Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11845
Peer-review started: April 13, 2022
First decision: May 30, 2022
Revised: June 18, 2022
Accepted: October 12, 2022
Article in press: October 12, 2022
Published online: November 16, 2022
Processing time: 208 Days and 13.6 Hours
Radiofrequency ablation (RFA) is gaining popularity as an additional therapy for pancreatic ductal adenocarcinoma. RFA appears to be an attractive treatment option for patients with unresectable, locally advanced and nonmetastatic pancreatic cancer.
A 60-year-old woman with 2 mo intermittent upper abdominal pains was admitted to hospital. She had undergone radical gastrectomy (Billroth II) for gastric antral cancer. Contrast-enhanced computed tomography (CECT) and abdominal ultrasound displayed a primary tumor in the neck of the pancreas. Pathological examination showed that the lesion was a pancreatic ductal ade
RFA of locally advanced, nonresectable, nonmetastatic, pancreatic tumor is characterized by feasibility-based treatment giving rise to tumor reduction based on improvement of quality of life.
Core Tip: A 60-year-old woman who had previously undergone radical gastrectomy (Billroth II) for gastric antral cancer was found to have a primary tumor on the neck of the pancreas. Pathological examination showed that the lesion was pancreatic ductal adenocarcinoma. Open approach radiofrequency ablation (RFA) was selected to treat the primary tumor. A local recurrent tumor was discovered in the follow-up contrast-enhanced computed tomography after 8 mo with the performance of another open RFA. The patient still enjoys her 9-year survival following the first RFA. It is promising that some cases still have long-term survival based on repeated open RFA.
- Citation: Zhang JY, Ding JM, Zhou Y, Jing X. Nine-year survival of a 60-year-old woman with locally advanced pancreatic cancer under repeated open approach radiofrequency ablation: A case report. World J Clin Cases 2022; 10(32): 11845-11852
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11845.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11845
Despite substantial breakthroughs in the diagnosis and treatment of pancreatic ductal adenocarcinoma (PDAC), disease-oriented oncological outcomes present a poor profile[1]. PDAC, as the fourth leading cause of cancer related death in 2016[2], is diagnosed approximate 340000 times each year worldwide[3], with a total 5-year survival rate < 10%[4]. Radical resection remains the only possibility for cure of PDAC[5]; comparatively, only < 20% of patients present with suitability for resection at diagnosis; and nearly 70% present with locally advanced or metastatic illness. The median survival of untreated patients fluctuates in the range of 3 to 4 mo; < 5% of patients survive 1 year following diagnosis[6]. No consensus has been reached upon the most appropriate treatment for patients with local advanced pancreatic cancer (LAPC). Chemotherapy and chemoradiotherapy have been applied as the most-valued frequently suggested treatments as they offer modest survival benefits for patients who have LAPC[7]. Additionally, as revealed, locally destructive illness accounts for half of the mortality of LAPC patients, despite that distant metastasis is one of the most common forms of disease progression[8], indicating the importance of local destructive therapies. Considering the limited success of present therapy in controlling the disease by prolonging the survival of LAPC patients, novel locally destructive therapies have been piloted and are considered to be increasingly important therapeutic approaches[9].
Radiofrequency ablation (RFA) is gaining popularity as an additional therapy for PDAC. RFA, a local thermal therapy, has been enjoying broad application to treat solid parenchymal tumors. At present, RFA is a welcome treatment option oriented to patients with unresectable, locally progressed, and nonmetastatic pancreatic cancer. To analyze the safety and efficacy of open RFA for LAPC, we report a patient with locally advanced pancreatic cancer treated with open RFA who achieved > 9-years’ survival.
A 60-year-old woman presented with intermittent upper abdominal discomfort lasting 2 mo.
The symptoms included postprandial fullness in the upper abdomen, dull pains in the upper abdomen, and radiating pains in the lower back. The patient, who had lost 5 kg in weight, had been experiencing poor appetite and poor sleep since the beginning of the disease.
The patient had experienced 4 years of maximum 150/90 mmHg (1 mmHg = 0.133 kPa) hypertension and coronary heart disease with no systemic treatment, presented with type II diabetes, and had been treated with metformin for 4 years. She had gone through radical gastrectomy (Billroth II) for gastric antral cancer 4 years ago, after which, she underwent up to three chemotherapy sessions; however, in the absence of known tumor recurrence or metastasis, she has enjoyed a satisfactory physical status, which has lasted for almost 4 years.
The patient had no personal and family history.
Physical examination: temperature: 36°C; pulse: 76 beats/min; blood pressure: 135/80 mmHg; respiratory rate: 18 breaths/min; no cutaneous or sclera icterus.
Laboratory findings displayed a normal total bilirubin count and conjugated bilirubin count with marked elevations in carbohydrate antigen (CA)19-9 level of 74.57 (reference range, 0–30 U/mL), CA-50 level of 111.92 (reference range, 0.6–16.3 U/mL).
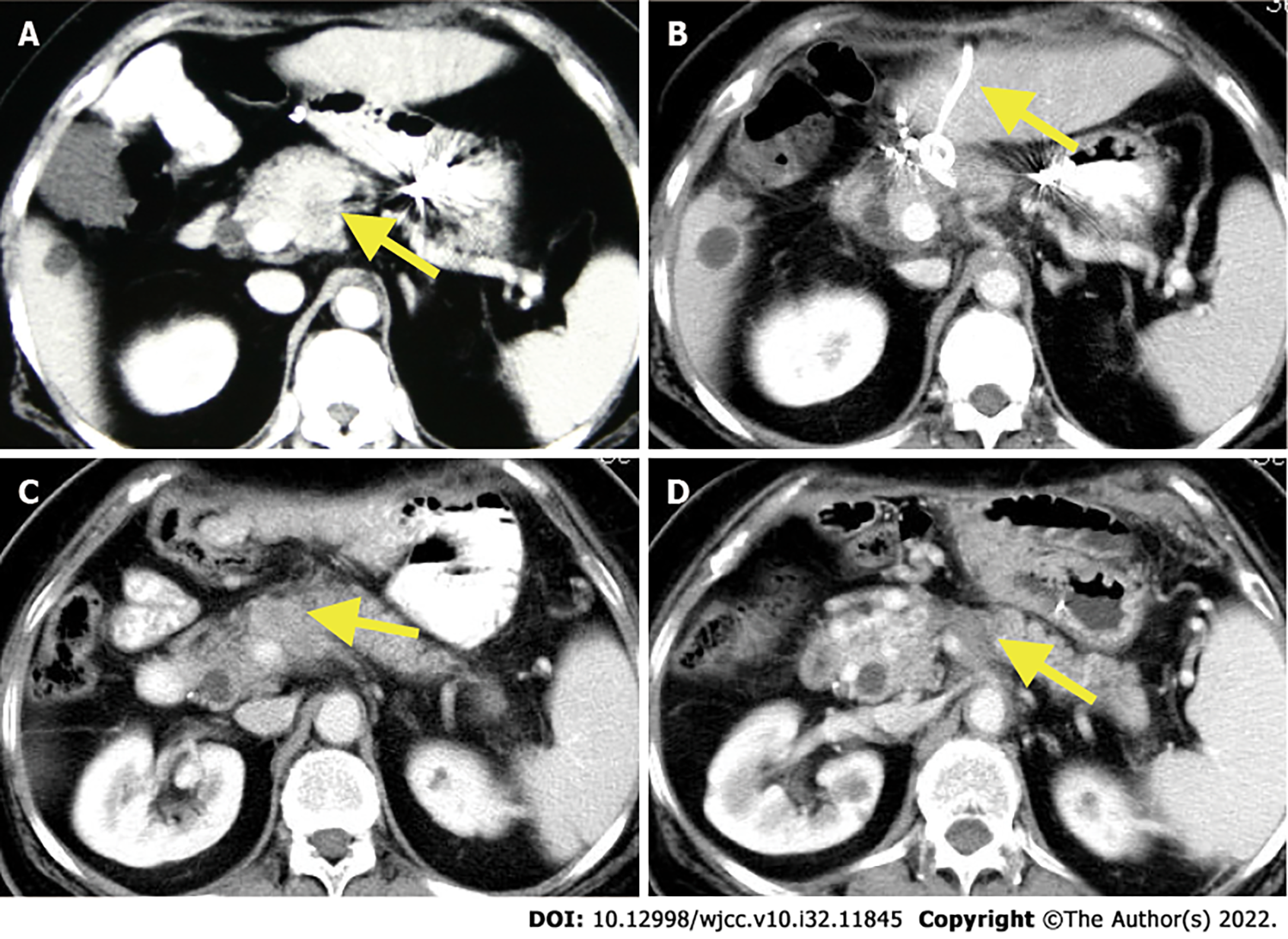
Abdominal ultrasound showed a 3.6 cm × 2.9 cm hypoechoic mass with unclear borders and irregular shape in the anterior superior part of the pancreatic neck, the diameter of the common bile duct was 0.9 cm, and gallstones with cholecystitis. According to contrast-enhanced computed tomography (CECT), heterogeneous enhancing masses were present at the neck of the pancreas, which was closely connected to the common hepatic artery (Figure 1A).
In virtue of body surface lymph node examination, chest CT and gastroscopy, gastric cancer recurrence and metastasis were ruled out. Taking into combined consideration the imaging reports and auxiliary examination, the patient was initially diagnosed with pancreatic cancer and gallstone cholecystitis. Because she was referred to our hospital that is oriented toward surgical treatment, the decision was made to perform open laparotomy.
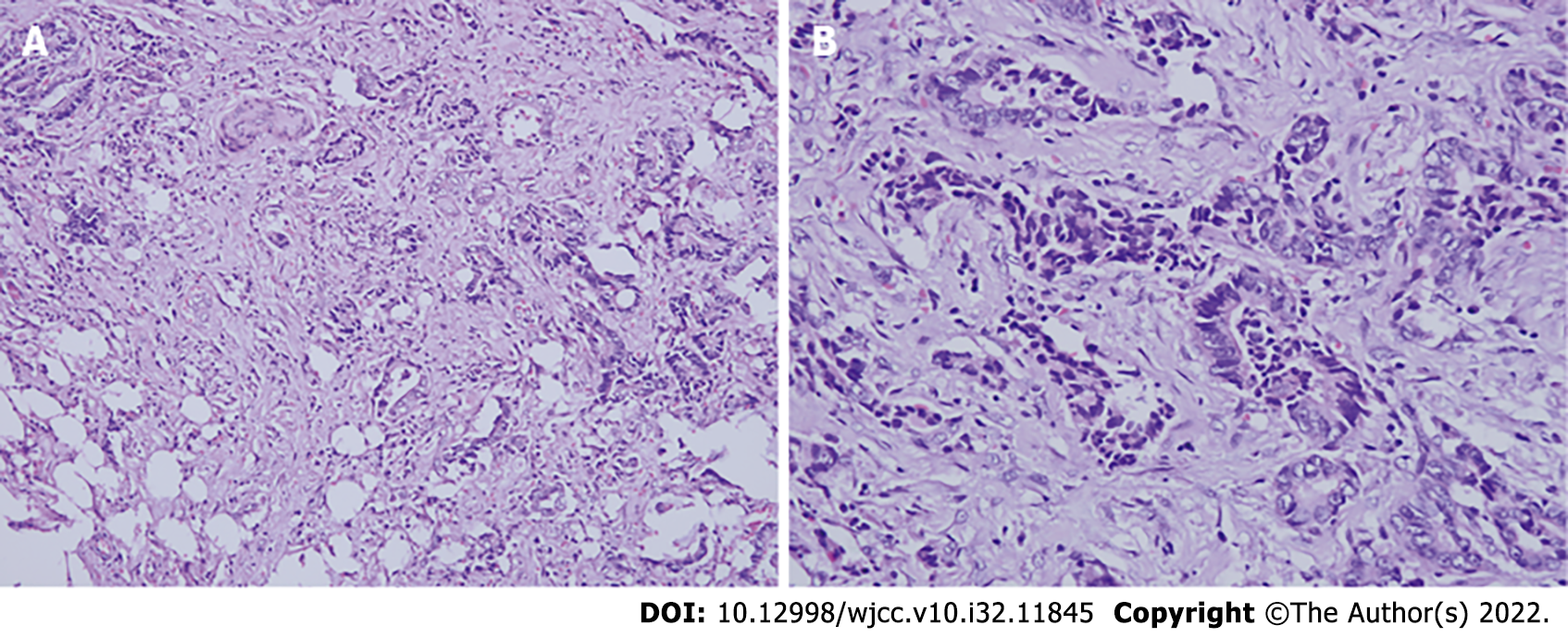
Biopsy confirmed PDAC.
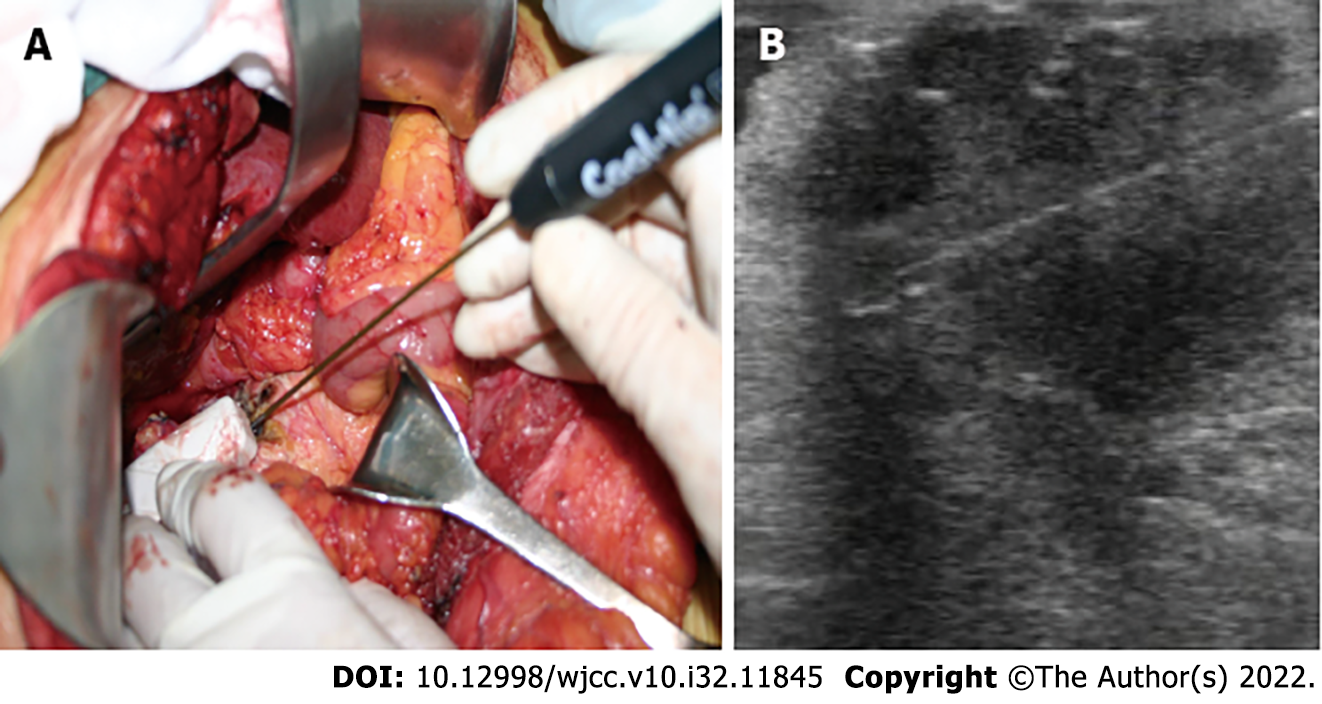
Laparotomy revealed a firm mass in the neck of the pancreas, protruding from the upper edge of the pancreas. Intraoperative ultrasound (IOUS) showed a tumor of 4.0 cm × 3.5 cm that encased and invaded the root of the common hepatic artery; biopsy confirmed the lesion as PDAC (Figure 2). In combination with the intraoperative conditions, the patient displayed no signs of recurrence of gastric cancer; therefore, we conducted RFA according to the plan, because radical resection based on revascularization was once considered impossible. Mobilization of the pancreatic head and duodenum was performed using a Kocher incision. The patient underwent radical gastrectomy and mobilization of the upper edge of the pancreas for membrane adhesions; the separation was performed at the right edge of the hepatoduodenal ligament before the surrounding tissues and organs were covered with wet gauze and the head and neck of the pancreas were completely exposed. Our group used the Cool-tip™ RFA system (Radionics, USA) along with a cooled electrode (17-gauge) (Figure 3A). The RF needle was set precisely into the tumor under IOUS (Figure 3B). The tumor was ablated starting at 40 W and the energy delivery rate was increased to 10 W/min, with 90 W as the maximum energy output. The ablation was carried out three times (6–10 min each). A tube was placed in the body of the pancreas and Winslow hole for drainage (Figure 1B) before the patient received anti-infective treatment, acid suppression, and pancreatic secretion inhibition.
Postoperative day 11 witnessed the patient developing complications of pancreatic fistula. Moreover, the abdominal drainage tube continuously drained clear pancreatic fluid with a maximum volume of nearly 500 mL/d. The patient was treated while maintaining drainage patency, including supplemental percutaneous transhepatic tube drainage, food fasting in addition to water, and parenteral nutrition. The patient, who had a smooth recovery, was allowed to be discharged with a drainage tube at 44 d following the procedure. No sign of tumor recurrence could be found in the 1-mo follow-up CECT.
Around 8 mo after surgery, follow-up CECT revealed a local recurrent tumor in the body of the pancreas along with splenic vein involvement (Figure 1C). The patient was in good general condition with no distant metastasis of the tumor but underwent further laparotomy RFA on November 17, 2013. Guided by IOUS, the RF needle was precisely placed into the tumor and the manual RF conditions were set as previously. The RFA was performed at two points (8 and 6 min, respectively). Following the operation, the patient developed a pancreatic fistula with no obstruction of the drainage tube. Intraperitoneal hemorrhage occurred, which was stopped by hemostasis and alleviated by interventional therapy. The abdominal drainage had a gradual decrease without discomfort following removal of the drainage tube.
The patient was recovering and discharged from the hospital as well as being in good health for 108 mo postoperatively with no signs of tumor recurrence (Figure 1D).
Recent years have seen the success of several ablation treatment approaches for tumor mass reduction in patients with locally advanced pancreatic cancer with no distant metastases, including RFA, microwave ablation (MWA), cryotherapy, irreversible electroporation (IRE, NanoKnife®), high-intensity focused ultrasound (HIFU), and stereotactic radiation therapy (Gamma-Knife®, CyberKnife®)[10-14]. These techniques consist of two main categories: thermal ablation, and nonthermal ablation causing direct damage to neoplastic cells. The therapeutic effects exerted by those palliative treatments are of relevance to the induction of intralesional necrosis, cytolysis, and cell death, ultimately leading to tumor cytoreduction[15]. Data in terms of the clinical application of RFA and MWA, IRE, cryoablation, and radiotherapy coupled with HIFU, show that these procedures have a relative safety profile for the (temporary) local control of inoperable pancreatic cancer[16]. Due to the thinner electrodes and the nonthermal action mechanism, IRE might be advantageous in protecting neighboring large vessels and nerves[17]. Nevertheless, the growth in tumor size has hindered effectiveness of the treatment. Meanwhile, the process has evolved into a more demanding task based on the growing electrode numbers. Hence, it has been suggested that IRE should be limited to the largest tumors with a diameter of 5.0 cm[18]. Direct comparison of the local ablation processes is currently not possible ever since the publishing of the corresponding research based on distinctively explained and unbalanced populations of patient groups and indications; the controlled comparative researches are currently not available.
According to the present case, good exposure via an open approach overcame the damage to adjacent organs such as the jejunum, colon and omentum. Spiliotis et al[19] revealed good results in their report of open RFA in five patients with inoperable pancreatic cancer. Varshney et al[20] demonstrated the feasibility and safety of intraoperative RFA in three patients with LAPC. From a technical point of view, reports can be seen on the significance of US guidance of the ablation process, which majorly occurred in the process of the needle positioning into the part of the lesion. Additionally, IOUS was able to ensure the security of ablation on the largest scale. It is worth mentioning that the complete removal of tumors around large vessels is difficult due to the cooling effect of blood flow. This case revealed that the conducts of higher power were made to defeat heat sink effect on the greatly vascularized pancreas to avoid incomplete ablation. Furthermore, the present investigations were on RFA in the open setting; in the meanwhile, larger numbers of recent researches have also reported the feasibility of minimal invasive ablation [11,21]. As a result, it is suggested that the safety of endoscopic-ultrasound guided or percutaneous RFA be applied to an in-depth investigation subject, which is meanwhile preceding the present effect research in the open setting.
The patient developed a pancreatic fistula twice after being treated, and hemorrhage after the second procedure, indicating severe symptoms of postablation complications. Considering this case, the postoperative pancreatic fistula came from the leakage of pancreatic fluid via a small pancreatic duct after RFA. Another suggestion is the importance for drainage when conducting open RFA. The occurrence of pancreatic fistula could be stopped by heating the needle, spraying protein gel onto the wounded surface, passing a drainage tube surrounding the pancreas, and somatostatin. The pancreatic fistula, resulting from RFA, could be under control in 1 mo on the basis of conservative treatment, on the condition that the main pancreatic duct is not blocked[22,23]. In general, two high-risk factors will bring about postoperative bleeding. First, the possible damage to the pancreatic duct during RFA, and second, the placement of the pancreatic duct around the tumor rupture, so that the pancreatic fluid activated during RFA treatment can erode the surrounding blood vessels. A major study of pancreatic RFA in 16 LAPC patients reportedly described high complication rates based on a 25% mortality rate. Ablation with temperatures exceeding 90°C at a safe distance of 5 mm from the probe to the main structure suggested by the investigation was studied. Each patient underwent 2–5 sessions of ablations[23]. The Verona group ensured the safety of the procedure by ablating more carefully, reducing the ablation temperature to a maximum of 90°C, to make the undefined peritumor margin a safe margin for the surrounding tissue[24]. Based on these measurements, physicians can create a decrease in morbidity rates of 40% towards a complete complication rate of 26% on 100 patients undergoing RFA. Of all the research, the temperature applied on the tumor was considered as the merely indispensable considered aspect. Thereby, it is suggested that future research with larger sample sizes should investigate other factors relevant to postablation complications.
It is commonly considered that recurrent pancreatic cancer is a systemic illness[25]. Therefore, patients can be treated with palliative chemotherapy. The lack of available literature may be only about the safety and outcome of repeat resection for isolated local recurrence. This case revealed that repeated RFA ought to be a practical option for locally recurrent pancreatic cancer. Progression-free survival following the first RFA treatment was 7 mo. After repeated committed RFA for the local recurrent tumor, no signs of tumor relapse were seen for > 100 mo. In-depth research still has an indispensable role in clinically validating repeated RFA.
In our patient, RFA was shown to be feasible, giving rise to tumor reduction and extending survival. IOUS features vital significance as it powerfully confirms the property of safety and feasibility of the RFA procedure. Constant RFA for local tumor recurrence is likely to lengthen survival, according to our case. More research is necessary to validate therapeutic approaches and associations oriented with the ideal survival results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chrcanovic BR, Sweden; Garg P, India S-Editor: Wu YXJ L-Editor: Kerr C P-Editor: Wu YXJ
| 1. | Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford). 2008;10:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Lambert A, Schwarz L, Borbath I, Henry A, Van Laethem JL, Malka D, Ducreux M, Conroy T. An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol. 2019;11:1758835919875568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 3. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20493] [Article Influence: 2049.3] [Reference Citation Analysis (20)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9956] [Article Influence: 995.6] [Reference Citation Analysis (0)] |
| 5. | Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 799] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 6. | Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4489] [Cited by in RCA: 4285] [Article Influence: 214.3] [Reference Citation Analysis (0)] |
| 7. | Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Peixoto RD, Speers C, McGahan CE, Renouf DJ, Schaeffer DF, Kennecke HF. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. 2015;4:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:2267-2278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | D'Onofrio M, Ciaravino V, De Robertis R, Barbi E, Salvia R, Girelli R, Paiella S, Gasparini C, Cardobi N, Bassi C. Percutaneous ablation of pancreatic cancer. World J Gastroenterol. 2016;22:9661-9673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | D'Onofrio M, Crosara S, De Robertis R, Butturini G, Salvia R, Paiella S, Bassi C, Mucelli RP. Percutaneous Radiofrequency Ablation of Unresectable Locally Advanced Pancreatic Cancer: Preliminary Results. Technol Cancer Res Treat. 2017;16:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Ierardi AM, Lucchina N, Bacuzzi A, Marco de C, Bracchi E, Cocozza E, Dionigi G, Tsetis D, Floridi C, Carrafiello G. Percutaneous ablation therapies of inoperable pancreatic cancer: a systematic review. Ann Gastroenterol. 2015;28:431-439. [PubMed] |
| 13. | Scheffer HJ, Vroomen LG, de Jong MC, Melenhorst MC, Zonderhuis BM, Daams F, Vogel JA, Besselink MG, van Kuijk C, Witvliet J, de van der Schueren MA, de Gruijl TD, Stam AG, van den Tol PM, van Delft F, Kazemier G, Meijerink MR. Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology. 2017;282:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Shi J, Zeng J, Alnagger M, Zhou L, Fang G, Long X, Pan Z, Li Y, Chen J, Xu K, Qian W, Niu L. Percutaneous Irreversible Electroporation for Ablation of Locally Advanced Pancreatic Cancer: Experience From a Chinese Institution. Pancreas. 2017;46:e12-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Saccomandi P, Lapergola A, Longo F, Schena E, Quero G. Thermal ablation of pancreatic cancer: A systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia. 2018;35:398-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Marinova M, Wilhelm-Buchstab T, Strunk H. Advanced Pancreatic Cancer: High-Intensity Focused Ultrasound (HIFU) and Other Local Ablative Therapies. Rofo. 2019;191:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Vogel JA, van Veldhuisen E, Agnass P, Crezee J, Dijk F, Verheij J, van Gulik TM, Meijerink MR, Vroomen LG, van Lienden KP, Besselink MG. Time-Dependent Impact of Irreversible Electroporation on Pancreas, Liver, Blood Vessels and Nerves: A Systematic Review of Experimental Studies. PLoS One. 2016;11:e0166987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Martin RC 2nd, Durham AN, Besselink MG, Iannitti D, Weiss MJ, Wolfgang CL, Huang KW. Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J Surg Oncol. 2016;114:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Varshney S, Sewkani A, Sharma S, Kapoor S, Naik S, Sharma A, Patel K. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74-78. [PubMed] |
| 21. | Seicean A, Tefas C, Ungureanu B, Săftoiu A. Endoscopic ultrasound guided radiofrequency ablation in pancreas. Hepatogastroenterology. 2014;61:1717-1721. [PubMed] |
| 22. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2007;96:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Girelli R, Frigerio I, Giardino A, Regi P, Gobbo S, Malleo G, Salvia R, Bassi C. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch Surg. 2013;398:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Hashimoto K, Ueno H, Ikeda M, Kojima Y, Hagihara A, Kondo S, Morizane C, Okusaka T. Do recurrent and metastatic pancreatic cancer patients have the same outcomes with gemcitabine treatment? Oncology. 2009;77:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |