Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11861
Peer-review started: May 29, 2022
First decision: August 21, 2022
Revised: September 1, 2022
Accepted: October 13, 2022
Article in press: October 13, 2022
Published online: November 16, 2022
Processing time: 162 Days and 18.8 Hours
Cardiac arrest after noncardiac surgery is a dangerous complication that may contribute to mortality. Because of the high mortality rate and many complications of cardiac arrest, it is very important to identify and correct a reversible etiology early. By reporting the treatment process of this case, we aimed to bro
A 69-year-old man visited our hospital complaining of low back pain on July 12, 2021. Magnetic resonance imaging showed lumbar disc herniation. Two hours after lumbar disc herniation surgery, the patient developed cardiac arrest. Cardiopulmonary resuscitation was performed, and ECMO was started 60 min after the initiation of cardiopulmonary resuscitation. Regarding the etiology of early cardiac arrest after surgery, acute myocardial infarction and pulmonary embolism were considered first. Based on ultrasound evaluation, acute myo
For early postoperative cardiac arrest, acute myocardial infarction should be considered first, and heparin should be used with caution.
Core Tip: Cardiac arrest after noncardiac surgery has high mortality and many complications. Therefore, it is important to identify and correct a reversible etiology early. We treated a 69-year-old patient who developed cardiac arrest 2 h after lumbar disc herniation surgery. Coronary angiography under extracorporeal membrane oxygenation was performed 1 h after cardiopulmonary resuscitation, and coronary artery stenting was performed after confirming occlusion of the left anterior descending branch. The patient was finally discharged in good clinical condition. This case showed that acute myocardial infarction should be considered first, and heparin should be used cautiously in patients with early postoperative cardiac arrest.
- Citation: Wang QQ, Jiang Y, Zhu JG, Zhang LW, Tong HJ, Shen P. Survival of a patient who received extracorporeal membrane oxygenation due to postoperative myocardial infarction: A case report. World J Clin Cases 2022; 10(32): 11861-11868
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11861.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11861
Postoperative myocardial infarction (POMI) after noncardiac surgery is a dangerous complication that occurs in 1%-4% of patients[1]. POMI leading to cardiac arrest is very serious and greatly affects the prognosis. Cardiopulmonary resuscitation (CPR) using extracorporeal membrane oxygenation (ECMO) is performed for patients with cardiac arrest deemed to be refractory to CPR. This case report describes a patient who developed cardiac arrest 2 h after lumbar intervertebral disc surgery and underwent coronary stent implantation with the support of ECMO.
A 69-year-old man visited our hospital complaining of low back pain on July 12, 2021. The symptoms had been present for 10 d.
The patient developed lumbar pain 10 d ago, and the pain radiated to the right hip, the posterior aspect of the right thigh, and the posterior aspect of the calf. The pain was relieved by rest and aggravated by exertion. The patient was unable to walk normally.
The patient had no previous medical history.
The patient’s vital signs were stable. He had lumbar tenderness, with obvious gaps between L4/5 and L5/S1. The right lower limb straight leg raising test was positive, while the bilateral femoral nerve pull test was negative. The bilateral quadriceps femoris muscle strength was normal, but the right great toe extensor muscle strength was weakened. The knee reflexes were normal, but the bilateral ankle reflexes were not elicited. Physiological reflexes were present, while pathological reflexes were not elicited. All other signs were negative.
The laboratory tests showed no abnormalities.
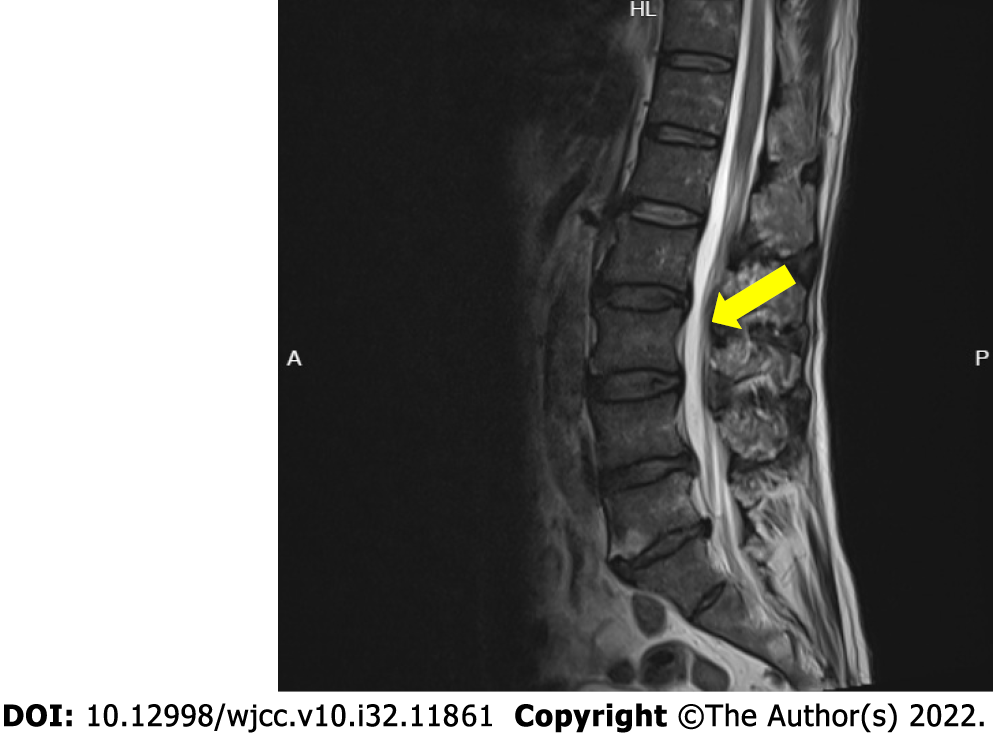
Magnetic resonance imaging showed L3/4, L4/5, and L5/S1 disc herniations (Figure 1).
After excluding surgical contraindications, lumbar fusion + pedicle screw internal fixation + lumbar discectomy with laminectomy + nerve root canal lysis were performed via the posterolateral approach at 15:20 on July 19, 2021. The duration of the whole operation was 110 min, with stable intraoperative vital signs and blood loss volume of 10 mL. At 19:15, the patient developed cardiac arrest in the resuscitation room. He had no palpable carotid or femoral pulse, and no spontaneous respiration. At 19:35, the patient still had not recovered a spontaneous heart rate, so the ECMO team was immediately called. Venoarterial ECMO was started 60 min after the initiation of CPR; the return of spontaneous circulation was 15 min later. A total of 6250 U of heparin was used during the procedure. Multidisciplinary collaboration discussion considered acute myocardial infarction as a possible cause of cardiac arrest, but pulmonary embolism could not be ruled out. At 22:00, percutaneous coronary intervention (PCI) was performed to investigate the cause of the cardiac arrest.
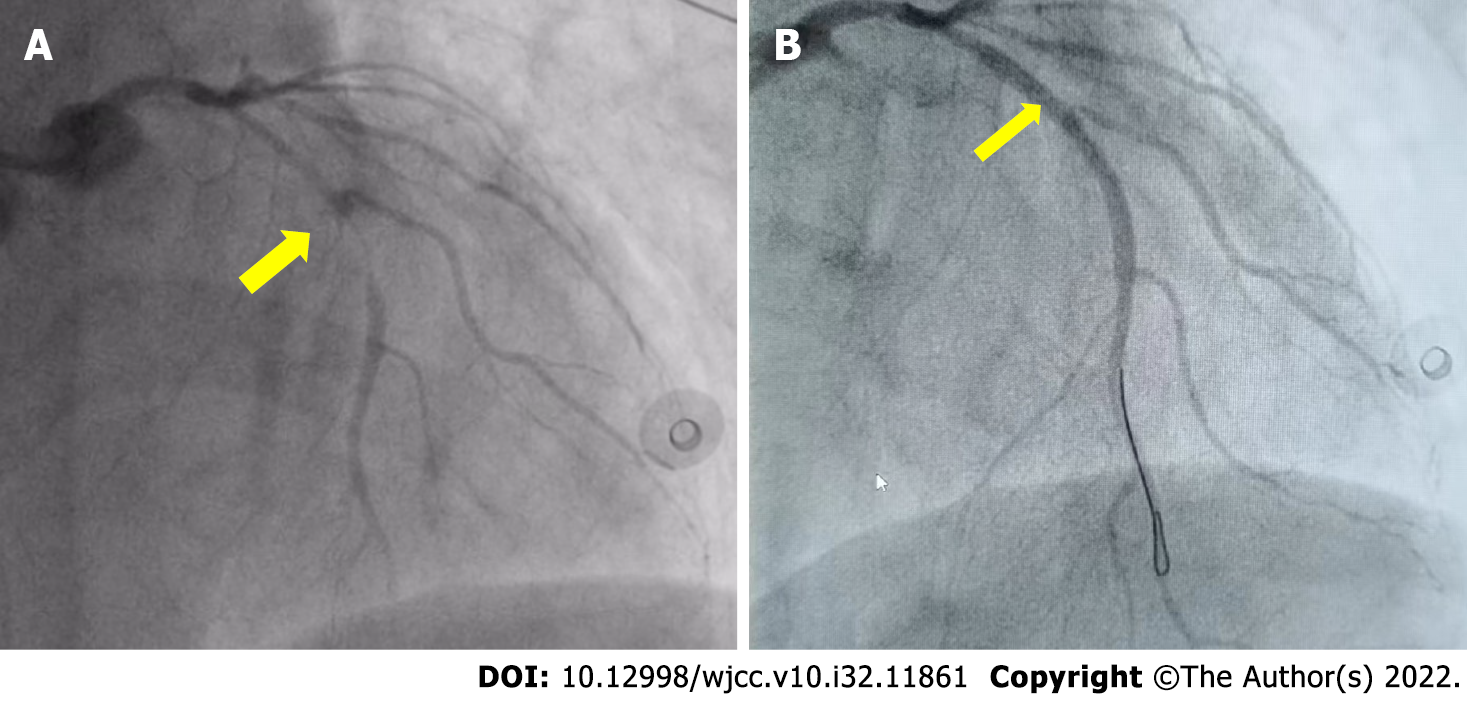
We confirmed subtotal occlusion of the middle part of the left anterior descending artery (Figure 2A) and performed PCI of the left anterior descending artery.
At 22:50, we confirmed partial recovery of the coronary circulation to the mid-left anterior descending artery (Figure 2B). Subsequently, pulmonary angiography was performed. The results showed that both pulmonary arteries were unobstructed, and pulmonary embolism was excluded. A total of 2500 U of heparin was administered during the operation. At 23:25, the patient was transferred to the Intensive Care Unit (ICU) and treated with vasoactive drugs, mild hypothermia, continuous renal replacement therapy, etc (Figure 3).
Echocardiography revealed akinesia of the mid-anteroseptal and anterior left ventricle, with severe left ventricle failure (ejection fraction 20%). The troponin I concentration was greater than 80 ng/mL. Due to the two previous administrations of heparin, the activated clotting time (ACT) was increased (479 s) when he was admitted to the ICU, and the negative pressure ball drainage rate of the incision was about 300 mL/h in the first 2 h. Even after the infusion of 5.5 U of suspended red blood cells, the hemoglobin concentration still decreased from 97 g/L after orthopedic surgery to 77 g/L. Therefore, we chose to use non-heparinized ECMO at that time. Due to insufficient blood volume, the ECMO flow was only 1.6-2.0 L/min, and the doses of noradrenaline and epinephrine were as high as 0.36 and 0.73 µg/kg/min, respectively. Blood gas analysis suggested obvious metabolic acidosis, with a lactic acid concentration of 14.8 mmol/L. During this period, repeated blood transfusion and coagulation correction were performed.
Four hours later (at 04:00 on July 20, 2021), the ACT decreased to 180 s, the incision negative pressure ball drainage rate slowed to less than 100 mL/h, and the hemoglobin concentration increased to 88 g/L. The ECMO flow was increased to 3 L/min, and the noradrenaline and epinephrine doses were gradually decreased. The lactic acid concentration showed a downward trend.
After 10 h (at 22:00 on July 20, 2021), the incision negative pressure bulb was almost without drainage, the hemoglobin concentration rose to 105 g/L, and the ACT decreased to 162 s. Therefore, we started anticoagulation with heparin, and the ACT target value was set as 160-180s. The total drainage volume of the postoperative incision negative pressure bulb was about 1000 mL, and the total infusion was 13 U of suspended red blood cells, 2700 mL of plasma, and 10 U of cryoprecipitate. On ICU day 6, the patient’s left ventricle function was restored (ejection fraction 62%). As the patient had made a full neurologic recovery and his general condition had been restored, he was weaned off ECMO.
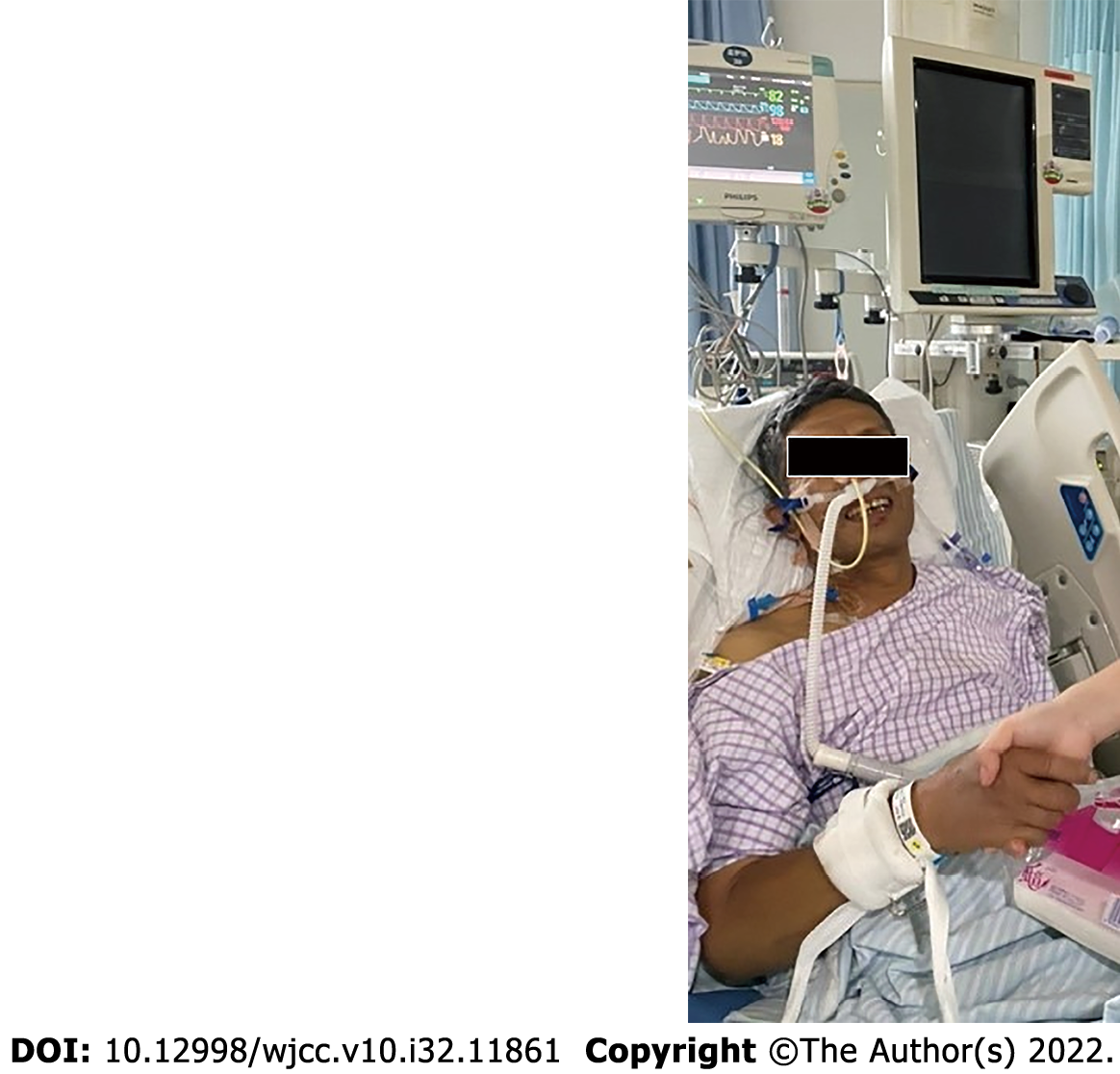
On ICU day 8, the patient’s tracheal intubation was successfully removed (Figure 4). He was transferred to the Department of Cardiology on ICU day 11 and discharged in good clinical condition 5 d later (Table 1).
| Date | Time | Event |
| July 12, 2021 | Admission to hospital | |
| July 12, 2021 | 15:20 | Posterolateral approach lumbar fusion + pedicle screw internal fixation + lumbar discectomy with laminectomy + nerve root canal lysis |
| 19:10 | End of the surgery | |
| 19:15 | Cardiac arrest | |
| 19:35 | Call on ECMO team | |
| 19:45 | ECMO team arrives at the scene | |
| 20:15 | VA ECMO | |
| 20:30 | Transferred to ICU | |
| 22:00-22:50 | PCI of the LAD | |
| 22:50-23:00 | Pulmonary angiography | |
| 23:25 | Returned to ICU | |
| July 19 -20, 2021 | 23:25-00:00 | ACT was 479, non-heparinized ECMO chosen |
| July 20, 2021 | 00:00-01:00 | The negative pressure ball drainage of the incision was about 300 mL; hemoglobin was 97 g/L |
| 01:00-02:00 | The negative pressure ball drainage of the incision was about 300 mL; hemoglobin was 77 g/L | |
| 02:00-03:00 | The negative pressure ball drainage of the incision was about 200 mL | |
| 03:00-04:00 | ACT was 180; the negative pressure ball drainage of the incision was about 100 mL; hemoglobin was 88 g/L | |
| 04:00-10:00 | ACT was 162; the negative pressure ball drainage rate of the incision gradually decreased; hemoglobin was 105 g/L | |
| 10:00 | Heparinized ECMO; the ACT target was 160-180 | |
| 00:00-10:00 | Repeated blood transfusion | |
| July 25, 2021 | Weaned off of ECMO | |
| July 27, 2021 | Extubated tracheal intubation | |
| July 30, 2021 | Transferred to the Department of Cardiology | |
| August 4, 2021 | Discharged |
Currently, postoperative adverse cardiovascular events are still the leading cause of morbidity and mortality after noncardiac surgery, resulting in an in-hospital mortality of 15%–25%[1-4]. Given that the annual worldwide number of adults undergoing major noncardiac surgery is 200 million, at least 2-8 million of these patients will likely develop POMI, making this a substantial public health problem[5]. Different causes of POMI require different treatments. Therefore, it is important to identify and correct a reversible etiology as soon as possible. In this case, the patient developed sudden cardiac arrest with unknown etiology 2 h after lumbar disc herniation surgery. Postoperative adverse cardiovascular events, including acute myocardial infarction and malignant arrhythmia, should be considered first. Therefore, we used ECMO to perform coronary angiography, confirmed POMI, and treated this with PCI. We excluded pulmonary embolism at the same time.
It is generally believed that plaque rupture and myocardial oxygen supply-demand imbalance contribute approximately equally to the risk of perioperative myocardial infarction. However, post-mortem studies have found that only 7% of patients who died had plaque rupture, and there was no evidence of plaque rupture in 83% of patients who died within the first 3 d postoperatively. Thus, the imbalance of oxygen supply and demand in the early postoperative period may be the cause of fatal POMI[6], which may be related to pain and sympathetic nerve stimulation. Based on the above factors, we considered that the POMI in this patient was MI type 2.
In the postoperative period, there are increases in the concentrations of all coagulation factors; fibrinogen increases by 50%–100%, causing an increase in plasma viscosity, platelet aggregability, and platelet sensitivity to catecholamine[4]. Hypercoagulability is even more marked when alterations in perioperative hemodynamics and metabolism are significant. Furthermore, research has shown that inflammatory mediators can induce coagulation. A recent study showed that perioperative triggers that may contribute to perioperative myocardial ischemia include tissue trauma, fluctuations in fluid status, the way in which anesthesia is administered, preoperative fasting, airway manipulation, pain, bleeding, and body temperature[7]. These triggers may result in perioperative inflammation, hypercoagulability, hemodynamic changes, hypothermia, stress response, hypoxia, and anemia, all of which play potentially prominent roles in the pathophysiology of POMI.
Due to the high mortality of POMI, early detection is particularly important. Even though routine electrocardiogram is a potentially low-yield test, it remains a potentially valuable, low-risk, and low-cost tool used in the diagnosis of POMI. Some research recommends postoperative 12-lead electrocardiogram screening in high-risk or symptomatic patients[8]. Thus, it is important to understand the pathophysiology of POMI to reduce its incidence and increase early detection. This may ultimately result in a decrease in mortality, shorter hospitalization, decrease in medical costs, and fewer long-term cardiac complications.
According to the consensus of the Extracorporeal Life Support Professional Committee of the Chinese Medical Doctor Association: For patients with cardiac arrest in the hospital, if the routine CPR rescue continues for 10 min and still fails to restore effective spontaneous circulation, and there is no contraindication to ECMO assistance, the electrically calibrated pyroelectric radiometer rescue process can be started immediately. The patient in this case had no serious underlying diseases. He suffered sudden cardiac arrest witnessed in the operating resuscitation room and immediately underwent CPR. The etiology was reversible and was in line with the indications for electrically calibrated pyroelectric radiometer. Therefore, ECMO was given after comprehensive consideration and the consent of the family.
It is well known that bleeding is a common complication of ECMO management. According to the Extracorporeal Life Support Organization database report, the incidence of hemorrhage is 14%, 11%, 6%, and 4% for cannulation site, surgical site, gastrointestinal bleeding, and cerebral bleeding, respectively[9]. However, there is a lack of consensus among studies about when to start anticoagulation or in which situations it is better to avoid any anticoagulation[10-13]. In this case, we were not aware of the possible high risk of postoperative bleeding and still used conventional heparin loading-dose anticoagulation during ECMO. Although we reduced the use of heparin during the PCI procedure, there was still massive bleeding at the surgical site. Therefore, heparin should be administered with caution. Recently, several reports have shown that patients can be successfully managed on heparin-free ECMO without increasing the extent of the bleeding[14-16]. These patients may benefit from the technological advancements in ECMO, including the use of more efficient membrane oxygenators, centrifugal pumps, miniaturization of circuits, and heparin-bonded circuitry, which have allowed ECMO use with little or no anticoagulation. Therefore, anticoagulation should be individually tailored, considering the severity of the trauma, the timing, and active bleeding[17].
POMI remains a serious complication after major surgery, with a considerable associated mortality rate. It is important to understand the pathophysiology of POMI to select appropriate therapies, which in turn have important public health implications. Clinicians should be cautious when selecting anticoagulation strategies for patients in the early postoperative period or those with a high risk of bleeding during ECMO therapy. Heparin should be used with caution in the early postoperative period. In cases of early postoperative cardiac arrest, the possibility of acute myocardial infarction should be considered first, and it is critical to identify and correct the cause as soon as possible.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta L, Indonesia; Kharlamov AN, Netherlands S-Editor: Liu XF L-Editor: Filipodia P-Editor: Liu XF
| 1. | House LM, Marolen KN, St Jacques PJ, McEvoy MD, Ehrenfeld JM. Surgical Apgar score is associated with myocardial injury after noncardiac surgery. J Clin Anesth. 2016;34:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | van Waes JA, Nathoe HM, de Graaff JC, Kemperman H, de Borst GJ, Peelen LM, van Klei WA; Cardiac Health After Surgery (CHASE) Investigators. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127:2264-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 3. | Yu J, Lim B, Lee Y, Park JY, Hong B, Hwang JH, Kim YK. Risk factors and outcomes of myocardial injury after non-cardiac surgery in high-risk patients who underwent radical cystectomy. Medicine (Baltimore). 2020;99:e22893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: A review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 401] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Khan J, Alonso-Coello P, Devereaux PJ. Myocardial injury after noncardiac surgery. Curr Opin Cardiol. 2014;29:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Biccard BM, Rodseth RN. The pathophysiology of peri-operative myocardial infarction. Anaesthesia. 2010;65:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Smit M, Coetzee AR, Lochner A. The pathophysiology of myocardial ischemia and perioperative myocardial infarction. J Cardiothorac Vasc Anesth. 2020;34:2501-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Longhitano S, Coriat P, Agrò F. Postoperative myocardial infarction: Pathophysiology, new diagnostic criteria, prevention. Minerva Anestesiol. 2006;72:965-983. [PubMed] |
| 9. | Conrad SA, Rycus PT, Dalton H. Extracorporeal life support registry report 2004. ASAIO J. 2005;51:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Della Torre V, Robba C, Pelosi P, Bilotta F. Extra corporeal membrane oxygenation in the critical trauma patient. Curr Opin Anaesthesiol. 2019;32:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Zonies D, Merkel M. Advanced extracorporeal therapy in trauma. Curr Opin Crit Care. 2016;22:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ried M, Bein T, Philipp A, Müller T, Graf B, Schmid C, Zonies D, Diez C, Hofmann HS. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: A 10-years institutional experience. Crit Care. 2013;17:R110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Wu MY, Chou PL, Wu TI, Lin PJ. Predictors of hospital mortality in adult trauma patients receiving extracorporeal membrane oxygenation for advanced life support: A retrospective cohort study. Scand J Trauma Resusc Emerg Med. 2018;26:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Amos T, Yeung M, Gooi J, Fitzgerald M. Survival following traumatic thoracic compartment syndrome managed with VV-ECMO. Trauma Case Rep. 2019;24:100249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Matsumoto S, Morizane M, Matsuo K, Yamazaki M, Kitano M. Pitfalls when using extracorporeal life support in trauma patients. Trauma Surg Acute Care Open. 2019;4:e000298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Louro J, Weir JJ, Brozzi NA, Dudaryk R. Treatment of refractory intraoperative hypoxemia after trauma with venovenous extracorporeal membrane oxygenation: A case report. A A Pract. 2018;11:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Wang C, Zhang L, Qin T, Xi Z, Sun L, Wu H, Li D. Extracorporeal membrane oxygenation in trauma patients: A systematic review. World J Emerg Surg. 2020;15:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |