Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11812
Peer-review started: August 5, 2022
First decision: September 23, 2022
Revised: September 28, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 16, 2022
Processing time: 94 Days and 20.2 Hours
Prostate artery embolization (PAE) is a promising minimally invasive therapy that improves lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH). Transurethral resection of the prostate (TURP) is the gold standard therapy for LUTS/BPH.
To evaluate the efficacy and safety of PAE vs TURP on LUTS related to BPH.
A literature review was performed to identify all published articles on PAE vs TURP for LUTS/BPH. Sources included PubMed, Embase, Cochrane library databases, and Chinese databases before June 2022. A systematic review and meta-analysis were conducted. Outcome measurements were combined by calculating the mean difference with a 95% confidence interval. Statistical analysis was carried out using Review Manager 5.3.
Eleven studies involving 1070 participants were included. Compared with the TURP group, the PAE group had a similar effect on the International Index of Erectile Function (IPSS) score, Peak urinary flow rate (Qmax), postvoid residual volume (PVR), Prostate volume (PV), prostatic specific antigen (PSA), The International Index of Erectile Function short form (IIEF-5) scores, and erectile dysfunction during 24 mo follow-up. Lower quality of life (QoL) score, lower rate of retrograde ejaculation and shorter hospital stay in the PAE group. There was no participant death in either group. A higher proportion of haematuria, urinary incontinence and urinary stricture was identified in the TURP group.
PAE may be an appropriate option for elderly patients, patients who are not candidates for surgery, and patients who do not want to risk the potential adverse effects of TURP. Studies with large cases and long follow-up time are needed to validate results.
Core Tip: Prostate artery embolization (PAE) is a promising minimally invasive therapy that improves lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH). Transurethral resection of the prostate (TURP) is the gold standard therapy for LUTS/BPH. This article uses a meta-analysis to evaluate the efficacy and safety of PAE compared with TURP on LUTS related to BPH. In our conclusion, PAE may be an appropriate option for elderly patients, patients who are not candidate for surgery, and patients who do not want to risk the potential adverse effects of TURP.
- Citation: Wang XY, Chai YM, Huang WH, Zhang Y. Prostate artery embolization on lower urinary tract symptoms related to benign prostatic hyperplasia: A systematic review and meta-analysis. World J Clin Cases 2022; 10(32): 11812-11826
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11812.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11812
Benign prostatic hyperplasia (BPH) is a very common disease in aging males and is positively correlated with age[1]. The morbidity rate of BPH is approximately half of all men aged 60 years or older[2]. Lower urinary tract symptoms (LUTS) are ordinarily secondary to BPH and are not usually life-threatening but often compromise the quality of life (QoL).
The transurethral resection of the prostate (TURP) has been considered a surgical reference standard for LUTS/BPH. Nevertheless, TURP is associated with significant postoperative complications, including hematuria, urinary retention, incontinence, urinary stricture, retrograde ejaculation and erectile dysfunction[3]. Therefore, a growing number of nonresective techniques, such as prostate artery embolization (PAE), have been developed.
PAE is an interventional radiological technique that involves unilaterally or bilaterally injecting small particles directly into the prostatic arteries, which leads to a progressive decrease in prostatic volume due to devascularization. Treatment of LUTS/BPH by PAE offers some advantages, including the continuation of anticoagulant drugs, local anesthesia, and a quick return to normal activities[4].
Although PAE is considered a therapeutic option for LUTS/BPH in the European Association of Urology guidelines and National Institute for Health and Care Excellence, TURP is the traditional gold standard[5], and controversy persists regarding PAE in the treatment of LUTS/BPH. Therefore, we performed a meta-analysis to evaluate the efficacy and safety of PAE compared with TURP, which may help urologists make better choices.
A comprehensive literature search was carried out by two independent reviewers. We searched Reference Citation Analysis (https://www.referencecitationanalysis.com/), PubMed, Embase, the Cochrane library databases and Chinese databases, such as the Chinese National Knowledge Infrastructure (CNKI), Wanfang data and the Chinese Science and Technology Periodicals (VIP) database, before June 2022. The search terms consisted of "BPH", " LUTS", "PAE" and "TURP", and confined fields in the title/abstract. Additionally, the reference lists of the retrieved studies were checked manually.
The inclusion criteria for this meta-analysis were as follows: (1) The study was a clinical controlled trial, prospective study or retrospective study; (2) the study subjects were BPH patients with LUT; (3) the intervention measures were PAE in the experimental group and TURP in the control group; (4) at least one of the following outcomes was reported at different follow-up times: International Prostate Symptom Score (IPSS), QoL score, prostate volume (PV), prostate-specific antigen (PSA), peak urine flow rate (Qmax), postvoid residual volume (PVR) and International Index of Erectile Function (IIEF) short form score; and (5) the full text was available; 6. if an identical study was published a different time point in a different journal, the most recently published study was included. If these inclusion criteria were not met, then the study was excluded from this meta-analysis.
The quality assessment was carried out jointly by all of the authors using the methodological index for nonrandomized studies (MINORS)[6]. Twelve items received 2 points for each item. The study received 2 points if it reported the item. If not intact, it received 1 point, and if absent, it received 0 points. Fourteen points was defined as a golden line. All authors agreed with the final results.
Two reviewers independently participated in the study screening and data extraction. The differences were resolved through discussion. The following data were extracted from the retrieved studies: (1) Basic information of the included studies: authors, publication time, country, sample size and inclusive criteria; (2) detailed materials used in the PAE group and energy sources in the TURP group; (3) follow-up duration and outcome measures; (4) procedure time, hospital time and the number of participants with complications; and (5) study quality evaluation of the relevant information.
The RevMan 5.3 software was used to conduct the meta-analysis. Outcome measurements were combined by calculating the mean difference (MD) with a 95% confidence interval (CI), and P < 0.05 was considered statistically significant. The statistical heterogeneity among studies was analyzed with the I2heterogeneity test. If I2 was less than 50%, a fixed-effects model was used; if not, we analyzed the source of heterogeneity. If heterogeneity was detected, the heterogeneity could be improved after a subset analysis and a sensitivity analysis. The evaluation of publication bias was based on funnel plots.
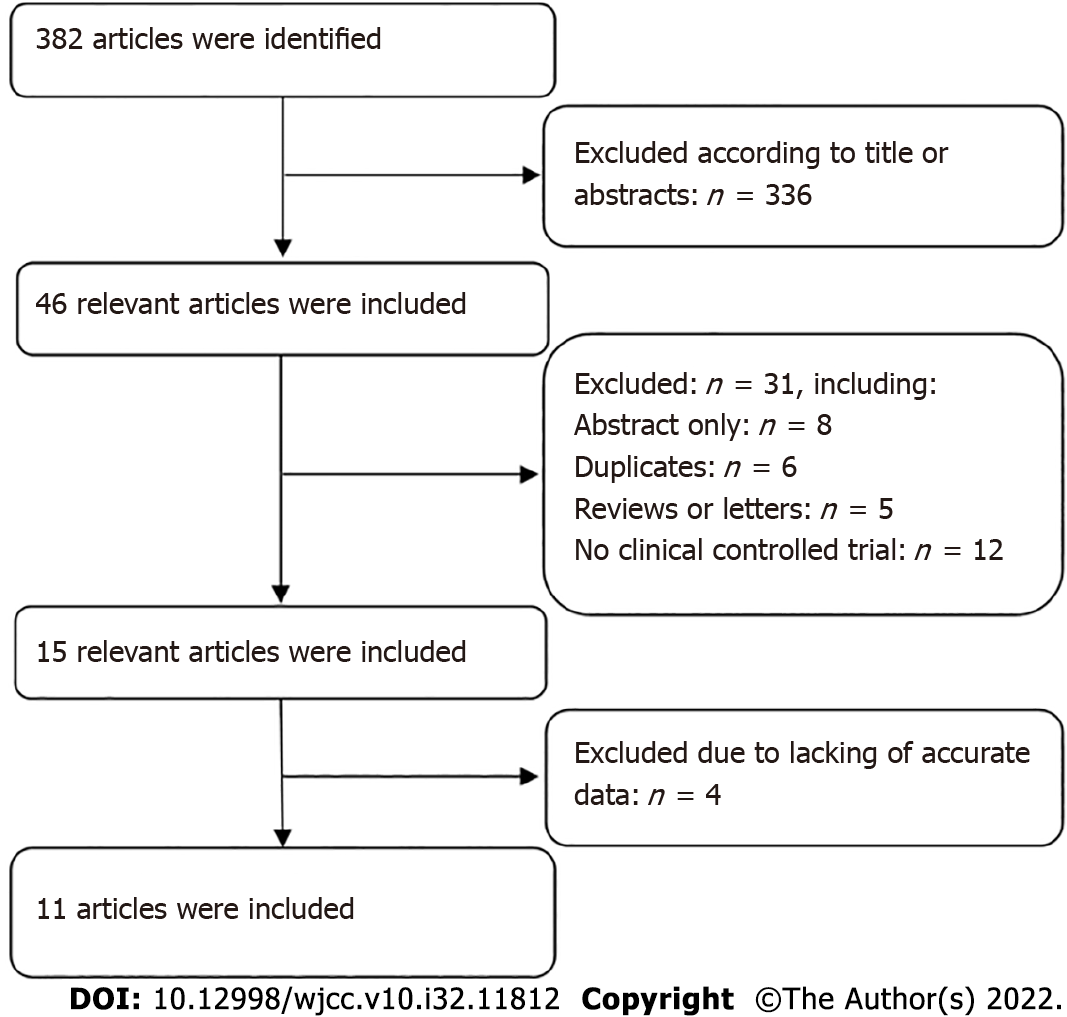
Altogether, 382 articles were selected through the search procedure. Finally, 11 articles involving 1070 BPH participants (582 in the PAE group and 488 in the TURP group) were eligible for this meta-analysis[7-17]. Figure 1 shows the flow diagram of the study inclusion process. The main characteristics and quality assessment of eligible studies are presented in Table 1.
| Studies | Study design | Country | Sample size | Inclusion criteria | Interventions | Follow-up (mo) | Outcome measuresa | Quality assessmentb | ||
| PAE | TURP | PAE | TURP | |||||||
| Abt 2021 | Prospective study | Switzer--land | 48 | 51 | Age ≥ 40 yr; PV 25-80 mL; IPSS ≥ 8; QoL ≥ 3; Qmax ≤ 12 mL/s | Bilateral (36); unilateral (12); 250-400 µm microspheres | Monopolar | 3, 6, 12, 24 | 1, 2, 3, 4, 5, 6, 7, 8, 9 | 19 |
| Carnevale 2016 | Prospective study | Brazil | 15 | 15 | Age ≥ 45yr; PV 30-90 mL; IPSS ≥ 19 | Bilateral (13); unilateral (2); 300-500µm microspheres | Monopolar | 12 | 1, 2, 3, 4, 5, 6, 7, 8 | 21 |
| Gao 2014 | Prospective study | China | 54 | 53 | PV 20-100 mL; IPSS > 7; Qmax ≤ 15 mL/s | Bilateral (48); unilateral (6); 355-500 µm polyvinyl alcohol microspheres | Bipolar | 1, 3, 6, 12, 24 | 1, 2, 3, 4, 5, 6, 8, 9 | 21 |
| Gu 2018 | Prospective study | China | 50 | 50 | Age > 55 yr; PV 70-150 mL; IPSS ≥ 25; QoL ≥ 5 | Bilateral or unilateral; BioSphere Medical S.A 100-300 µm | Bipolar | 6 | 1, 2, 3, 4, 5 | 19 |
| Hou 2016 | Retrospective study | China | 31 | 39 | Age ≥ 49 yr; PV 60-110 mL; IPSS > 7; QoL > 3; Qmax < 12 mL/s | Bilateral; polyvinyl alcohol microspheres | Bipolar | 3, 6, 12, 24 | 1, 2, 3, 4, 5 | 17 |
| Insausti 2020 | Prospective study | Spain | 23 | 22 | Age > 60yr; IPSS > 19; QoL > 3; Qmax ≤ 10 mL/s | Bilateral; 300-500 µm polyvinyl alcohol microspheres | Bipolar | 3, 6, 12 | 1, 2, 3, 4, 5, 6, 8, 9 | 21 |
| Qiu 2017 | Retrospective study | China | 17 | 40 | Age > 60 yr; PV > 50mL; IPSS > 7; QoL > 3; Qmax < 13 mL/s | Bilateral or unilateral; 90-180 µm embosphere microspheres | Bipolar | 3, 6, 12 | 1, 2, 3, 5 | 17 |
| Ray 2018 | Prospective study | British | 216 | 89 | Age > 60 yr; PV > 50mL; IPSS > 7; QoL > 3; Qmax < 15 mL/s | Bilateral; polyvinyl alcohol microspheres | Monopolar (45) Bipolar (44) | 1, 3, 6, 12 | 1, 2, 3, 4, 5, 7 | 19 |
| Tan 2018 | Prospective study | China | 47 | 47 | Age ≥ 50 yr; PV > 60 mL; IPSS > 19; QoL > 4; Qmax < 13 mL/s | Bilateral; polyvinyl alcohol microspheres | Bipolar | 3, 6, 12 | 1, 2, 3, 4, 5, 6, 9 | 21 |
| Wang 2018 | Prospective study | China | 61 | 62 | Age > 55 yr; PV > 45 mL; IPSS > 19; QoL > 3; Qmax < 10 mL/s | Bilateral; polyvinyl alcohol microspheres | Bipolar | 3 | 1, 2, 3, 4, 7, 8, 9 | 20 |
| Zhu 2018 | Prospective study | China | 20 | 20 | Age ≥ 49 yr; PV>60mL; IPSS>7; QoL>3; Qmax < 12 mL/s | Bilateral; 100–300 or 310–500µm Microspheres | Bipolar | 3, 6, 12 | 1, 2, 3, 4, 5, 6 | 19 |
Changes in IPSS: Subgroup and sensitivity analyses were carried out to examine sources of heterogeneity. Study Insausti2020[12] was eliminated at postoperative 3 mo. Study Gu2018[10] and study Insausti2020[12] were eliminated at postoperative 6 mo. Study Carnevale2016[8] was eliminated at postoperative 12 mo.
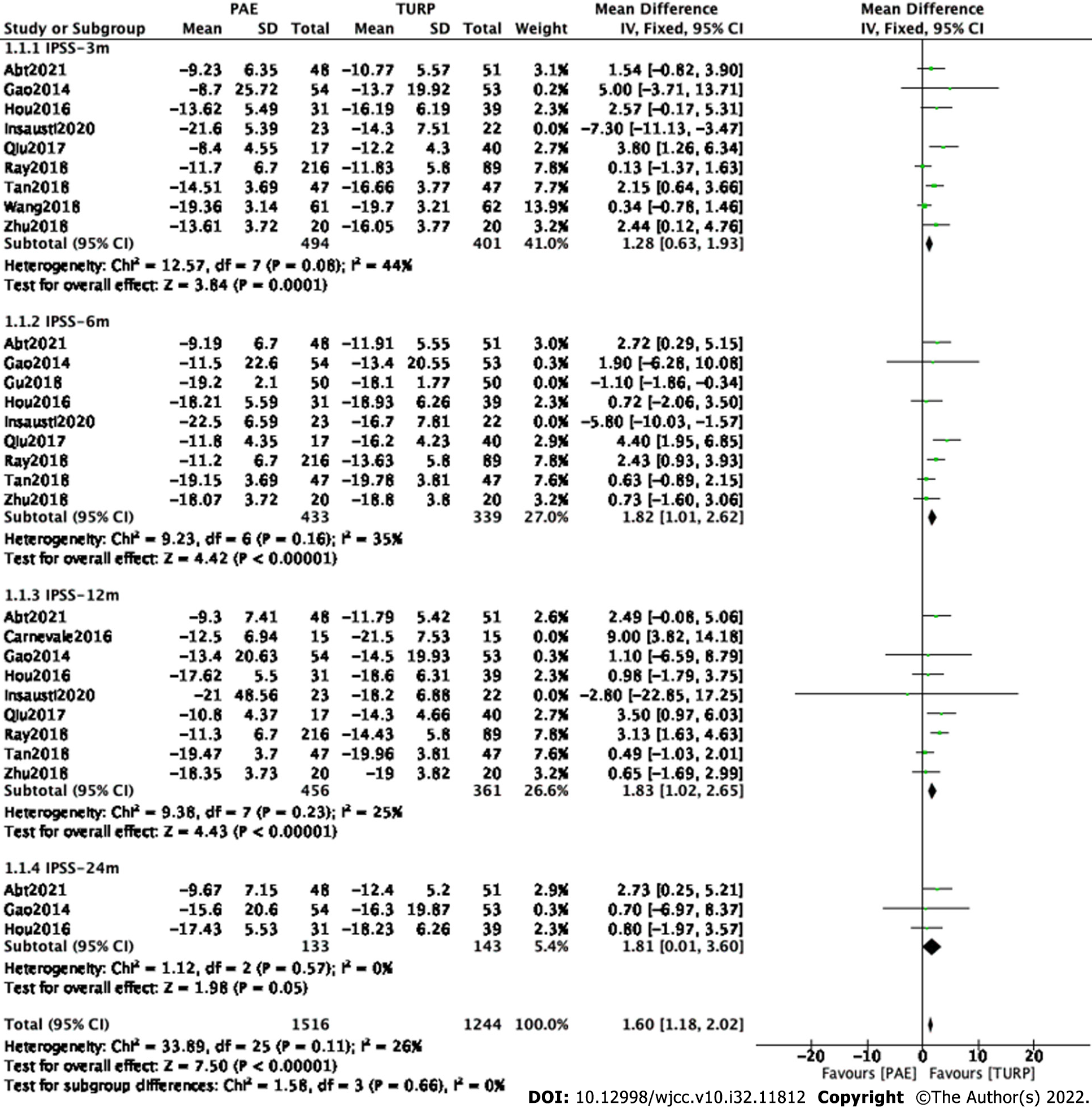
Finally, eight studies[7,9,11,13-17] involving 895 participants, seven studies[7,9,11,13-15,17] involving 772 participants, eight studies[7,9,11-15,17] involving 817 participants and three studies[7,9,11] involving 276 participants were enrolled in the analysis of IPSS changes at postoperative 3, 6, 12 and 24 mo, respectively (Figure 2).
The forest plot demonstrated that the difference in IPSS changes between the PAE group and the TURP group was statistically significant at postoperative 3 mo (MD 1.28; 95%CI: 0.63 to 1.93; P = 0.0001), 6 mo (MD 1.82; 95%CI: 1.01 to 2.62; P < 0.00001) and 12 mo (MD 1.83; 95%CI: 1.02 to 2.65; P < 0.00001) but was not statistically significant at postoperative 24 mo (MD 1.81; 95%CI: 0.01 to 3.60; P = 0.05).
Subgroup and sensitivity analyses were carried out to examine sources of heterogeneity. Study Insausti2020[12] and study Wang2018[16] were eliminated at postoperative 3 mo. Study Gu2018[10] and study Insausti2020[12] were eliminated at postoperative 6 mo. Study Insausti2020[12] and study Ray2018[14] were eliminated at postoperative 12 mo.
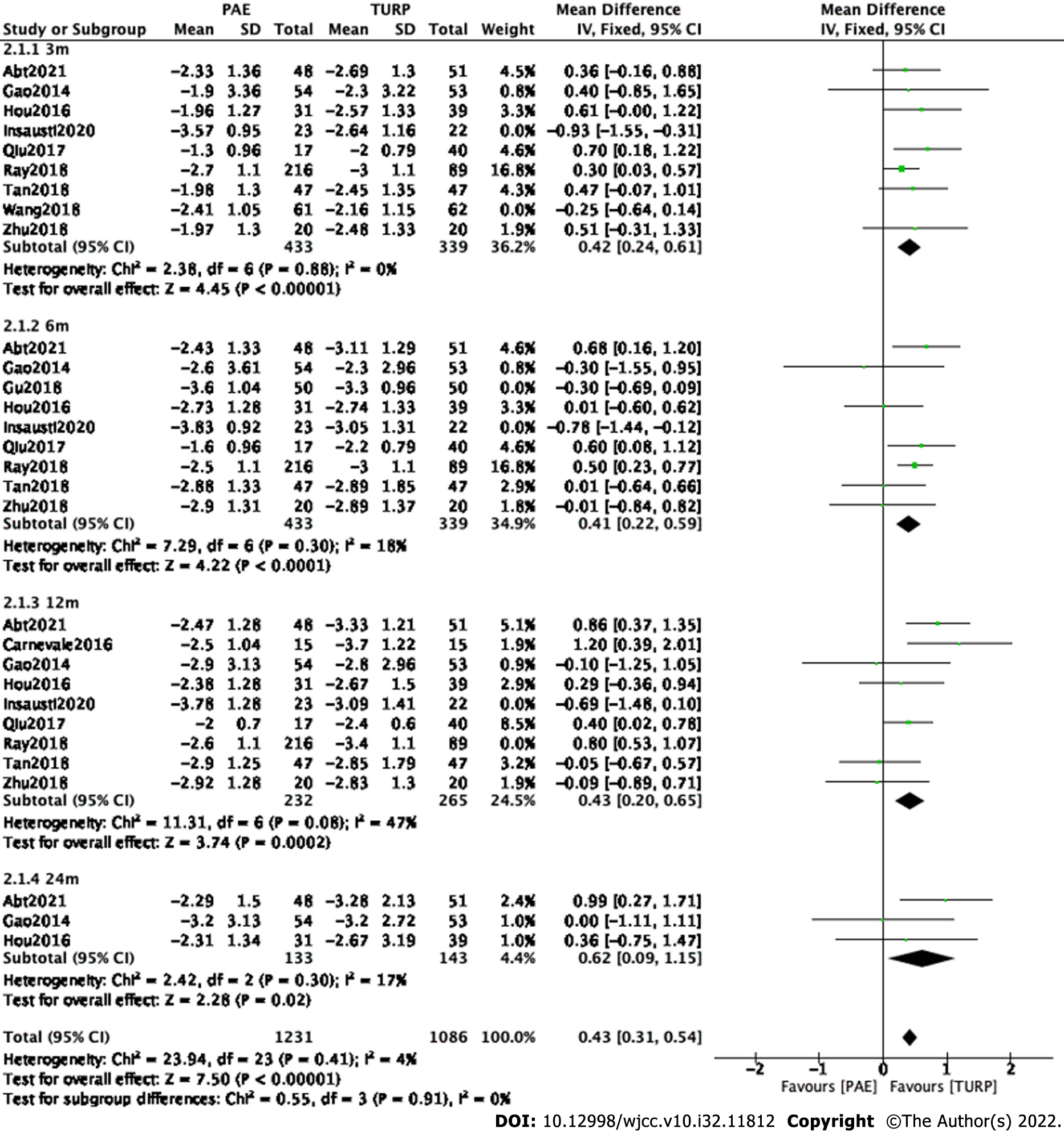
Finally, seven studies[7,9,11,13-15,17] involving 772 participants, seven studies[7,9,11,13-15,17] involving 772 participants, seven studies[7-9,11,13,15,17] involving 497 participants and three studies[7,9,11] involving 276 participants were enrolled in the analysis of QoL changes at postoperative 3 mo, 6 mo, 12 mo and 24 mo, respectively (Figure 3).
The forest plot demonstrated that the difference in QoL changes between the PAE group and the TURP group was statistically significant at postoperative 3 mo (MD 0.42; 95%CI: 0.24 to 0.61; P < 0.00001), 6 mo (MD 0.41; 95%CI: 0.22 to 0.59; P < 0.0001), 12 mo (MD 0.43; 95%CI: 0.20 to 0.65; P = 0.0002) and 24 mo (MD 0.62; 95%CI: 0.09 to 1.15; P = 0.02).
Subgroup and sensitivity analyses were carried out to examine sources of heterogeneity. Study Gu2018[10] was eliminated at postoperative 6 mo. Study Carnevale2016[8] was eliminated at postoperative 12 mo.
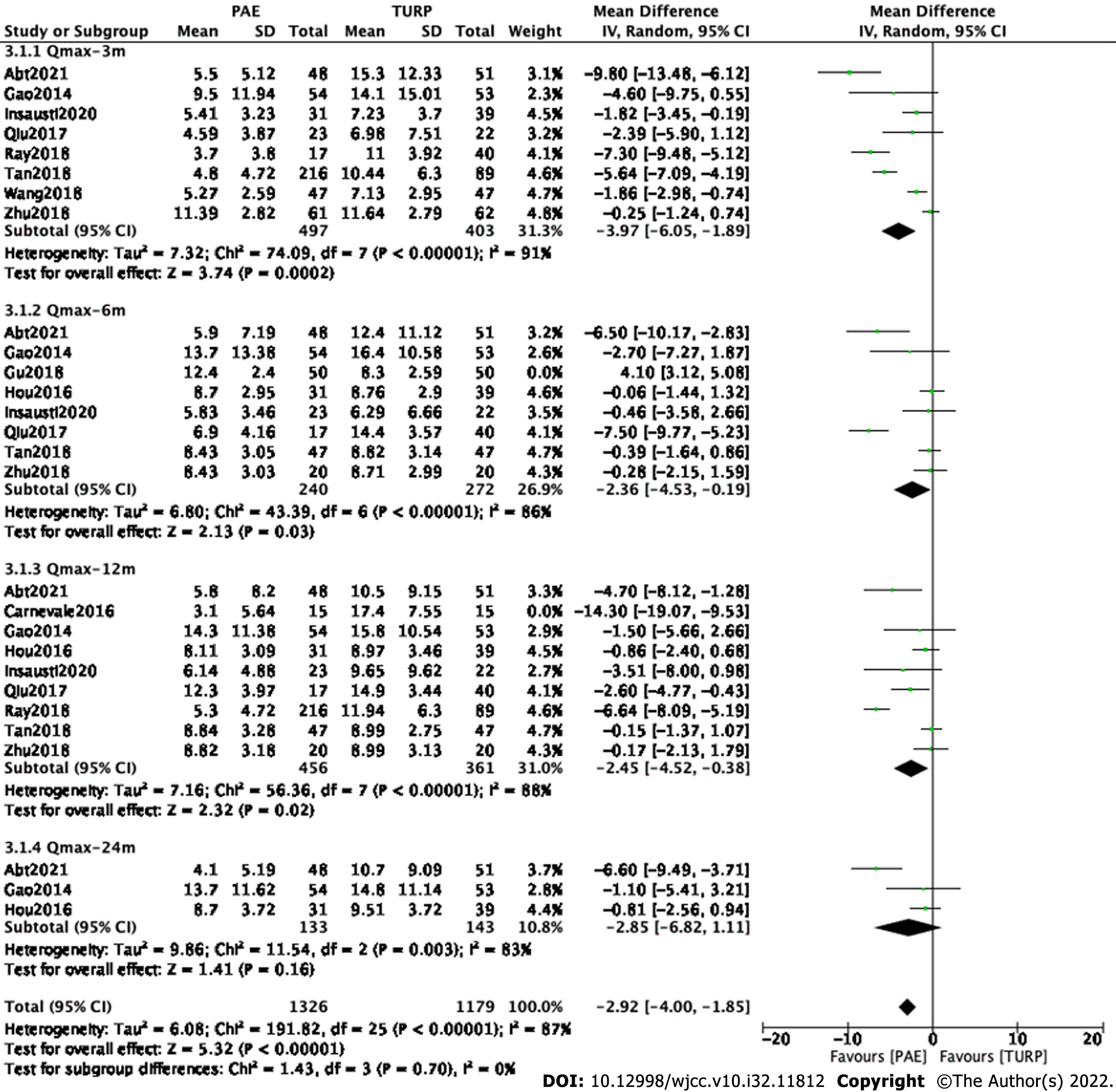
Finally, eight studies[7,9,12,13-17] involving 900 participants, seven studies[7,9,11-13,15,17] involving 512 participants, eight studies[7,9,11-15,17] involving 817 participants and three studies[7,9,11] involving 276 participants were enrolled in the analysis of Qmax changes at postoperative 3 mo, 6 mo, 12 mo and 24 mo, respectively (Figure 4).
The forest plot demonstrated that the difference in Qmax changes between the PAE group and the TURP group was statistically significant at postoperative 3 mo (MD -3.97; 95%CI: -6.05 to -1.89; P = 0.002), 6 mo (MD -2.36; 95%CI: -4.53 to -0.19; P = 0.03) and 12 mo (MD -2.45; 95%CI: -4.52 to -0.38; P = 0.02) but was not statistically significant at postoperative 24 mo (MD -2.85; 95%CI: -6.82 to 1.11; P = 0.16).
Subgroup and sensitivity analyses were carried out to examine sources of heterogeneity. Study Abt2021[7] and study Ray2018 [14] were eliminated at postoperative 3 mo. Study Abt2021[7] was eliminated at postoperative 6 mo. Study Abt2021[7] and study Ray2018[14] were eliminated at postoperative 12 mo. Study Abt2021[7] was eliminated at postoperative 24 mo.
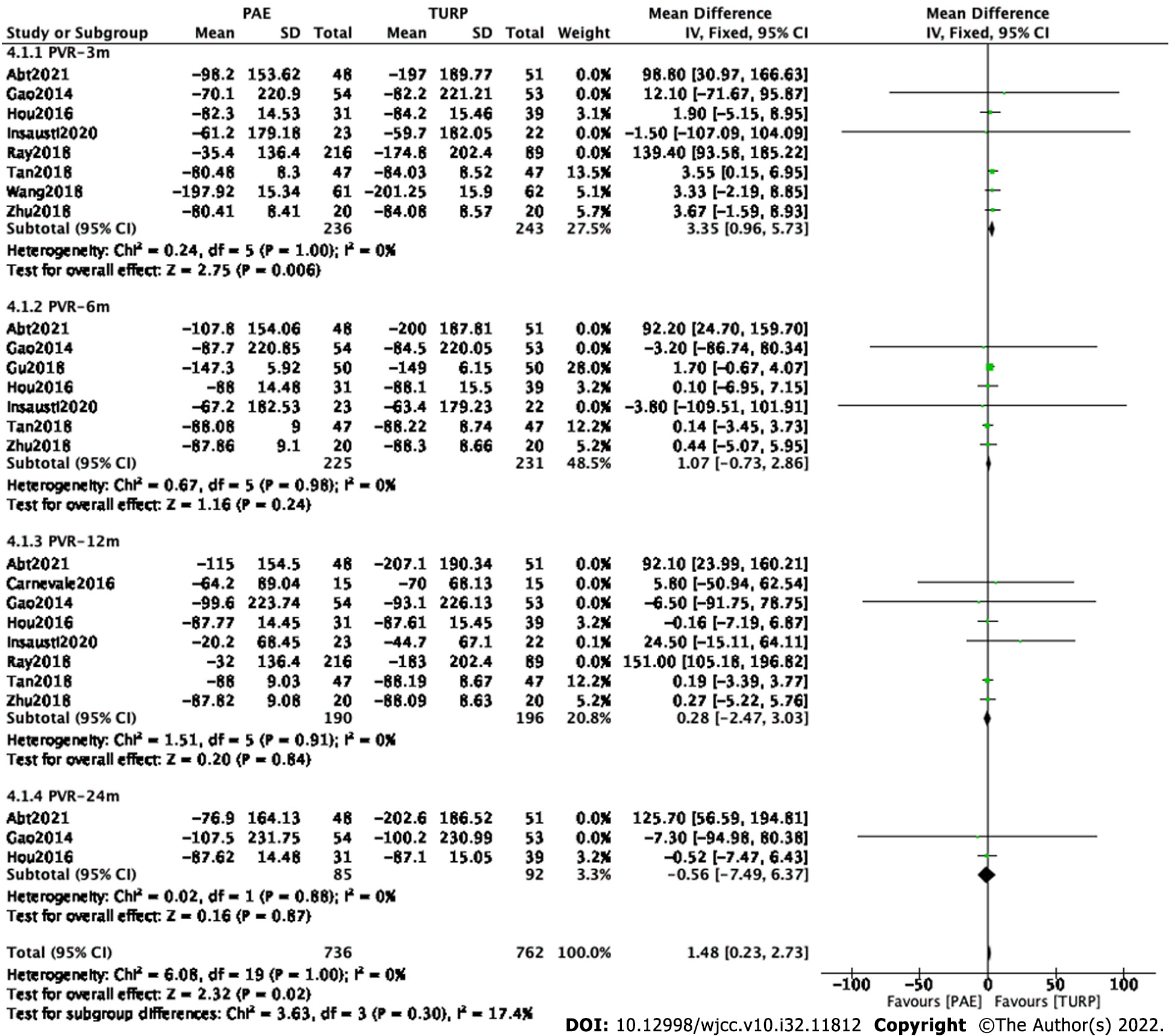
Finally, six studies[9,11,12,15-17] involving 479 participants, six studies[9-11,12,15,17] involving 456 participants, six studies[8,9,11,12,15,17] involving 386 participants and two studies[9,11] involving 177 participants were enrolled in the analysis of PVR changes at postoperative 3 mo, 6 mo, 12 mo and 24 mo, respectively(Figure 5).
The forest plot demonstrated that the difference in PVR changes between the PAE group and the TURP group was statistically significant at postoperative 3 mo (MD 3.35; 95%CI: 0.96 to 5.73; P = 0.006) but was not statistically significant at postoperative 6 mo (MD 1.07; 95%CI: -0.73 to 2.86; P = 0.24), 12 mo (MD 0.28; 95%CI: -2.47 to 3.03; P = 0.84) and 24 mo(MD -0.56; 95%CI: -7.49 to 6.37; P = 0.87).
Subset and sensitivity analysis were carried out to improve the heterogeneity.
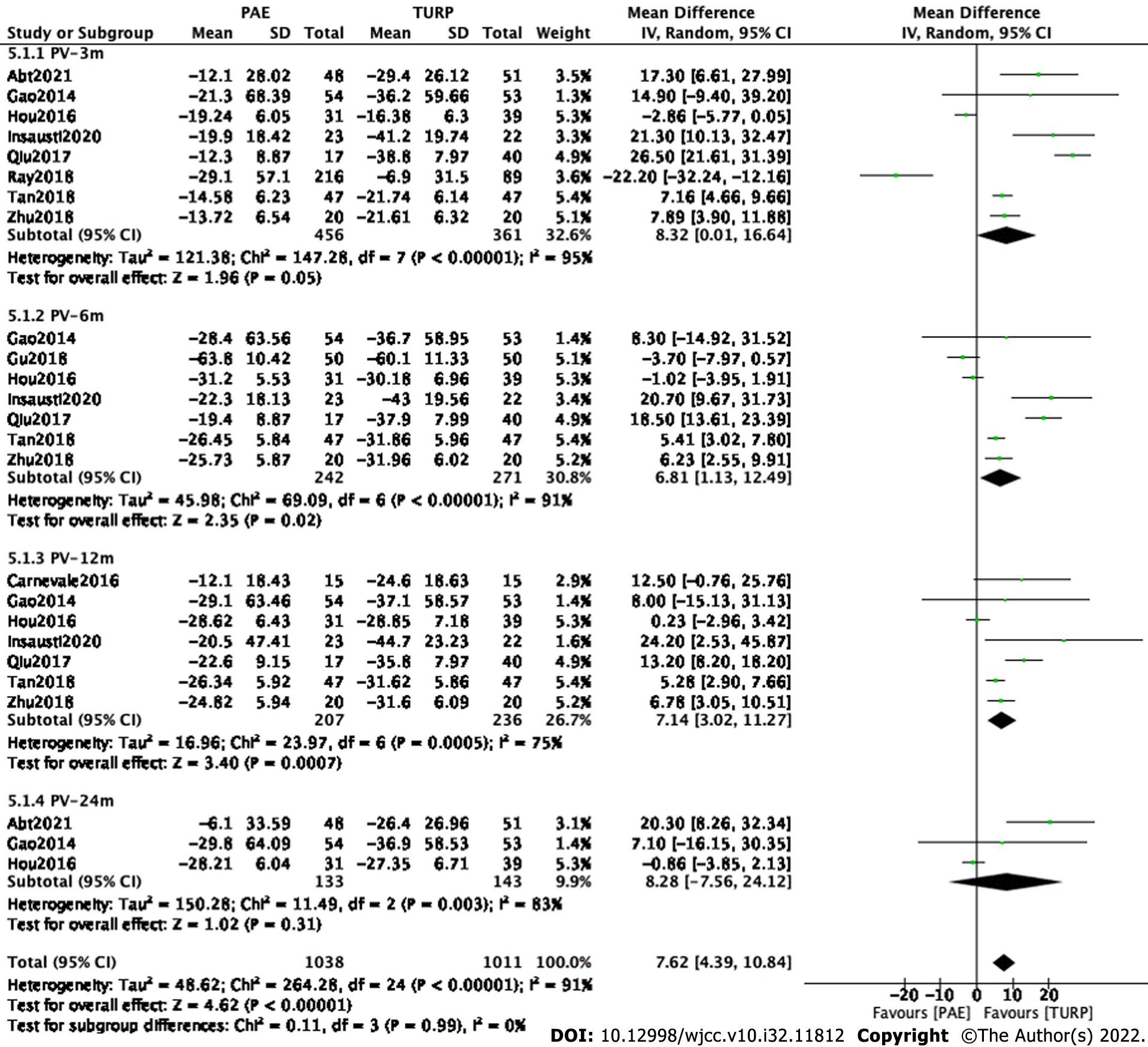
Finally, eight studies[7,9,11-15,17] involving 817 participants, seven studies[9-13,15,17] involving 513 participants, seven studies[8,9,11-14,17] involving 443 participants and three studies[7,9,11] involving 276 participants were enrolled in the analysis of PV changes at postoperative 3 mo, 6 mo, 12 mo and 24 mo, respectively (Figure 6).
The forest plot demonstrated that the difference in PV changes between the PAE group and the TURP group was statistically significant at postoperative 6 mo (MD 6.81; 95%CI: 1.13 to 12.49; P = 0.02) and 12 mo (MD 7.14; 95%CI: 3.02 to 11.27; P = 0.0007) but was not statistically significant at postoperative 3 mo (MD 8.32; 95%CI: 0.01 to 16.64; P = 0.05) and 24 mo (MD 8.28; 95%CI: -7.56 to 24.12; P = 0.31).
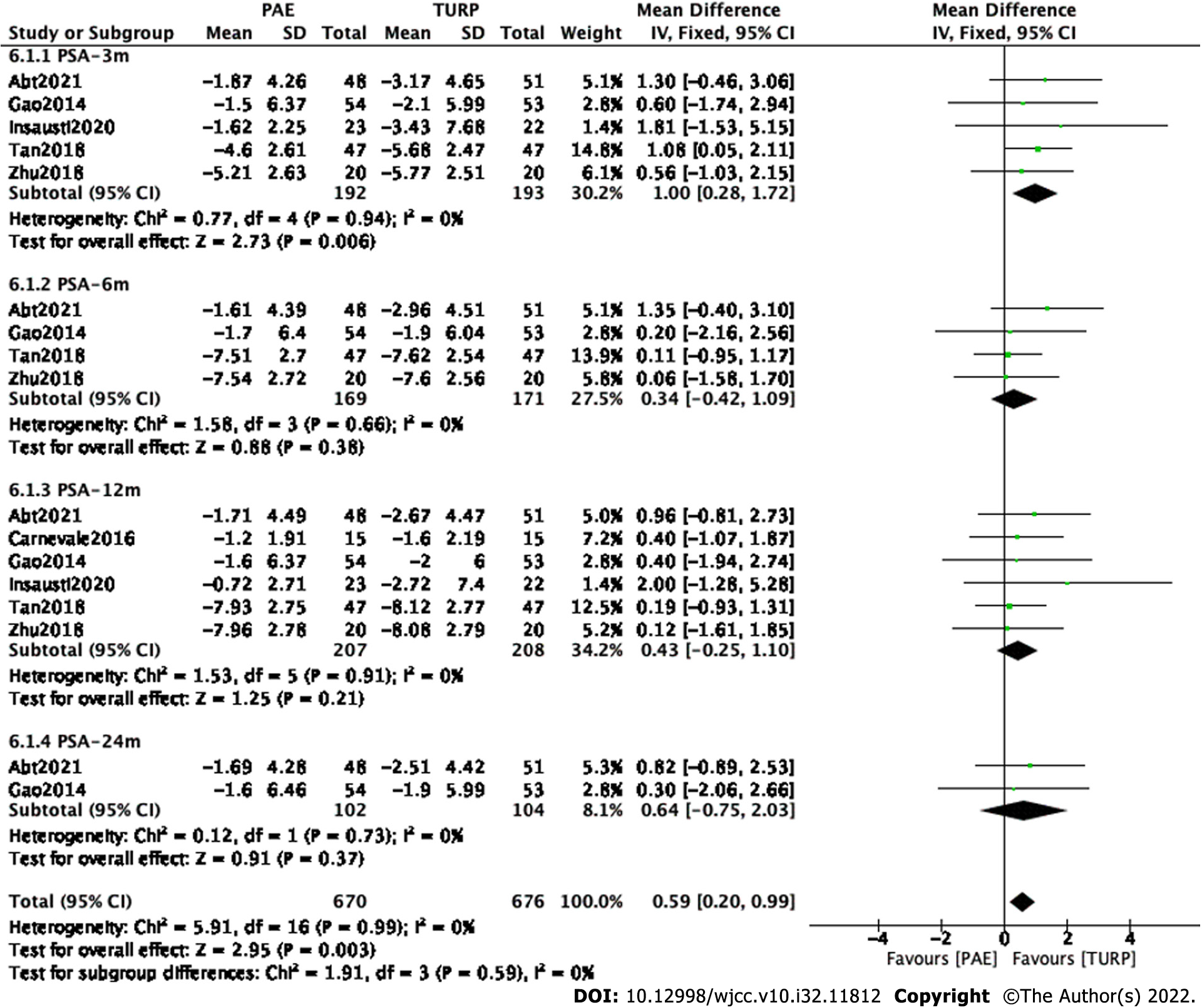
Subgroup and sensitivity analyses were carried out to examine sources of heterogeneity. Finally, five studies[7,9,12,15,17] involving 385 participants, four studies[7,9,15,17] involving 340 participants, six studies[7,8,9,12,15,17] involving 415 participants and two studies[7,9] involving 206 participants were enrolled to analyze PSA changes at postoperative 3 mo, 6 mo, 12 mo and 24 mo (Figure 7).
The forest plot demonstrated that the difference in PSA changes between the PAE group and the TURP group was statistically significant at postoperative 3 mo postoperatively (MD 1.00; 95%CI: 0.28 to 1.72; P = 0.006) but was not statistically significant at postoperative 6 mo(MD 0.34; 95%CI: -0.42 to 1.09; P = 0.38), 12 mo (MD 0.43; 95%CI: -0.25 to 1.10; P = 0.21) or 24 mo (MD 0.64; 95%CI: -0.75 to 2.03; P = 0.37).
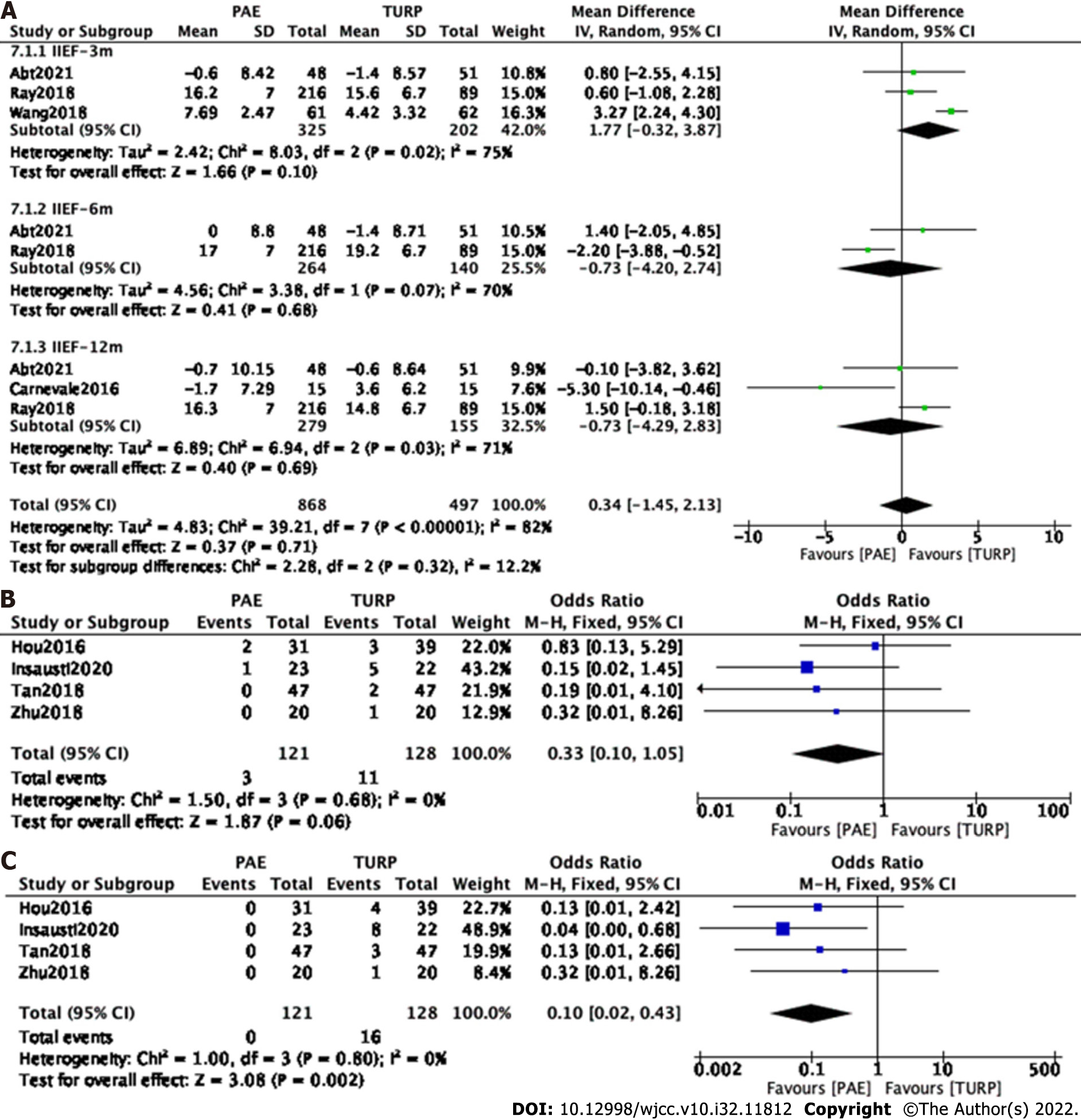
Subgroup and sensitivity analyses were carried out to examine sources of heterogeneity. Finally, three studies[7,14,16] involving 527 participants, two studies[7,14] involving 414 participants and three[7,8,14] studies involving 434 participants were enrolled to analyze IIEF changes at postoperative 3 mo, 6 mo and 12 mo, respectively (Figure 8A).
The forest plot demonstrated that the difference in IIEF changes between the PAE group and the TURP group was not statistically significant at postoperative 3 mo (MD 1.77; 95%CI: -0.32 to 3.87; P = 0.10), 6 mo (MD -0.73; 95%CI: -4.20 to 2.74; P = 0.68) and 12 mo (MD -0.73; 95%CI: -4.29 to 2.83; P = 0.69).
Four studies[11,12,15,16] involving 249 participants were enrolled to analyze postoperative erectile dysfunction. The forest plot demonstrated that the difference between the PAE group and the TURP group was not statistically significant (MD 0.33; 95%CI: 0.10 to 1.05; P = 0.06) (Figure 8B).
Four studies[11,12,15,16] involving 249 participants were enrolled to analyze postoperative retrograde ejaculation. The forest plot demonstrated that the difference between the PAE group and the TURP group was statistically significant (MD 0.10; 95%CI: 0.02 to 0.43; P = 0.002) (Figure 8C).
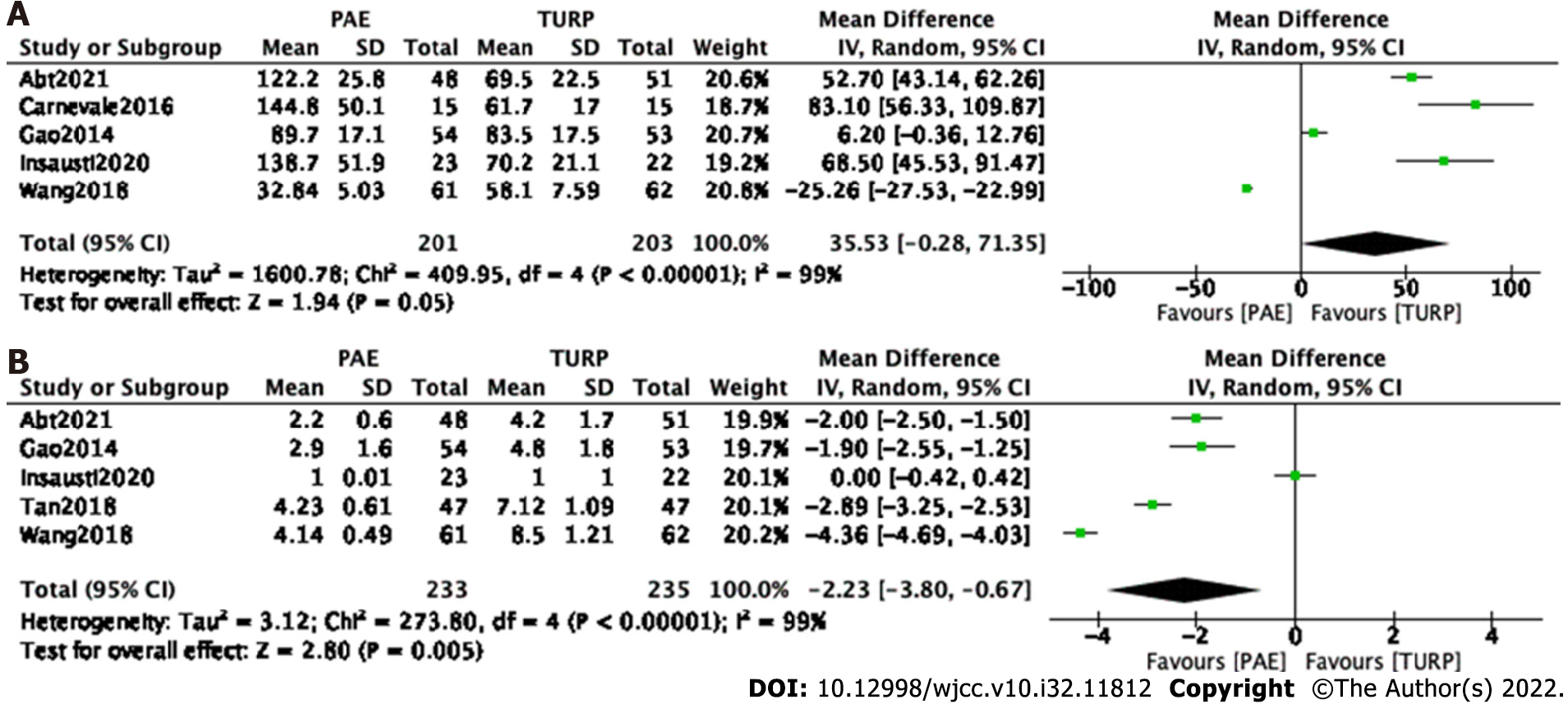
Procedure time: Five studies[7-9,12,16] involving 404 participants were enrolled to analyze the procedure time. The forest plot demonstrated that the difference between the PAE group and the TURP group was not statistically significant (MD 35.53; 95%CI: -0.28 to 71.35; P = 0.05) (Figure 9A).
Five studies[7,9,12,15,16] involving 468 participants were enrolled for an analysis of the hospital stay. The forest plot demonstrated that the difference between the PAE group and TURP group was statistically significant (MD -2.23; 95%CI: -3.80 to -0.67; P = 0.005) (Figure 9B).
The TURP group experienced more complications (80.8%, P < 0.00001); however, the differences in the rates of major events (Clavien–Dindo grade ≥ 3) between the two groups were not statistically significant (P = 0.23), such as blood transfusion and sepsis in the TURP group (3.35%) or groin hematoma and bladder ischemia in the PAE group (2.12%). Participant deaths did not occur in either group (Table 2).
| Complications | Haematuria | Irritation or pain | Urinary retention | Urinary incontinence | Urinary tract infection | Urinary stricture | Major events (clavien ≥ 3) | Total |
| PAE | 46 (8.14%) | 222 (39.29%) | 27 (4.78%) | 2 (0.35%) | 30 (5.31%) | 1 (0.18%) | 12 (2.12%) | 349 (61.77%) |
| TURP | 88 (19.64%) | 149 (33.26%) | 15 (3.35%) | 18 (4.02%) | 35 (7.81%) | 14 (3.13%) | 15 (3.35%) | 362 (80.8%) |
| P value | < 0.00001 | 0.05 | 0.26 | 0.001 | 0.11 | 0.005 | 0.23 | < 0.00001 |
Urinary irritation or local pain was the main complication in both groups, but the difference between the PAE group (39.29%) and the TURP group (33.26%) was not statistically significant (P = 0.05). A higher proportion of hematuria (19.64%), urinary incontinence (4.02%) and urinary stricture (3.13%) was identified in the TURP group (P < 0.00001, P = 0.001 and P = 0.005) (Table 2).
In the early 1970s, PAE was primarily used to treat refractory hematuria. The treatment of LUTS/BPH with PAE was gradually introduced into clinical practice until 2000[18]. This meta-analysis presented changes in different outcomes at 3, 6, 12 and 24 mo postoperatively and a summary of the latest comparisons between PAE and TURP in patients with LUTS/BPH.
In the present study, we observed more significant changes in IPSS, Qmax, PVR, PV and PSA in the TURP group at 3, 6 and 12 mo postoperatively than in the PAE group, although both groups achieved comparable results at 24 mo postoperatively. Thus, no differences were observed at 24 mo postoperatively, suggesting that PAE eventually achieves similar clinical efficacy during long-term follow-up, although the results of this procedure are slow to emerge.
This delay may be caused by the different mechanisms of these two procedures. The mechanical obstruction of the urinary tract in prostatic hyperplasia is mainly due to the enlargement of the prostate volume and the protruding prostatic tissue, which in turn obstructs the urethra[19]. The direct removal of pathologically enlarged prostate tissue provides immediate relief of mechanical obstruction of the urethra with satisfactory urodynamic results. PAE does not significantly reduce PV in a short period of time and takes a long time to obtain histopathologic changes after the disruption of the blood supply to the prostate[20]. PAE disrupts the vasculature of the prostate and takes several months to complete the complex histopathologic changes.
In addition, two types of PAE, unilateral and bilateral embolization, have been employed. For PAE, bilateral embolization has been reported to be more effective than unilateral embolization[21]. Combined bilateral necrosis results in better overall shrinkage and lower regeneration rates. The size of the embolization particles and the embolization route are also important factors in the outcome of the procedure, and the appropriate embolization route and material should be selected intraoperatively[22].
QoL scores supported a greater improvement in the TURP group than in the PAE group at all follow-up time points. We reviewed the included trials and found that the inclusion criteria for these studies varied. Thus, patient selection bias is a possible cause of heterogeneity. For example, the baseline data for IPSS scores in Gu's study were no less than 25, whereas they were no less than 8 in Abt's study and Gao's study.
The preservation of sexual function is an important point for many patients with BPH and should be preserved as much as possible during treatment. Epidemiological evidence suggests a clear and clinically meaningful association between LUTS and sexual dysfunction that is independent of age and comorbidity[23]. Continued improvement in LUTS was accompanied by the beginning of an increase in IIEF-5 scores. Regarding changes in sexual function, we assessed 3 indicators, including IIEF-5 scores, erectile dysfunction and retrograde ejaculation. For both groups, the degree of improvement in IIEF-5 scores and the incidence of erectile dysfunction postoperatively did not significantly differ, but the incidence of retrograde ejaculation was significantly higher in the TURP group than in the PAE group. Several studies have explained that postoperative erectile dysfunction is closely associated with injury to the prostatic capsule through which the cavernous nerve passes and the heating effect of the electrode occurs during TURP[24]. However, penile artery weakness may be the direct cause of erectile dysfunction after nontargeted embolization with PAE[25]. The main pathogenic mechanism of retrograde ejaculation is related to the removal of the bladder neck (internal sphincter) that occurs during TURP[26].
PAE uses endovascular surgery rather than transurethral surgery and does not cause urethral injury or necessitate bladder irrigation. Thus, the risk of transurethral resection syndrome, urethral stricture and bladder neck contracture is eliminated[27]. For anesthesia, prostate embolization is performed under local anesthesia. Local anesthesia is a safer form of anesthesia for frail patients, and it reduces the risks associated with general anesthesia. Therefore, the PAE group had fewer complications and shorter hospital stays than the TURP group. However, we found that the operation time in the PAE group was similar to that in the TURP group. The longer procedure duration of PAE was often due to difficult anatomy, including tortuosity and atherosclerotic changes of the iliac arteries[28].
The disadvantages of PAE include radiation exposure and a lack of tissue sampling for histopathological analysis[29]. Due to a lack of data, PAE radiation exposure was not evaluated in our analysis. Laborda described a case of radiation dermatitis in an obese patient after 72 minutes and 8023949 mGy cm2 of fluoroscopy exposure during a PAE procedure[30]. The radiation dose was usually decreased after approximately 10 cases were performed by interventional radiologists. In addition, the use of cone-beam CT (CBCT) reduces the risk of nontargeted embolism[31].
In the UK Register of Prostate Embolization study, PAE had a reoperation rate of 19.9% within 2 years, whereas only 5% of men who had undergone an initial TURP procedure needed repeat surgery[32]. Furthermore, patients with suboptimal outcomes after PAE were more likely to receive escalation, such as resective techniques, whereas patients were more likely to receive pharmacological treatment after TURP[33]. PAE may fill a therapeutic gap between pharmacological and surgical treatment in the treatment pathway of patients with LUTS/BPH or even replace pharmacological treatment in selected patients.
Nevertheless, our study had some limitations. The main limitation was the heterogeneity generated by different participant selections, embolization patterns, and embolization materials. Subgroup analysis, sensitivity analysis, or the use of random-effects models may reduce this heterogeneity but cannot eliminate it. In addition, the small sample sizes of some of the included studies and the absence of long-term follow-up studies added to the bias.
In our conclusion, PAE can be performed on an outpatient basis with local anesthesia as an alternative to medication and surgery. It may be an appropriate option for elderly patients, patients who are not candidates for surgery, and patients who do not want to risk the potential adverse effects of TURP, such as urinary incontinence, urinary stricture or retrograde ejaculation. Studies with large numbers of cases and long follow-up times are needed to validate the results.
Prostate artery embolization (PAE) is a promising minimally invasive therapy that improves lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH). Transurethral resection of the prostate (TURP) is the gold standard therapy for LUTS/BPH.
Although PAE is considered a therapeutic option for LUTS/BPH in the European Association of Urology guidelines and National Institute for Health and Care Excellence, controversy persists regarding PAE in the treatment of LUTS/BPH.
A literature review was performed to identify all published articles on PAE vs TURP for LUTS/BPH. Sources included PubMed, Embase, Cochrane library databases, and Chinese databases before June 2022. A systematic review and meta-analysis were conducted.
Therefore, we performed a meta-analysis to evaluate the efficacy and safety of PAE compared with TURP, which may help urologists make better choices.
Eleven studies involving 1070 participants were included. Compared with the TURP group, the PAE group had a similar effect on the International Index of Erectile Function (IPSS) score, Peak urinary flow rate (Qmax), postvoid residual volume (PVR), Prostate volume (PV), prostatic specific antigen (PSA), The International Index of Erectile Function short form (IIEF-5) scores, and erectile dysfunction during 24 mo follow-up. Lower quality of life (QoL) score, lower rate of retrograde ejaculation and shorter hospital stay in the PAE group. A higher proportion of haematuria, urinary incontinence and urinary stricture was identified in the TURP group.
PAE may be an appropriate option for elderly patients, patients who are not candidates for surgery, and patients who do not want to risk the potential adverse effects of TURP.
Studies with large cases and long follow-up time are needed to validate results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anuar MFM; Duijn M S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline Amendment 2020. J Urol. 2020;204:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Carrero-López VM, Cózar-Olmo JM, Miñana-López B. Benign prostatic hyperplasia and lower urinary tract symptoms. A review of current evidence. Actas Urol Esp. 2016;40:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Karavitakis M, Kyriazis I, Omar MI, Gravas S, Cornu JN, Drake MJ, Gacci M, Gratzke C, Herrmann TRW, Madersbacher S, Rieken M, Speakman MJ, Tikkinen KAO, Yuan Y, Mamoulakis C. Management of Urinary Retention in Patients with Benign Prostatic Obstruction: A Systematic Review and Meta-analysis. Eur Urol. 2019;75:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Pisco JM, Bilhim T, Costa NV, Torres D, Pisco J, Pinheiro LC, Oliveira AG. Randomised Clinical Trial of Prostatic Artery Embolisation Versus a Sham Procedure for Benign Prostatic Hyperplasia. Eur Urol. 2020;77:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | Malde S, Umbach R, Wheeler JR, Lytvyn L, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, Mamoulakis C, Rieken M, Speakman MJ, Gravas S, Drake MJ, Guyatt GH, Tikkinen KAO. A Systematic Review of Patients' Values, Preferences, and Expectations for the Diagnosis and Treatment of Male Lower Urinary Tract Symptoms. Eur Urol. 2021;79:796-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5636] [Article Influence: 256.2] [Reference Citation Analysis (0)] |
| 7. | Abt D, Müllhaupt G, Hechelhammer L, Markart S, Güsewell S, Schmid HP, Mordasini L, Engeler DS. Prostatic Artery Embolisation Versus Transurethral Resection of the Prostate for Benign Prostatic Hyperplasia: 2-yr Outcomes of a Randomised, Open-label, Single-centre Trial. Eur Urol. 2021;80:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 8. | Carnevale FC, Iscaife A, Yoshinaga EM, Moreira AM, Antunes AA, Srougi M. Transurethral Resection of the Prostate (TURP) Versus Original and PErFecTED Prostate Artery Embolization (PAE) Due to Benign Prostatic Hyperplasia (BPH): Preliminary Results of a Single Center, Prospective, Urodynamic-Controlled Analysis. Cardiovasc Intervent Radiol. 2016;39:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Gao YA, Huang Y, Zhang R, Yang YD, Zhang Q, Hou M, Wang Y. Benign prostatic hyperplasia: prostatic arterial embolization vs transurethral resection of the prostate--a prospective, randomized, and controlled clinical trial. Radiology. 2014;270:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Gu C, Wu Z. Application Effect of Interventional Embolization of Prostate Artery in the Patients with Hyperplasia of Prostate Gland. Chinese and Foreign Medical Treatment. 2018;37 (36):33-35. |
| 11. | Hou HY, Yang BZ. Comparison of Short-term and Long-term Effects of Prostatic Arterial Embolization, Transurethral Resection of Prostate and Conservative Treatment in Treatment of Benign Prostatic Hyperplasia. Medical & Pharmaceutical Journal of Chinese People's Liberation Army. 2016;28 (12):91-95. |
| 12. | Insausti I, Sáez de Ocáriz A, Galbete A, Capdevila F, Solchaga S, Giral P, Bilhim T, Isaacson A, Urtasun F, Napal S. Randomized Comparison of Prostatic Artery Embolization vs Transurethral Resection of the Prostate for Treatment of Benign Prostatic Hyperplasia. J Vasc Interv Radiol. 2020;31:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Qiu Z, Zhang C, Wang X, Cheng K, Liang X, Wang D, Hou S. Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: a single-center retrospective study. Wideochir Inne Tech Maloinwazyjne. 2017;12:409-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Ray AF, Powell J, Speakman MJ, Longford NT, DasGupta R, Bryant T, Modi S, Dyer J, Harris M, Carolan-Rees G, Hacking N. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int. 2018;122:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 15. | Tan X. Comparison of prostatic arterial embolization on patients with benign prostatic hyperplasia after transurethral resection of the prostate. Chinese Journal Modern Drug Application. 2018;12(14): 56-57. |
| 16. | Wang you, TANG Hua, LIN Hai, CHEN Jian, Yu-Ting, GUO. Changping SU Zhuying.Effect of prostatic arterial embolization on postoperative sexual ability in patients with benign prostatic hyperplasia after transurethral resection of the prostate. Chinese Journal of Human Sexuality. 2018;27 (8):18-21. |
| 17. | Zhu C, Huang W, Huang Z, Cai J. Prostate artery embolization and transurethral resection of the prostate for benign prostatic hyperplasia: a prospective randomized controlled trial. Chinese Journal Intervention Imaging Therapy. 2018;15 (3):134-138. |
| 18. | Pisco JM, Bilhim T, Pinheiro LC, Fernandes L, Pereira J, Costa NV, Duarte M, Oliveira AG. Medium- and Long-Term Outcome of Prostate Artery Embolization for Patients with Benign Prostatic Hyperplasia: Results in 630 Patients. J Vasc Interv Radiol. 2016;27:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 19. | Zhang J, Zhang M, Tang J, Yin G, Long Z, He L, Zhou C, Luo L, Qi L, Wang L. Animal models of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2021;24:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Gupta NK, Gange SN, McVary KT. New and Emerging Technologies in Treatment of Lower Urinary Tract Symptoms From Benign Prostatic Hyperplasia. Sex Med Rev. 2019;7:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Bilhim T, Pisco J, Rio Tinto H, Fernandes L, Campos Pinheiro L, Duarte M, Pereira JA, Oliveira AG, O'Neill J. Unilateral vs bilateral prostatic arterial embolization for lower urinary tract symptoms in patients with prostate enlargement. Cardiovasc Intervent Radiol. 2013;36:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Hwang JH, Park SW, Chang IS, Jung SI, Jeon HJ, Lho YS, Kim HG, Paick SH, Park HK. Comparison of Nonspherical Polyvinyl Alcohol Particles and Microspheres for Prostatic Arterial Embolization in Patients with Benign Prostatic Hyperplasia. Biomed Res Int. 2017;2017:8732351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Calogero AE, Burgio G, Condorelli RA, Cannarella R, La Vignera S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. 2019;22:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Chen LK, Lai YW, Chiu LP, Chen SS. Significant relationship between parameters measured by transrectal color Doppler ultrasound and sexual dysfunction in patients with BPH 12 mo after TURP. BMC Urol. 2021;21:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Müllhaupt G, Hechelhammer L, Diener PA, Engeler DS, Güsewell S, Schmid HP, Mordasini L, Abt D. Ejaculatory disorders after prostatic artery embolization: a reassessment of two prospective clinical trials. World J Urol. 2020;38:2595-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Couteau N, Duquesne I, Frédéric P, Thiounn N, Timsit MO, Mejean A, Pinar U, Audenet F. Ejaculations and Benign Prostatic Hyperplasia: An Impossible Compromise? J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Teichgräber U, Aschenbach R, Diamantis I, von Rundstedt FC, Grimm MO, Franiel T. Prostate Artery Embolization: Indication, Technique and Clinical Results. Rofo. 2018;190:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Moreira AM, de Assis AM, Carnevale FC, Antunes AA, Srougi M, Cerri GG. A Review of Adverse Events Related to Prostatic Artery Embolization for Treatment of Bladder Outlet Obstruction Due to BPH. Cardiovasc Intervent Radiol. 2017;40:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Geevarghese R, Harding J, Parsons N, Hutchinson C, Parsons C. The relationship of embolic particle size to patient outcomes in prostate artery embolisation for benign prostatic hyperplasia: a systematic review and meta-regression. Clin Radiol. 2020;75:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Laborda A, De Assis AM, Ioakeim I, Sánchez-Ballestín M, Carnevale FC, De Gregorio MA. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Cadour F, Tradi F, Habert P, Scemama U, Vidal V, Jacquier A, Bartoli JM, Moulin G, Bessayah A. Prostatic artery embolization using three-dimensional cone-beam computed tomography. Diagn Interv Imaging. 2020;101:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Eredics K, Wachabauer D, Röthlin F, Madersbacher S, Schauer I. Reoperation Rates and Mortality After Transurethral and Open Prostatectomy in a Long-term Nationwide Analysis: Have We Improved Over a Decade? Urology. 2018;118:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Carnevale FC, McClure T, Cadour F, Vidal V, de Assis AM, Moreira AM, Rocha ADD, Rebet A, Nutting C. Advanced image guidance for prostatic artery embolization - a multicenter technical note. CVIR Endovasc. 2021;4:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |