core Tip: Pulmonary hypertension (PH) is difficult to manage as a serious complication of bronchopulmonary dysplasia (BPD) in preterm infants. Mortality is high, especially when it is complicated by heart failure. Valuable and noninvasive biomarkers, such as B-type natriuretic peptide, should be routinely measured during hospitalization to evaluate the risk and prognosis of BPD-associated PH in preterm infants. Inhaled nitric oxide can effectively improve the respiratory and circulatory status of infants with PH associated with premature BPD. Tadalafil combined with bosentan for the treatment of PH associated with premature BPD was better than sildenafil in this case.
- Citation: Li J, Zhao J, Yang XY, Shi J, Liu HT. Successful treatment of pulmonary hypertension in a neonate with bronchopulmonary dysplasia: A case report and literature review. World J Clin Cases 2022; 10(32): 11898-11907
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11898.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11898
Bronchopulmonary dysplasia (BPD) is one of the most common complications in premature infants[1] and is associated with poor outcomes involving multiple systems including pulmonary dysfunction, neurodevelopmental impairments, and cardiac dysfunction[2,3]. Approximately one-quarter of infants with moderate-to-severe BPD develop pulmonary hypertension (PH) associated with cardiac dysfunction[4]. The incidence of PH increases with the decrease of gestational age[3]. In recent years, the prevalence of PH related to BPD has increased, along with the increased survival rate of extremely preterm infants. PH due to BPD is gradually becoming an important component of PH in children[5]. PH can worsen the prognosis of premature infants with BPD considerably. Infants with BPD and PH have much higher morbidity and mortality rates than those with BPD only[3,5,6]. PH increases the mortality of infants with BPD by up to 50%[7].
Inhaled nitric oxide (iNO) is the first-line drug for the treatment of PH[8]; however, it is not conducive for long-term medication because of its high cost and the need for endotracheal intubation. Sildenafil is the most commonly used drug for BPD complicated by PH[9]. Recently, it has been reported that refractory PH can also be treated with bosentan[10]. If the curative effect is poor, the choice of the drug is difficult. The European guidelines for PH in children suggest that tadalafil can be added to the treatment[11]. However, there are few reports on the use of this drug in children with a low gestational age[12].
This study reported a case of an extremely low birth weight (ELBW) infant with severe BPD-associated PH that achieved better efficacy after treatment.
The baby was born prematurely.
The age of the baby was 4 h with a gestational age of 26+5 wk. The baby was delivered because of a threatened miscarriage. The birth weight of the baby was 830 g, and Apgar scores were 6 and 8 at 1 and 5 min, respectively. The mother was referred to a local hospital for premature rupture of the membranes by approximately 9 h at 26+5 wk of gestation and twin pregnancy. The amniotic fluid was clear, with an unknown volume. The baby was intubated with a surfactant, supported with mechanical ventilation, and then transported to the neonatal intensive care unit.
There was no significant abnormality in the patient’s previous history.
The father and mother of the patient were in good health. Pre-gestational workup, including ultrasound screening of the mother, did not indicate any abnormalities.
An immature appearance; poor responsiveness; and petechiae and ecchymosis scattered over the body. Cardiopulmonary examination showed no abnormal features except for positive three concave signs. The abdomen was soft, and the liver and spleen were not touched under the ribs. The newborn exhibited a decreased muscle tone, and the primitive reflex was not observed.
There was no significant abnormality in the laboratory examinations on admission.
Chest radiography on admission demonstrated neonatal respiratory distress syndrome.
Severe BPD-associated PH; ELBW infant.
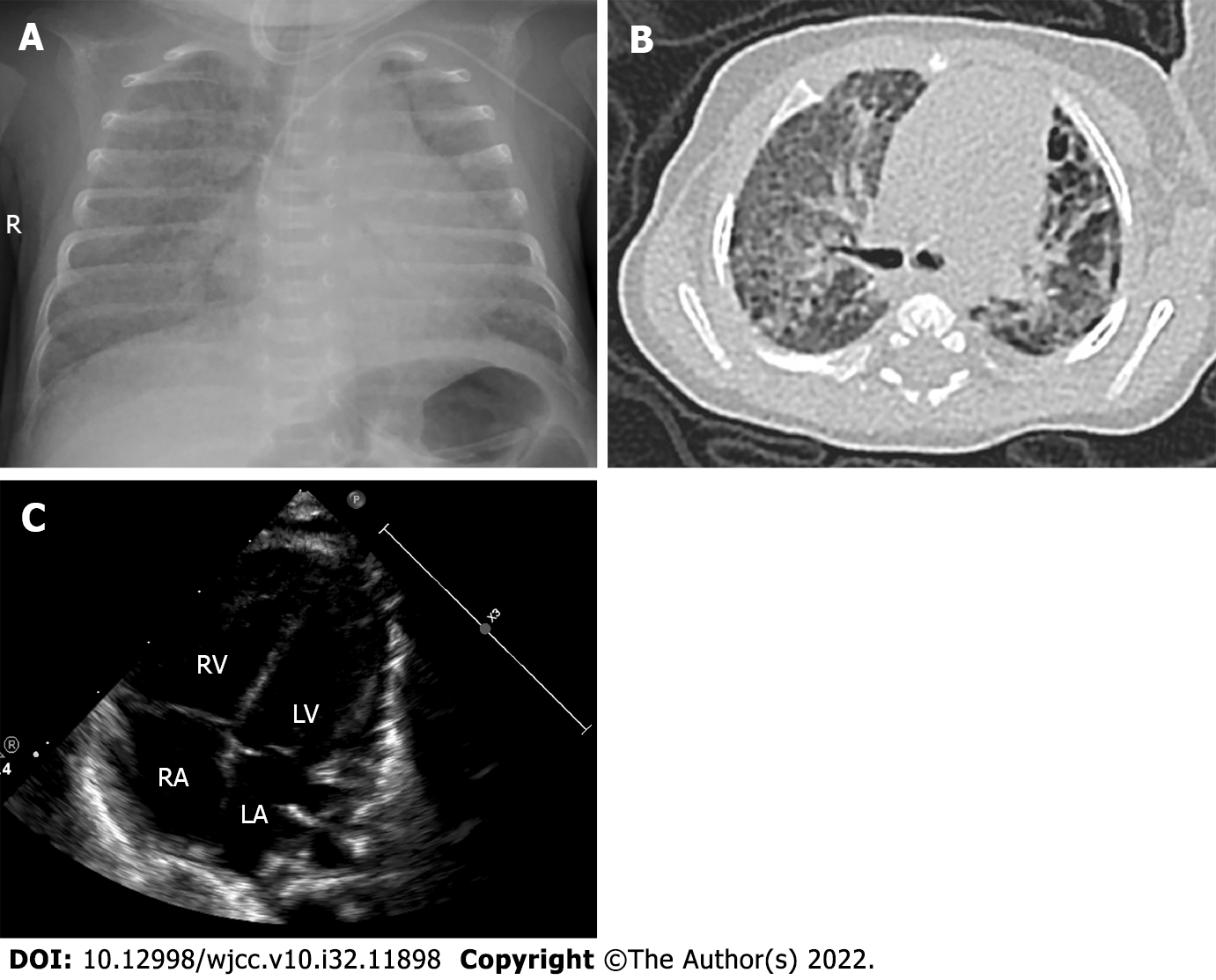
The baby had a pulmonary hemorrhage shortly after admission, and the difference in oxygen saturation (SpO2) between the right hand and foot was over 10% at 3 d after birth. Echocardiography indicated persistent PH of the newborn (PPHN) (a width of 2 mm right-to-left shunt at the macrovascular level and a width of 2 mm right-to-left shunt at the obovular foramen) with an oxygen index (oxygen index = fraction of inspired oxygen mean airway pressure 100/partial pressure of oxygen) of 34. We also measured a B-type natriuretic peptide (BNP) value of 1306 pg/mL (Centaur XPT automatic chemiluminescence analyzer; Siemens, Munich, Germany). Thus, the baby was mechanically ventilated with 10 ppm iNO. At 12 d after birth, she was weaned off iNO, and her BNP level dropped to 60 pg/mL. Echocardiography was performed 3 wk after birth and showed a patent ductus arteriosus with a diameter of 1 mm (left-to-right shunt). Systemic dexamethasone was administered on the 14th d after birth, and the infant’s condition improved after treatment. At 65 d after birth (36 wk of corrected gestational age), her weight increased to 2500 g, but the baby still required high-flow nasal cannula oxygen therapy with an inhaled oxygen concentration of 30% at a flow rate of 5 L/min. She was diagnosed with BPD based on chest radiography and computed tomography examination (Figure 1A and B). Echocardiography revealed acleistocardia, atrial septal defect (bidirectional shunt), patent ductus arteriosus (right-to-left shunt), and PH (tricuspid regurgitation velocity Vmax = 3.0 m/s, enlarged right atrium (RA = 19 mm) and right ventriculus (RV = 12 mm), thickened RV anterior wall (4 mm), pulmonary artery slightly widened [main pulmonary artery (MPA) = 12 mm, the estimated pulmonary artery pressure was 47 mmHg] (Figure 1C). Considering the severe BPD combined with PH, she was treated with oral sildenafil (1 mg/kg three times a day) combined with oral diuretic (hydrochlorothiazide and spironolactone) therapy and maintained SpO2 at 92%-95%.
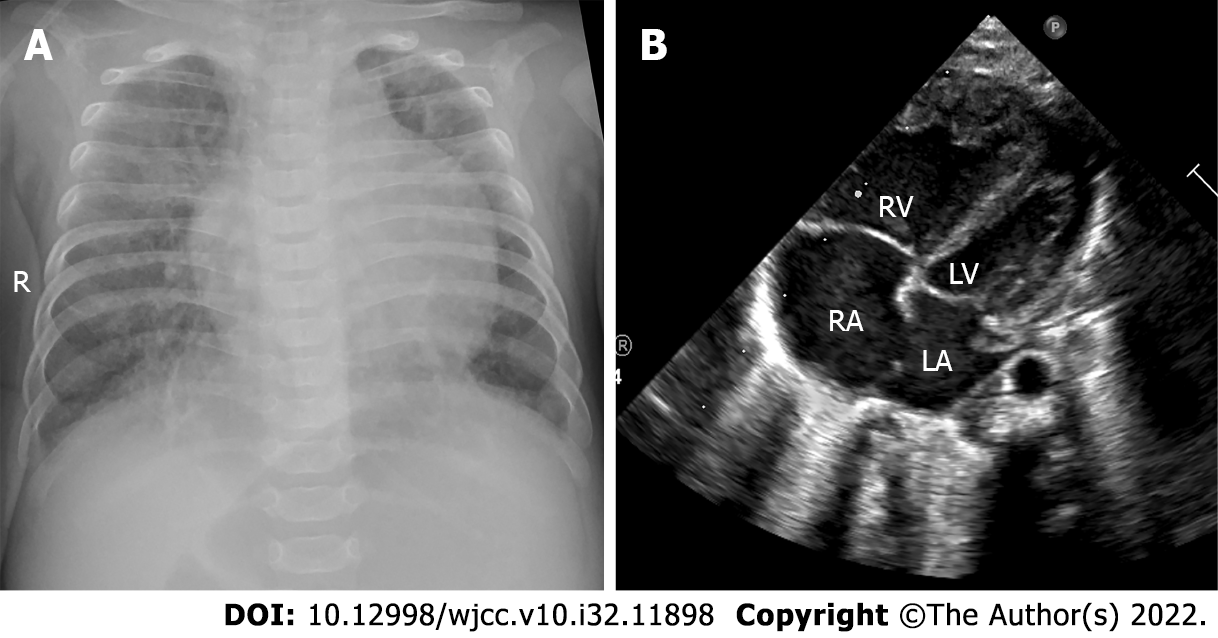
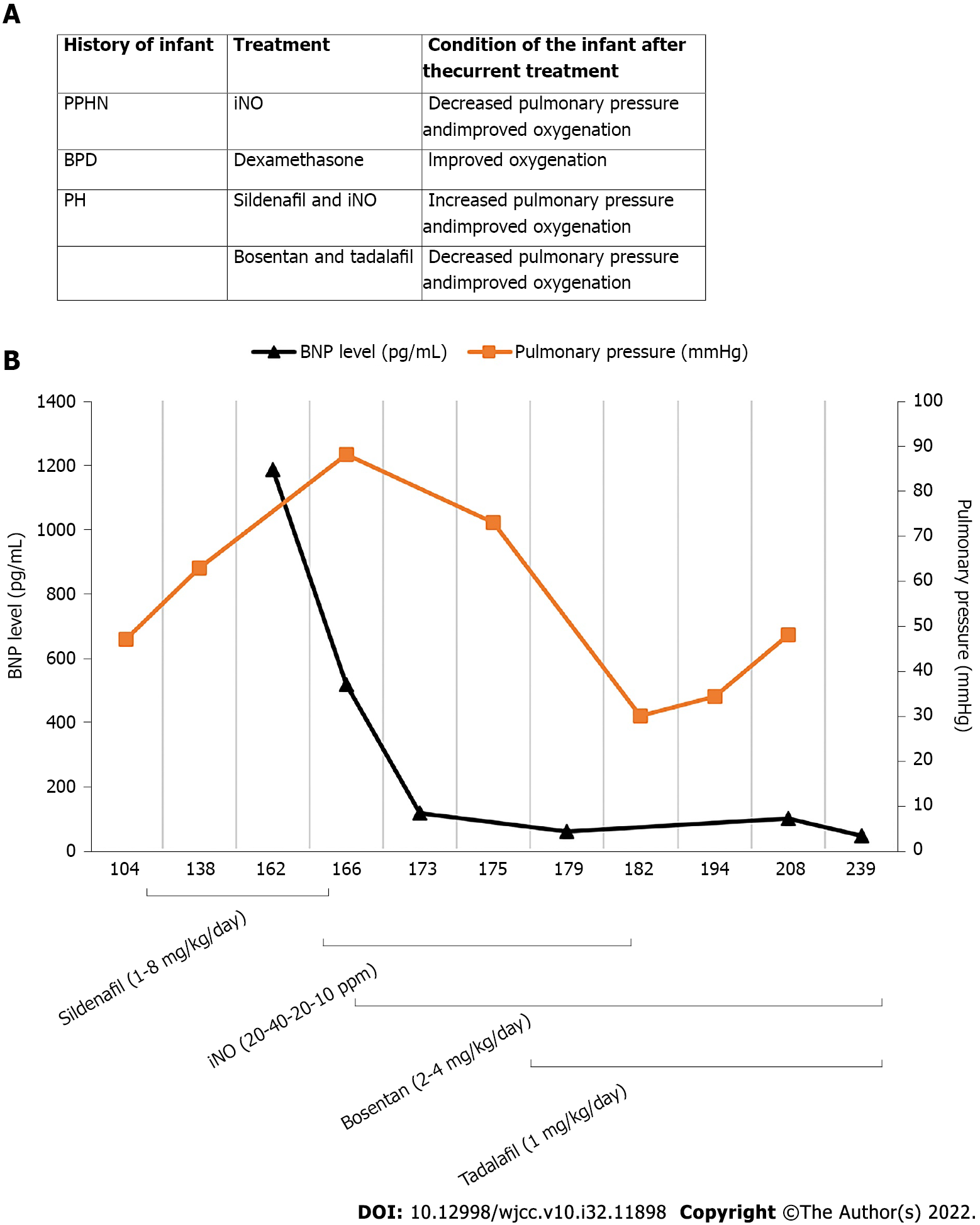
On day 151, she suddenly developed fever with irritability, and her breathing and heart rate increased significantly. An enlarged liver was palpated 4 cm below the right rib cage. Chest radiography showed the new appearance of an infiltrative shadow in the bilateral lungs, and the echocardiogram revealed an ejection fraction of 53%, atrial septal defect (bidirectional shunt), and severe PH (tricuspid regurgitation velocity Vmax = 4.3 m/s, enlarged RA (25 mm) and RV (17 mm), thickened RV anterior wall (6 mm), smaller left atrium (LA = 13 mm) and left ventriculus (LV = 16 mm), pulmonary artery slightly widened (MPA = 13 mm), the estimated pulmonary artery pressure was 88 mmHg) (Figure 2). Sputum culture revealed Klebsiella pneumoniae (ESBL-negative, sensitive to cefoperazone sulbactam). We measured a BNP value of 1186.65 pg/mL. The baby developed a pulmonary hypertensive crisis, and her SpO2 decreased to 60%, followed by cardiopulmonary arrest. Cardiopulmonary resuscitation was performed successfully immediately, and we treated her with mechanical ventilation, iNO at 20 ppm, antibiotics (cefoperazone/sulbactam), depressants (phenobarbital and midazolam), diuretics (hydrochlorothiazide, spironolactone, and furosemide), and upregulated the dose of oral sildenafil to 8 mg/kg/d. Her respiratory condition improved, and the SpO2 level increased after these treatments, with the BNP level decreasing to 116.04 pg/mL at 173 d of age. However, the echocardiogram revealed that her PH did not improve significantly [tricuspid regurgitation velocity Vmax = 3.8 m/s, enlarged RA (24 mm) and RV (15 mm), thickened RV anterior wall (6 mm), smaller LA and LV, the estimated pulmonary artery pressure was 73 mmHg], and we could not discontinue iNO and wean her from the mechanical ventilation. Therefore, oral sildenafil was gradually reduced, and oral bosentan (2 mg/kg twice a day) and tadalafil (1 mg/kg once daily) were added to her treatment regimen. Her condition became increasingly stable, with her BNP level decreasing to 59.53 pg/mL at 179 d of age. At 182 d of age, her PH improved [atrial septal defect (left-to-right shunt), tricuspid regurgitation velocity Vmax = 2.0 m/s, enlarged RA (25 mm) and RV (15 mm), thickened RV anterior wall (6 mm), the volume of LA and LV were normal, the estimated pulmonary artery pressure was 30 mmHg], and we were able to discontinue the iNO and wean her from mechanical ventilation. At 239 d of age, her BNP value was 47.39 pg/mL. The patient was weaned from oxygen and discharged with bosentan and tadalafil at 246 d of age. The clinical course of the patient is shown in Figure 3. Other laboratory examinations, including serum bilirubin, liver enzymes, alkaline phosphatase, serum creatinine, serum electrolytes, and complete blood count, did not show any abnormalities during treatment.
The baby recovered well when discharged home, and all intracranial color Doppler examinations performed during hospitalization did not show any abnormalities. An auditory brainstem examination revealed normal results, and no obvious abnormality was found in retinal screening. After returning home, the baby was followed-up regularly and monitored with echocardiography and BNP levels every 3 mo. No side effects of drugs were found, and the laboratory examination of liver and renal function was normal during the follow-up. The echocardiography parameters did not indicate PH when she was 1.5-years-old, and bosentan and tadalafil were discontinued. Her pulmonary artery pressure and BNP levels were normal at the age of 2 years. The parents were satisfied with our treatment.
In this case, we report an ELBW infant that experienced early PH, followed by late PH with severe BPD during hospitalization. Our patient experienced serious heart failure with severe PH induced by pulmonary infection. We detected serial changes in serum BNP levels, which showed a good correlation with changes in pulmonary arterial pressure detected by echocardiography. In addition, iNO effectively improved the pulmonary condition of the infant. Several vasodilators were used in this case, of which bosentan plus tadalafil seemed to perform better than sildenafil in decreasing pulmonary arterial pressure. This case may make a significant contribution to the practice or Clinical Practice Guidelines because PH is difficult to manage as a serious complication of BPD in preterm infants.
BPD is one of the major complications of prematurity. In one-third of ELBW infants, BPD is the most common form of chronic lung disease, and PH is seen in one-fifth of ELBW infants, mainly in infants with moderate-to-severe BPD[13]. The feature of PH associated with BPD includes the following process: Alveolar diffusion impairment, abnormal vascular remodeling, and pulmonary vessels growth arrest. These processes increase the pulmonary vascular resistance and the risk of right-sided heart failure[14]. Compared with other causes of PH in children, BPD-related PH may improve with age and the development of lungs and vessels[15]. In normal fetal circulation, pulmonary artery pressure is the same as systemic pressure but falls rapidly after birth, and at 2–3 mo of age, it reaches levels similar to those in adults. However, there is no conclusion when the pulmonary artery pressure of preterm infants reaches the adult level at different gestational weeks.
BPD-associated PH can be divided into early, late, and chronic PH[16]. Early PH occurs within the initial postnatal week. This is mainly due to pathological factors that lead to a delayed transition from fetal circulation to neonatal circulation. The clinical manifestations include hypoxemia and a continuous right-to-left shunt of blood. Studies have shown that the incidence of PH is inversely related to gestational age[17], and the incidence of early onset PH can be as high as 26% among extremely preterm infants[13]. Other studies indicated a significant correlation between PH and BPD-related PH 4-7 d after birth[7,18].
The gold standard for PH diagnosis is cardiac catheterization[5]. However, it is rarely used in preterm infants because it is invasive and difficult to perform in preterm infants. Transthoracic echocardiography is the most commonly used modality for the clinical diagnosis of PH in preterm infants since it is noninvasive and easy to perform at the bedside. In this case, the diagnosis of PH was mainly based on echocardiography. Point-of-care ultrasound screening is recommended as a helpful modality for the assessment of PH in neonates and children[19]. High-risk factors for early PH include oligohydramnios, premature rupture of membranes, and infection[20]. Therefore, the strategy of our neonatal intensive care unit is to conduct routine echocardiography in ELBW infants at 3 and 7 d after birth. Our patient developed hypoxemia 3 d after birth with PPHN, which was probably caused by the high risk of PPHN during pregnancy. When the gestational age was corrected to 36 wk, echocardiography showed PH, which indicated that PH within 1 wk after birth was a risk factor for BPD-related PH.
In this case, changes in the pulmonary artery pressure were monitored using echocardiography. In addition, we measured the level of serum BNP in infants. BNP is an amino acid hormone that is synthesized and released from cardiomyocytes in response to ventricular pressure and volume changes. It is a valuable biomarker for predicting ventricular function and prognosis in patients with suspected heart failure[21]. A prospective observational case-control study including 60 preterm infants reported elevated BNP levels in infants with severe BPD and speculated that BNP level is a marker associated with the severity of BPD[22]. BNP has also been suggested as a useful biomarker for PH in preterm infants with BPD. Kandasamy[23] retrospectively analyzed the BNP levels in 25 extremely preterm infants with BPD. The median BNP levels of infants with PH, based on echocardiographic diagnosis, were higher than those of infants without PH. Their research suggested that BNP is a useful predictor in the screening of PH in preterm infants with BPD. A threshold BNP value of 117 pg/mL, with 93.8% sensitivity and 100% specificity, was identified for predicting PH[23]. Another retrospective cohort study involving 36 ELBW infants with BPD and PH showed that the peak BNP level was much lower in surviving infants than in those who did not, which suggests that BNP can predict the mortality of ELBW infants with BPD-associated PH. A threshold BNP value of 220 pg/mL with 90% sensitivity and 65% specificity was identified for predicting the mortality of ELBW with BPD and PH[24]. König et al[25] conducted a prospective observational cohort study of 83 extremely preterm infants with BPD born at less than 28 wk gestational age and survived to 36 wk corrected gestational age. A combined echocardiogram and serum BNP test revealed that the serum BNP level was significantly higher in infants with tricuspid regurgitation, indicating a potential role of BNP as a valuable biomarker for PH in infants with BPD[25]. A meta-analysis revealed the diagnostic accuracy of BNP for diagnosing and monitoring BPD-associated PH with a sensitivity of 0.94 [95% confidence interval (CI): 0.70-1.00] and specificity of 1.00 (95%CI: 0.66-1.00)[26]. Therefore, BNP has emerged as a predictive and prognostic biomarker of PH in infants. In this case, the serial changes in BNP levels detected in the infant correlated well with the respiratory condition and pulmonary arterial pressure detected by echocardiography.
PH-targeted therapy is currently focused on three main molecular pathways: The nitric oxide, endothelin, and prostacyclin pathways. The lack of related clinical trials and therapies for children with PH is mostly based on adult PH treatments. Moreover, there is no effective and safe treatment for PH due to BPD in infants[5]. Although data from randomized clinical trials did not suggest the routine use of early iNO to prevent BPD, and early iNO is not a Food and Drug Administration-approved treatment, the administration of iNO for preterm infants with early hypoxic respiratory failure caused by PPHN has been proved to be successful in several cases. Based on these reports and expert opinions, the American Heart Association and American Thoracic Society published guidelines for the treatment of pediatric PH in 2015, which recommend the use of iNO for preterm infants with severe hypoxemia mainly owing to PPHN physiology, especially in the case of suspected preterm premature rupture of membranes and oligohydramnios[8]. Although iNO has no significant effect on reducing the incidence of BPD[27,28], studies have shown that iNO may improve oxygenation and hemodynamics in PPHN[29] or BPD-related PH crisis[30]. We have used iNO to treat children with PPHN in the early stage and PH crisis in the late stage and achieved good results. However, the disadvantages of iNO are the requirement for intubation and its high cost[3]. We performed iNO on postnatal day 3 for 10 d at 10 ppm, and from day 164, the respiratory condition of this infant improved with a decrease in BNP levels. The duration of iNO in this case was approximately 2 wk with a peak NO ppm of 40. The dosing of nitric oxide for the treatment of PH crisis varies among different studies. Experts have suggested 10-20 ppm of nitric oxide for the treatment of acute exacerbation of BPD-associated PH, including a PH crisis[31,32]. Lower doses of iNO (< 10 ppm) are helpful for improving gas exchange. There may be no additional benefits for PH when the doses of iNO > 20 ppm. Oxygenation or other outcomes will not be enhanced, and the risk of methemoglobinemia and other complications will be increased when the dose of iNO is over 20 ppm.
Several vasodilator medications were used in this case. Sildenafil, a selective phosphodiesterase type 5 (PDE5) inhibitor, can induce vascular relaxation. It is effective in the treatment of children and adults with idiopathic PH. Sildenafil is considered a first-line medicine for the treatment of children with PH[33]. In infants with BPD, the addition of sildenafil is also believed to be beneficial in managing PH. A considerable dose is up to 2 mg/kg every 6 h[9]. Wardle and Tulloh[34] suggested that the dose of sildenafil for neonates is started at 250-500 μg/kg every 4-6 h with a maximum dose of 30 mg/d[34]. A retrospective study involving 22 infants diagnosed with BPD with a mean gestational age of 25.6 wk and birth weight of 631 g revealed that sildenafil administration was well tolerated and was associated with an improvement in echocardiographic markers of PH[35]. A similar finding was also reported in a retrospective review of 23 premature infants with PH associated with BPD treated with sildenafil (mean gestational age 26 wk and birth weight 710 g), in which sildenafil improved the echocardiographic index[36]. Caputo et al[37] reported on the long-term (12 mo) treatment with sildenafil in an extremely preterm infant with a gestational age of 23 wk and birth weight of 520 g, which completely resolved PH. We added sildenafil at an initial dose of 1 mg/kg per day to our patient 112 d after the echocardiogram revealed PH (the estimated pulmonary artery pressure was 47 mmHg). However, sildenafil did not perform well in the present case. The pulmonary artery pressure detected by echocardiography increased during the period of sildenafil addition (from 47 to 63 mmHg). During the period of severe PH crisis, we upregulated the dose of sildenafil to 8 mg/kg per day; however, it did not improve the pulmonary condition and did not promote iNO downregulation. Therefore, in this case, we replaced sildenafil with tadalafil, another selective PDE5 inhibitor. Compared to sildenafil, tadalafil has a longer half-life and higher oral bioavailability[38]. Tadalafil is reported to be more effective and well tolerated than sildenafil in the treatment of children aged 2 mo to 5 years with PH[39]. Another observational study involving 391 children with PH confirmed the effectiveness and safety of tadalafil[40]. Recently, a phase-3 international randomized controlled trial compared the effect of tadalafil with placebo in pediatric patients with PH who received an endothelin receptor antagonist, including bosentan or ambrisentan[12]. The results showed a positive trend in improvement in cardiopulmonary function indexes, such as 6-min walk distance, NT-Pro-BNP, World Health Organization functional class for Period 1, and echocardiographic parameters in the tadalafil treatment group without significant side effects. Despite this, clinical research on the application of tadalafil in preterm infants with PH related to BPD is rare. Further research is required to investigate the effects of tadalafil in preterm infants.
Another oral medicine added in this case was bosentan, an endothelin receptor antagonist, which is widely and effectively used in adults with PH. In children and infants, bosentan is mainly used to treat PH associated with congenital heart disease and idiopathic or heritable PH[10,41,42]. In a randomized, double-blind, placebo-controlled, prospective study of PPHN in newborns, bosentan performed effectively and safely in the treatment of PPHN[43]. But little is known about the efficacy of bosentan in PH in infants with BPD. Rugolotto et al[44] reported the case of a 5-mo-old infant who presented with PH secondary to severe BPD. PH aggravated at 8 mo of age, and high-frequency oscillatory ventilation (HFOV) and iNO were used to maintain oxygenation. They found that the addition of epoprostenol and bosentan improved the oxygenation index, promoted the discontinuation of HFOV and iNO, and finally decreased the systolic right ventricular pressure. However, severe PH recrudesced after replacing epoprostenol and bosentan with sildenafil[44]. Although studies of bosentan in neonatal PH are limited, it is also suggested as a chronic therapy in infants with PH-associated BPD[45]. In our patient, iNO combined with tadalafil and bosentan showed a good therapeutic effect in improving the respiratory condition and reducing pulmonary arterial pressure in infants with PH related to BPD.
A combination of different drugs in the treatment of PH may be beneficial with an additive effect[45]. In adults, the combination of drugs from different biological pathways can improve the morbidity and mortality of pulmonary arterial hypertension[46]. In a recent Bayesian network meta-analysis about the efficacy and safety of different drugs in the treatment of pulmonary arterial hypertension in adults, it is concluded that a combination of PDE5 inhibitors, such as sildenafil and tadalafil, with endothelin receptor antagonists, such as bosentan, is a better therapy for pulmonary arterial hypertension[47]. However, successful treatment for cases of BPD-associated PH in infants with tadalafil combined with bosentan is rare and needs further clinical trials.
Side effects of tadalafil and bosentan were monitored during the treatment’s duration. Neither clinical adverse events, including hypotension, bleeding events, gastric intolerance, skin rashes, and respiratory infection, nor the laboratory examinations, including complete blood count, liver enzymes, serum bilirubin, alkaline phosphatase, serum creatinine, and serum electrolytes, revealed any abnormal findings.
The current guidelines recommend cardiac catheterization if a second drug needs to be added to treat PH or if the child has ventricular dysfunction[5]. We did not perform cardiac catheterization on this baby because the child's parents refused this procedure.
PH is difficult to manage as a serious complication of BPD in preterm infants. Mortality is high, especially when it is complicated by heart failure. Valuable and noninvasive biomarkers, such as BNP, should be routinely measured during hospitalization to evaluate the risk and prognosis of BPD-associated PH in preterm infants. Comprehensive treatment involving iNO combined with vasodilators, including tadalafil and bosentan, may be effective. A prospective study involving a large sample size is needed to validate the efficacy and safety of these treatments.
We are thankful to the patients’ parents for providing data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Deshwal H, United States; Pop TL, Romania; Sabetian S, Iran S-Editor: Liu XF L-Editor: Filipodia P-Editor: Liu XF
| 1. | Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Hwang JS, Rehan VK. Recent Advances in Bronchopulmonary Dysplasia: Pathophysiology, Prevention, and Treatment. Lung. 2018;196:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Bui CB, Pang MA, Sehgal A, Theda C, Lao JC, Berger PJ, Nold MF, Nold-Petry CA. Pulmonary hypertension associated with bronchopulmonary dysplasia in preterm infants. J Reprod Immunol. 2017;124:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33:553-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, Ivy DD, Berger RMF. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 390] [Article Influence: 65.0] [Reference Citation Analysis (1)] |
| 6. | Seki K, Iwashima S, Uchiyama H, Ohishi A, Ishikawa T. Successful Management of Pulmonary Arterial Hypertension by Monitoring N-Terminal Pro-B-Type Natriuretic Peptide Serum Levels in a Preterm Infant with Chronic Lung Disease: A Case Report. AJP Rep. 2019;9:e133-e137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 402] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 8. | Abman SH, Ivy DD, Archer SL, Wilson K; AHA/ATS Joint Guidelines for Pediatric Pulmonary Hypertension Committee. Executive Summary of the American Heart Association and American Thoracic Society Joint Guidelines for Pediatric Pulmonary Hypertension. Am J Respir Crit Care Med. 2016;194:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Herbert S, Tulloh R. Sildenafil, pulmonary hypertension and bronchopulmonary dysplasia. Early Hum Dev. 2016;102:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Carter NJ, Keating GM. Bosentan: in pediatric patients with pulmonary arterial hypertension. Paediatr Drugs. 2010;12:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, Budts W, D'Alto M, Gatzoulis MA, Hasan BS, Kozlik-Feldmann R, Kumar RK, Lammers AE, Latus H, Michel-Behnke I, Miera O, Morrell NW, Pieles G, Quandt D, Sallmon H, Schranz D, Tran-Lundmark K, Tulloh RMR, Warnecke G, Wåhlander H, Weber SC, Zartner P. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 12. | Ivy D, Bonnet D, Berger RMF, Meyer GMB, Baygani S, Li B; LVHV Study Group. Efficacy and safety of tadalafil in a pediatric population with pulmonary arterial hypertension: phase 3 randomized, double-blind placebo-controlled study. Pulm Circ. 2021;11:20458940211024955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Arjaans S, Zwart EAH, Roofthooft M, Kooi EMW, Bos AF, Berger RMF. Pulmonary hypertension in extremely preterm infants: a call to standardize echocardiographic screening and follow-up policy. Eur J Pediatr. 2021;180:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hansmann G, Sallmon H, Roehr CC, Kourembanas S, Austin ED, Koestenberger M; European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr Res. 2021;89:446-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 15. | Altit G, Bhombal S, Hopper RK, Tacy TA, Feinstein J. Death or resolution: the "natural history" of pulmonary hypertension in bronchopulmonary dysplasia. J Perinatol. 2019;39:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Malloy KW, Austin ED. Pulmonary hypertension in the child with bronchopulmonary dysplasia. Pediatr Pulmonol. 2021;56:3546-3556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Nakanishi H, Suenaga H, Uchiyama A, Kusuda S; Neonatal Research Network, Japan. Persistent pulmonary hypertension of the newborn in extremely preterm infants: a Japanese cohort study. Arch Dis Child Fetal Neonatal Ed. 2018;103:F554-F561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, Poindexter BB, Ingram DA, Abman SH. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 19. | Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, Sanchez-de-Toledo J, Brierley J, Colunga JM, Raffaj D, Da Cruz E, Durand P, Kenderessy P, Lang HJ, Nishisaki A, Kneyber MC, Tissieres P, Conlon TW, De Luca D. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 20. | Mandell E, Kinsella JP, Abman SH. Persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2021;56:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | De Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 756] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 22. | Kalra VK, Aggarwal S, Arora P, Natarajan G. B-type natriuretic peptide levels in preterm neonates with bronchopulmonary dysplasia: a marker of severity? Pediatr Pulmonol. 2014;49:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Kandasamy J. B-type natriuretic peptide is a biomarker for pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Res Rep Neonatol. 2013;3:33-36. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Cuna A, Kandasamy J, Sims B. B-type natriuretic peptide and mortality in extremely low birth weight infants with pulmonary hypertension: a retrospective cohort analysis. BMC Pediatr. 2014;14:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | König K, Guy KJ, Nold-Petry CA, Barfield CP, Walsh G, Drew SM, Veldman A, Nold MF, Casalaz DM. BNP, troponin I, and YKL-40 as screening markers in extremely preterm infants at risk for pulmonary hypertension associated with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1076-L1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Xiong T, Kulkarni M, Gokulakrishnan G, Shivanna B, Pammi M. Natriuretic peptides in bronchopulmonary dysplasia: a systematic review. J Perinatol. 2020;40:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2017;1:CD000509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Kinsella JP, Steinhorn RH, Krishnan US, Feinstein JA, Adatia I, Austin ED, Rosenzweig EB, Everett AD, Fineman JR, Hanna BD, Hopper RK, Humpl T, Ivy DD, Keller RL, Mullen MP, Raj JU, Wessel DL, Abman SH. Recommendations for the Use of Inhaled Nitric Oxide Therapy in Premature Newborns with Severe Pulmonary Hypertension. J Pediatr. 2016;170:312-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Hansmann G, Apitz C. Treatment of children with pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102 Suppl 2:ii67-ii85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, Hanna B, Romer L, Keller RL, Fineman J, Steinhorn R, Kinsella JP, Ivy DD, Rosenzweig EB, Raj U, Humpl T, Abman SH; Pediatric Pulmonary Hypertension Network (PPHNet). Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J Pediatr. 2017;188:24-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 33. | Cohen JL, Nees SN, Valencia GA, Rosenzweig EB, Krishnan US. Sildenafil Use in Children with Pulmonary Hypertension. J Pediatr. 2019;205:29-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Wardle AJ, Tulloh RM. Paediatric pulmonary hypertension and sildenafil: current practice and controversies. Arch Dis Child Educ Pract Ed. 2013;98:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Tan K, Krishnamurthy MB, O'Heney JL, Paul E, Sehgal A. Sildenafil therapy in bronchopulmonary dysplasia-associated pulmonary hypertension: a retrospective study of efficacy and safety. Eur J Pediatr. 2015;174:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Trottier-Boucher MN, Lapointe A, Malo J, Fournier A, Raboisson MJ, Martin B, Moussa A. Sildenafil for the Treatment of Pulmonary Arterial Hypertension in Infants with Bronchopulmonary Dysplasia. Pediatr Cardiol. 2015;36:1255-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Caputo S, Furcolo G, Rabuano R, Basilicata AM, Pilla LM, De Simone A, Pasquariello B, Ciampi Q, Vetrano G, Villari B. Severe pulmonary arterial hypertension in a very premature baby with bronchopulmonary dysplasia: normalization with long-term sildenafil. J Cardiovasc Med (Hagerstown). 2010;11:704-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Zhang W, Wu YE, Yang XY, Shi J, van den Anker J, Song LL, Zhao W. Oral drugs used to treat persistent pulmonary hypertension of the newborn. Expert Rev Clin Pharmacol. 2020;13:1295-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Shiva A, Shiran M, Rafati M, Zamani H, Babazadeh K, Saeedi M, Ala S. Oral Tadalafil in Children with Pulmonary Arterial Hypertension. Drug Res (Stuttg). 2016;66:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Yamazaki H, Kobayashi N, Taketsuna M, Tajima K, Suzuki N, Murakami M. Safety and effectiveness of tadalafil in pediatric patients with pulmonary arterial hypertension: a sub-group analysis based on Japan post-marketing surveillance. Curr Med Res Opin. 2017;33:2241-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Kuang HY, Wu YH, Yi QJ, Tian J, Wu C, Shou WN, Lu TW. The efficiency of endothelin receptor antagonist bosentan for pulmonary arterial hypertension associated with congenital heart disease: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e0075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | He J, Ren Y, Chen Y, Feng Y. Bosentan treatment for pulmonary arterial hypertension due to patent ductus arteriosus and Down's syndrome in an infant. Int J Cardiol. 2014;176:e117-e118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. 2012;32:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Rugolotto S, Errico G, Beghini R, Ilic S, Richelli C, Padovani EM. Weaning of epoprostenol in a small infant receiving concomitant bosentan for severe pulmonary arterial hypertension secondary to bronchopulmonary dysplasia. Minerva Pediatr. 2006;58:491-494. [PubMed] |
| 45. | Lakshminrusimha S, Mathew B, Leach CL. Pharmacologic strategies in neonatal pulmonary hypertension other than nitric oxide. Semin Perinatol. 2016;40:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Ruopp NF, Cockrill BA. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA. 2022;327:1379-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 333] [Article Influence: 111.0] [Reference Citation Analysis (2)] |
| 47. | Fu W, He W, Li Y, Chen Y, Liang J, Lei H, Fu L, Ren N, Jiang Q, Shen Y, Ma R, Wang T, Wang X, Zhang N, Xiao D, Liu C. Efficacy and safety of novel-targeted drugs in the treatment of pulmonary arterial hypertension: a Bayesian network meta-analysis. Drug Deliv. 2021;28:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |