Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11743
Peer-review started: August 28, 2022
First decision: October 4, 2022
Revised: October 7, 2022
Accepted: October 18, 2022
Article in press: October 18, 2022
Published online: November 16, 2022
Processing time: 72 Days and 2.9 Hours
Upper endoscopy is the gold standard for predicting esophageal varices in China. Guidelines and consensus suggest that patients with liver cirrhosis should undergo periodic upper endoscopy, most patients undergo their first upper endoscopy when esophageal variceal bleeds. Therefore, it is important to develop a non-invasive model to early diagnose esophageal varices.
To develop a non-invasive predictive model for esophageal varices based on liver and spleen volume in viral cirrhosis patients.
We conducted a cross-sectional study based on viral cirrhosis crowd in the Second Affiliated Hospital of Xi'an Jiaotong University. By collecting the basic infor
The portal vein diameter, the liver and spleen volume, and volume change rate were the independent risk factors of esophageal varices. We successfully used the factors to establish the predictive model [area under the curve (AUC) 0.87, 95%CI: 0.80-0.95], which showed better predictive value than other models. The model showed good discriminating ability, calibration ability and the clinical practicability in both modelling group and external validation group.
The developed non-invasive predictive model can be used as an effective tool for predicting esophageal varices in viral cirrhosis patients.
Core Tip: An effective novel non-invasive predictive model based on the standard liver and spleen volume formula for esophageal varices in individuals with viral cirrhosis was developed. The model's specificity, calibrability, and clinical efficacy were superior to other models.
- Citation: Yang LB, Zhao G, Tantai XX, Xiao CL, Qin SW, Dong L, Chang DY, Jia Y, Li H. Non-invasive model for predicting esophageal varices based on liver and spleen volume. World J Clin Cases 2022; 10(32): 11743-11752
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11743.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11743
Esophageal varices (EVs) frequently occur in patients with cirrhosis. Presently, upper endoscopy is the most effective approach and gold standard for predicting EVs[1]. For patients with cirrhosis, guidelines and consensus statements strongly recommend periodic upper endoscopy[2]. In China, upper endoscopy is usually not performed under sedation, thus, the procedure may result in discomfort and psychological burden on patients. As a result, some patients do not accept upper endoscopy until EVs bleed.
In individuals with cirrhosis and liver transplantation, the measurement of the liver volume and spleen volume is essential[3,4]. Despite the usage of computed tomography (CT) and magnetic resonance imaging could be used for estimating the liver and spleen volume, these techniques were tedious. In our previous study, we used 207 healthy Chinese patient’s body surface area (BSA) data, established the standard liver volume (SLV) and standard spleen volume formulas (SSV), as follow: SLV = 858.186 × BSA-393.349 (R2 = 0.350), SSV = 188.813 × BSA - 140.981 (R2 = 0.126)[5]. Based on the derived formulas, a non-invasive predictive model for high-risk EVs in viral cirrhosis patients was developed[5].
Until now, several models were established to predict EVs. The APRI (aspartate aminotransferase platelet ratio index)[6], the AAR (aspartate aminotransferase: Alanine aminotransferase ratio)[6], LSPS (liver stiffness/Spleen diameter Platelet Score)[7] and VRI (variceal risk index)[8] are the more traditional non-invasive prediction models used to predict EVs. Liver stiffness measure (LSM), spleen diameter, platelet, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were put in the models. The use of SLV and SSV calculation formulas to construct a non-invasive prediction model for EVs in patients with liver cirrhosis has not been reported. Bavono VI recommended that liver stiffness and platelets be used to predict EVs[9]. In China, most hospitals could not offer FibroScan or FibroTouch to detect LSM. For the preliminary prediction of EVs, the study was aimed to develop a non-invasive predictive model for EVs based on liver and spleen volume in viral cirrhosis patients.
We conducted a cross-sectional study in the Second Affiliated Hospital of Xi'an Jiaotong University from October 2017 to December 2018, each participant in our study was diagnosed as viral cirrhosis and underwent upper endoscopy and CT. The diagnosis of liver cirrhosis is based on symptoms, signs, biochemical tests and more than 2 kinds of imaging findings. Inclusion criteria: Hepatitis B or C viral cirrhosis positive; upper abdominal CT, biochemical, as well as upper endoscopy examinations; over 18 years old. Exclusion criteria: With other types of cirrhosis; suspected liver tumor; benign disorders which alter spleen and liver volume; patients having medium/Large ascites; history of spleen or liver resection; other conditions that can potentially affect the portal vein/splenic vein hemodynamic; unreliable LSM; other conditions that may have an effect on LSM, like BMI > 35 kg/m2; severe weight loss or malnutrition; history of esophageal varices bleeding and endoscopic/surgical treatment; hematological diseases altering spleen volume. Based on the upper endoscopy result, subjects were defined as EVs group, others were defined as non-EVs group. A total of 111 subjected was enrolled as the modeling group, including 86 subjects with EVs and 25 subjects without EVs. Our study was approved by the Ethics Committee of Xi’an Jiaotong University, Shaanxi, China (NO. 2017-445). Informed consent was waived by the Ethics Committee of Xi’an Jiaotong University as patients were identified retrospectively, according to institutional review board exempt protocols.
We collected sex, age, body weight (BW), body height (BH), etiology, course of cirrhosis, and Child-Pugh score of the subjects. The Mosteller’s formula was used to compute the patient’s BSA {BSA = √ [BW (kg) × BH (cm)/3600]}. The Child-Pugh scoring method was used to grade cirrhosis patient’s scores. Blood test results [aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBil), direct bilirubin (DBil) and platelets (PLT)] were also collected.
The actual liver volume and spleen volume were calculated by the multi-slice spiral CT scanner (GE 128-slice spiral CT scanner; Linux Medical System, United States) with a 5 mm reconstructed layer thickness and five seconds-time interval, and were defined as CTLV and CTSV, respectively. The SLV and SSV were calculated by our previously derived formulas [SSV = 188.813 × BSA-140.981 (R2 = 0.126), and SLV = 858.186 × BSA - 393.349 (R2 = 0.350)]. The volume change was equal to the different value between the volume calculated by formulas and CT. The volume change rate was equal to the volume change divide by the volume calculated by formulas. PVSA (portal vein surface area), PVD (portal vein diameter), and SVD (spleen vein diameter) were also recorded.
LSM was performed using FibroScan (Echosens, France) and FibroTouch (Haishkell Medical Technology Centre, China) equipment[10,11]. The TE results were obtained retrospectively and presented in kilopascals (kPa). We selected the lower interquartile/lower median variability values for patients having more LSM. Blood routine assessment, hepatitis detection, liver and renal function examination were done in all patients, using suitable equipment. Experienced doctors examined EVs by the help of an electronic upper endoscopy (Olympus, Tokyo, Japan) and then assigned them into two groups: EVs group and non-EVs group.
All data analysis were carried out using IBM SPSS Statistics 26.0 (SPSS, Inc., Chicago, IL, United States). It was considered statistically significant when P < 0.05 (two side test). We set EVs diagnosis as dependent variable, data collected as independent variables. Independent variables of P < 0.05 in the univariate analysis were selected in the subsequent multiple Logistic regression to identify independent risk factors. Predictive factors were chosen from independent risk factors. Finally, independent risk factors were put into new Logistic regression. We used Hosmer-Lemeshow Goodness of Fit Test and calibration plots to test classification accuracy. We evaluated the performance of our model and that of previously constructed models: LSPS = [LSM (kPa) × SLD (cm)]/PLT (× 109/L)[7]; AAR = AST/ALT[6]; VRI = -4.364 + 0.538 × SLD (cm)-0.049 × PLT (× 109/L)-0.044 × LSM (kPa) + 0.001 × (LSM × PLT)[8]; and APRI = [AST (U/L)/AST (normal upper limit)] × 100/PLT (× 109/L)[6].
Receiver operating characteristic (ROC) curve was analyzed, area under the curve (AUC) and its corresponding 95%CI was measured to evaluate prediction effect[12]. We calculated the sensitivity (SEN), specificity (SPE), positive predictive value (PPV), negative predictive value (NPV) and Youden Index (YDI) to evaluate the accuracy and select cut-off value of prediction model.
External validation consisted of recalculating of AUC, Hosmer-Lemeshow Chi-square statistics and calibration plots to evaluate the discrimination ability and accuracy of prediction rule[13,14]. 56 subjects who met the inclusion criteria were taken in as the external validation group to prove the validity and generalization of the predictive rule. Decision curve analysis (DCA) was used to assess the population impact of using this prediction rule on clinical decision making[15,16].
A total of 111 qualified subjects were enrolled in the modeling group, 86 (77.5%) were in EVs group. External validation group included 56 subjects. The characteristics of modeling group and the external validation group were shown in Tables 1 and 2.
| Parameter | Patients with EVs, n = 86 | Patients without EVs, n = 25 | All patients, n = 111 | P value |
| Age, yr | 53.55 ± 11.58 | 51.32 ± 12.03 | 53.05 ± 11.12 | 0.28 |
| Male (%) | 47 (54.7) | 17 (68.0) | 64 (55.7) | 0.47 |
| Etiology, HBV/HCV | 73/13 | 17/8 | 90/21 | 0.51 |
| Child-Pugh class, A/B/C | 45/34/7 | 21/4/0 | 66/38/7 | < 0.01 |
| Red Sign | 36 | 0 | 36 | < 0.01 |
| Parameter | Patients with EVs, n = 37 | Patients without EVs, n = 19 | All patients, n = 56 | P value |
| Age, yr | 51.46 ± 10.87 | 53.03 ± 11.41 | 54.68 ± 10.93 | 0.39 |
| Male (%) | 20 (54.1) | 10 (52.6) | 30 (53.6) | 0.43 |
| Etiology, HBV/HCV | 27/10 | 15/4 | 42/14 | 0.56 |
| Child-Pugh class, A/B/C | 13/21/3 | 12/7/0 | 25/28/3 | < 0.05 |
| Red sign | 14 | 0 | 14 | < 0.01 |
Indices were compared between EVs group and non-EVs group (Table 3). Nine showed statistically significance in univariate analysis were entered in Logistic regression. In multivariable analysis, spleen volume change rate, liver volume change rate, CTSV, PVD and CTLV were independently associated (P < 0.05) with EVs (Table 4). The 5 variables were put in the predictive model.
| Parameter | Patients with EVs, n = 86 | Patients without EVs, n = 25 | P value |
| PVSA (mm3) | 211.96 ± 69.98 | 175.16 ± 77.43 | 0.04 |
| PVD (mm) | 13.76 ± 2.48 | 12.94 ± 2.73 | < 0.05 |
| SVD (mm) | 9.54 ± 3.21 | 7.16 ± 3.36 | < 0.01 |
| CTLV (cm3) | 913.54 ± 312.89 | 1241.92 ± 34.83 | < 0.01 |
| CTSV (cm3) | 787.78 ± 399.46 | 439.23 ± 126.25 | < 0.01 |
| SSV (cm3) | 176.44 ± 31.21 | 195.71 ± 33.88 | 0.10 |
| SLV (cm3) | 1049.40 ± 188.53 | 1136.97 ± 174.13 | 0.10 |
| Rate of liver volume change | -0.12 ± 0.55 | 0.12 ± 0.48 | < 0.01 |
| Rate of spleen volume change | 3.46 ± 2.49 | 1.38 ± 2.73 | < 0.01 |
| Liver volume change (cm3) | 135.85 ± 334.26 | 112.05 ± 280.68 | 0.01 |
| Spleen volume change (cm3) | 243.52 ± 163.89 | 611.33 ± 293.17 | < 0.01 |
| ALT (IU/L) | 32.08 ± 5.44 | 49.44 ± 7.63 | 0.16 |
| AST (IU/L) | 45.05 ± 7.98 | 65.28 ± 8.14 | 0.26 |
| LSM (KPa) | 21.68 ± 5.96 | 23.01 ± 5.24 | 0.29 |
| Parameter | Patients with | Patients without | P value |
| EVs (n = 86) | EVs (n = 25) | ||
| PVSA (mm3) | 211.96 ± 69.98 | 175.16 ± 77.43 | 0.15 |
| PVD (mm) | 13.76 ± 2.48 | 12.94 ± 2.73 | 0.02 |
| SVD (mm) | 9.54 ± 3.86 | 7.16 ± 3.36 | 0.15 |
| CTLV (cm3) | 913.54 ± 312.89 | 1241.92 ± 34.83 | 0.01 |
| CTSV(cm3) | 787.78 ± 399.46 | 439.23 ± 126.25 | 0.02 |
| Rate of liver volume change | -0.12 ± 0.55 | 0.12 ± 0.48 | 0.11 |
| Rate of spleen volume change | 3.46 ± 2.49 | 1.38 ± 2.73 | 0.04 |
| Liver volume change (cm3) | 135.85 ± 334.26 | 112.05 ± 280.68 | 0.03 |
| Spleen volume change (cm3) | 243.52 ± 163.89 | 611.33 ± 293.17 | > 0.05 |
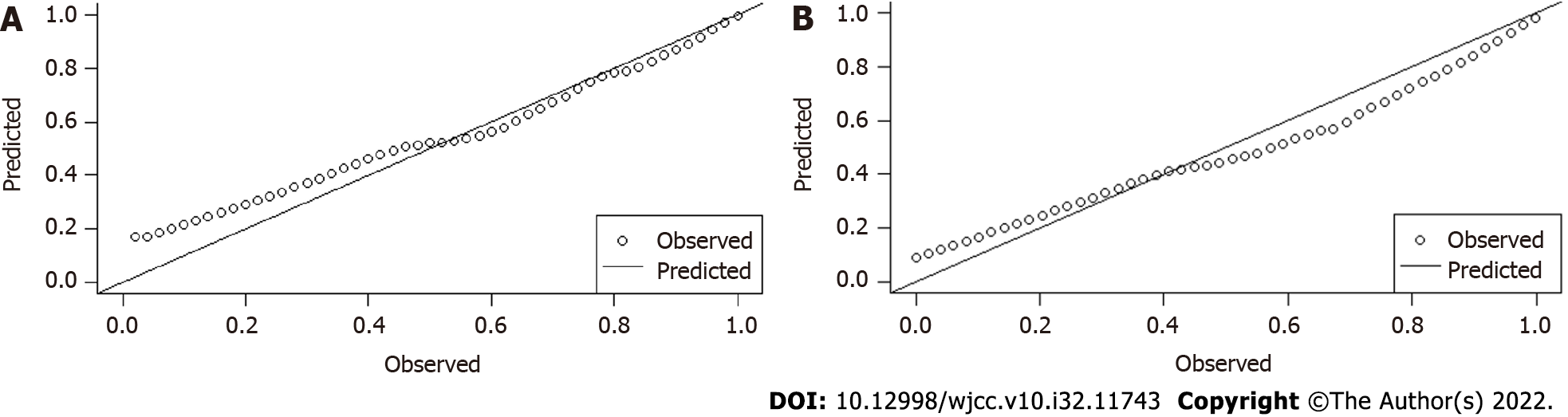
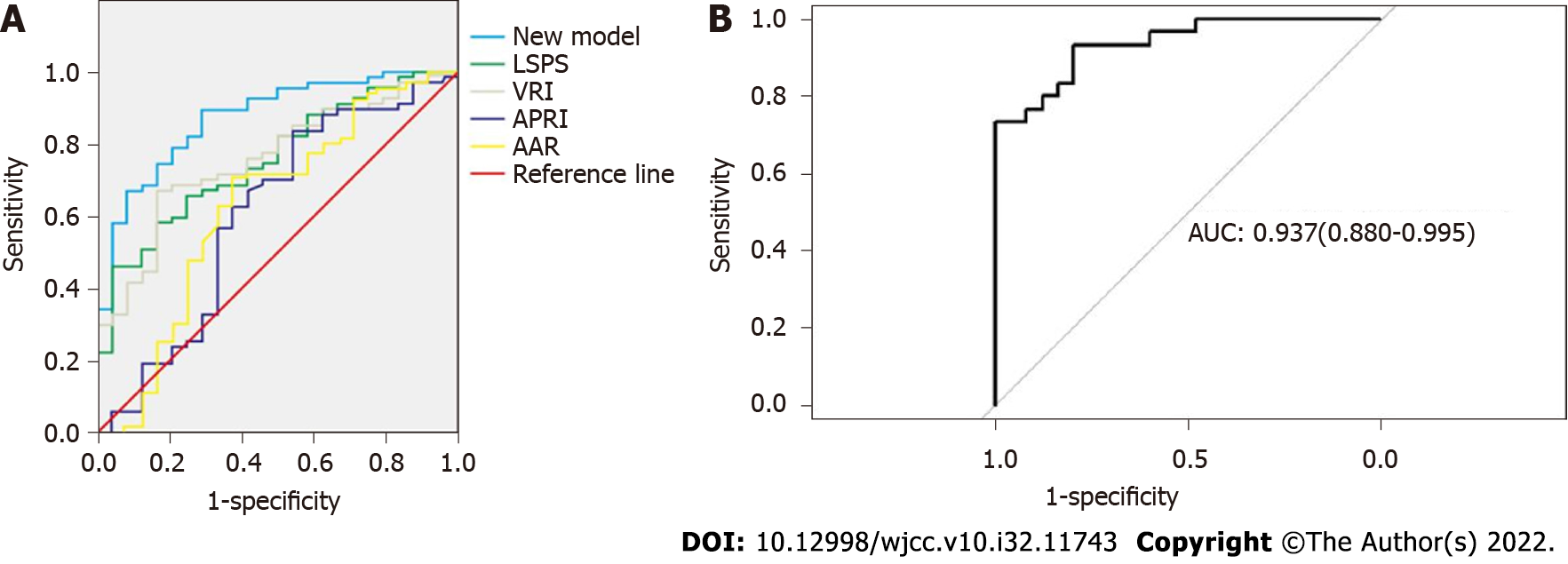
The 5 selected independent factors were input to Logistic regression (Table 5). The prediction model was determined as: ln[P/(1-P)] = 12.93-0.01 × CTLV (cm3)+0.02 × CTSV (cm3)+0.01 × (CTLV-SLV) (cm3)-1.93 × [(CTSV-SSV)/SSV]-0.22 × PVD (mm). The result of Hosmer-Lemeshow test was very good (P = 0.90), calibration plots were shown in Figure 1A. The AUC was 0.87 (95%CI: 0.80, 0.95), indicating the good discrimination ability (Figure 2A). The cut-off value was 0.85 on the ground of the maximum YDI (0.55); In light of the cut-off value, the SEN, SPE, PPV, NPV of the predictive model were 0.65, 0.90, 66.3%, 47%, respectively (Tables 6 and 7).
| Parameter | B | S.E | Wals | df | Sig. | Exp (B) | 95%CI of EXP (B) |
| PVD (mm) | -0.216 | 0.184 | 1.384 | 1 | 0.239 | 0.806 | 0.562-1.155 |
| CTLV (cm3) | -0.013 | 0.004 | 9.465 | 1 | 0.002 | 0.987 | 0.979-0.995 |
| CTSV (cm3) | 0.016 | 0.006 | 7.047 | 1 | 0.008 | 1.016 | 1.004-1.028 |
| Rate of spleen volume change | -1.929 | 0.895 | 4.646 | 1 | 0.031 | 0.145 | 0.025-0.839 |
| liver volume change (cm3) | 0.009 | 0.004 | 5.112 | 1 | 0.024 | 1.009 | 1.001-1.016 |
| Constant | 12.925 | 4.414 | 8.573 | 1 | 0.003 | 410393 |
| AUC | Standard error | Sig. | 95%CI of EXP(B) | |||||
| Lower limit | Upper limit | Sensitivity | Specificity | Youden index | ||||
| New model | 0.873 | 0.040 | 0.000 | 0.795 | 0.951 | 0.653 | 0.897 | 0.55 |
| LSPS | 0.757 | 0.053 | 0.000 | 0.652 | 0.861 | 0.667 | 0.759 | 0.426 |
| VRI | 0.757 | 0.053 | 0.000 | 0.653 | 0.862 | 0.647 | 0.828 | 0.475 |
| APRI | 0.607 | 0.074 | 0.120 | 0.462 | 0.753 | 0.696 | 0.552 | 0.248 |
| AAR | 0.627 | 0.073 | 0.067 | 0.483 | 0.770 | 0.588 | 0.793 | 0.381 |
| Accuracy, % | Positive predictive value, % | Negative predictive value, % | Cutoff value | |
| New model | 62.2 | 66.3 | 47.0 | 0.849 |
| LSPS | 52.2 | 52.2 | 36.0 | 3.77 |
| VRI | 47.8 | 52.3 | 38.5 | 0.029 |
| APRI | 53.2 | 62.8 | 20.0 | 1.17 |
| AAR | 45.0 | 41.9 | 44.0 | 1.43 |
The AUC of the developed model was 0.87, compared with, the AUCs of the VRI (0.76), LSPS (0.76), AAR (0.63), and APRI (0.61) (Figure 2A), indicating the prediction effect of the model was better than other models. The developed model posited a 62.20% accuracy and a 66.3% positive predictive value (Tables 6 and 7), which were also better than other models.
AUC of the external validation group was 0.94 (95%CI: 0.88-0.99) (Figure 2B), indicating the model’s good discrimination ability. The calibration in the external validation group was also good (P = 0.97), the calibration plots were shown in Figure 1B.
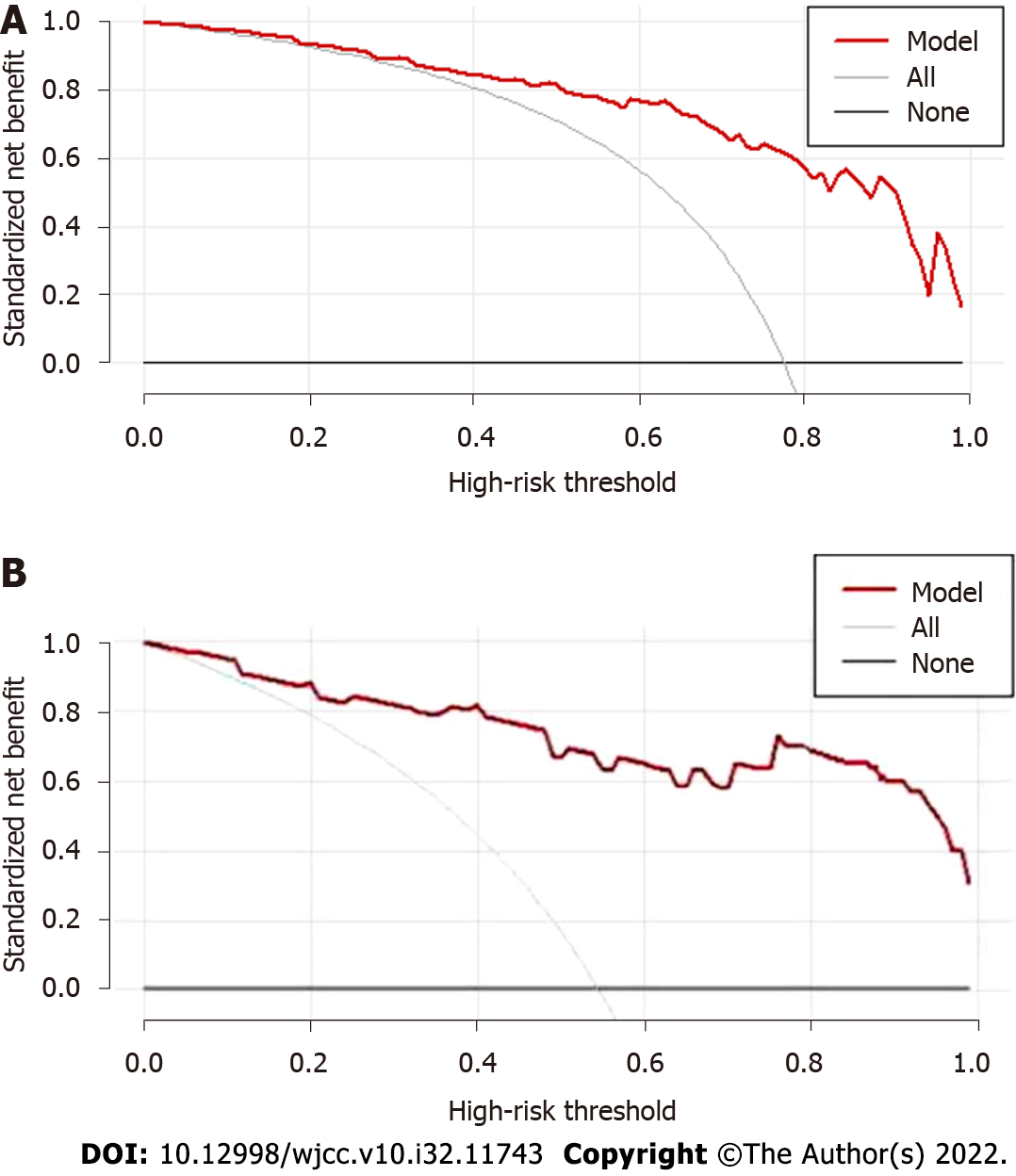
From 35% to 100% highest risk threshold, the DCA curve (Figure 3A) of predictive model was higher than both two extreme lines, showing that from 35% to 100% risk threshold, patients may benefit and the prediction model had good clinical practicability. DCA curve of external validation group showed even better clinical practicability (Figure 3B).
The non-invasive prediction of EVs can be used as an initial screening tool for cirrhosis patients with EVs. We set up a clinical prediction model based on 5 readily clinical data, including spleen volume change rate, liver volume change rate, CTSV, PVD and CTLV, to help virus cirrhosis patients screen EVs and guide upper endoscopy. This is the first prediction model established based on PVD, liver and spleen volume, liver and spleen volume change rate. Consistent with our previous study, we found spleen volume change rate is associated with high-risk EVs[5]. Kim and Berzigotti found PLT, LSM and spleen longest diameter (SLD) were independent risk factors for predicting EVs[7,8]. Baveno VI Consensus recommended LSM < 20 kPa combined with platelet count > 150 × 109/L can save 10% to 30% of upper endoscopy[17]. All the researchers put LSM in their model, which must be detected by FibroScan or FibroTouch. We approve of the above models. Actually, in China, there were no FibroScan or FibroTouch in many hospitals. In China, for patients with liver cirrhosis, CT was used as a routine examination to determine the degree of liver cirrhosis and exclude malignant lesions[18].
The AUC reflected the prediction effect of the model. Wang used APRI to predict EVs, and the AUC was 0.789, the sensitivity and specificity was 71% ans 76%[6], respectively. Which was similar to the result of our study, and was lower than our prediction model. A variety of statistical analysis were used to evaluate the predictve model in three sides: Calibration, discrimination and clinical practicability. Based on the DCA curve of modeling group, patients with a risk from 35% to 100% may benefit from the model. Cut-off value was another critical index derived from ROC analysis, and each corresponding a SEN and SPE. The cut-off value were chosen according to the maximal YDI. In our study, we set the cut-off value at 0.85, if the cut-off value was changed, sensitivity may increase while specificity decreases.
Our research has several highlights. First of all, it is applicable to hospitals without FibroScan or FibroTouch in China. The indexes included in the model can be obtained by CT and standard liver/spleen volume formula. Secondly, all the patients enrolled in the study were virus cirrhosis, with good uniformity, the results were true and reliable. Thirdly, similar to Kim’s study[7], our model aims at the prediction of EVs, and it provides an ideal quantitative tool for clinical early warning and diagnosis of EVs.
There were some limitations in our research: First, it is a retrospective study rather than cohort study and samples were collected in only one hospital, it may cause admission and selection bias; Second, this study was limited by the low sample size, and depended on CT, which has associated radiation risk. Third, due to the differences in spleen and liver volume changes between viral cirrhosis patients and other forms of cirrhosis the promotion and application of the developed model may be limited. Finally, with the discover of potential and unknown risk factors of EVs, the clinical efficacy of the prediction model may be reduced. In the future, we need to add new and potential factors to optimize and verify the predictive model.
Despite the limitations, the prediction model can help accurately and effectively predict EVs in patients with virus cirrhosis, which provides an ideal quantitative tool for clinical early warning and diagnosis of EVs.
In conclusion, based on the standard liver and spleen volume formula, a non-invasive predictive model with good discriminating ability, calibration ability and the clinical practicability was established.The model is a useful tool for early diagnosis of EVs in patients with viral cirrhosis.
Although there are reports of models predicting esophageal varices; however, there were no models based on the standard liver and spleen volume calculation formula.
It is highly important to identify virus patients with esophageal varices (EVs) and guide them for gastroscopy, and a non-invasive predictive model can be used to identify EVs.
A non-invasive predictive model for EVs based on liver and spleen volume in viral cirrhosis patients.
A cross-sectional study based on viral cirrhosis crowd were conducted in the Second Affiliated Hospital of Xi'an Jiaotong University. By collecting the participants’ basic information and clinical data of the, we derived the independent risk factors and established the prediction model of EVs. We compared the established model with others. Area under the receiver operating characteristic curve, calibration plot and decision curve analysis were used to test the discriminating ability, calibration ability and clinical practicability in both internal and external validation group.
The portal vein diameter, the liver and spleen volume, and volume change rate were successfully used to establish the predictive model, which showed better predictive value than other models. The model indicating good discriminating ability, calibration ability and clinical practicability in both modelling and external validation group.
The developed model is a credible predictor of EVs with high specificity, calibrability and clinical efficacy.
Further studies to confirm this model’s potential using larger sample sizes are recommended. Besides, there is need to develop predictive models with high diagnostic accuracy, while considering the limitations herein.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar R, India; Shen L, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Maydeo A, Patil G. How to Approach a Patient with Gastric Varices. Gastroenterology. 2022;162:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (36)] |
| 3. | Masuda Y, Yoshizawa K, Ohno Y, Mita A, Shimizu A, Soejima Y. Small-for-size syndrome in liver transplantation: Definition, pathophysiology and management. Hepatobiliary Pancreat Dis Int. 2020;19:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Ikegami T, Balci D, Jung DH, Kim JM, Quintini C. Living donor liver transplantation in small-for-size setting. Int J Surg. 2020;82S:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Yang LB, Xu JY, Tantai XX, Li H, Xiao CL, Yang CF, Zhang H, Dong L, Zhao G. Non-invasive prediction model for high-risk esophageal varices in the Chinese population. World J Gastroenterol. 2020;26:2839-2851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Wang JH, Chuah SK, Lu SN, Hung CH, Chen CH, Kee KM, Chang KC, Tai WC, Hu TH. Transient elastography and simple blood markers in the diagnosis of esophageal varices for compensated patients with hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol. 2012;27:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim DY. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102-111.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 394] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 9. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 10. | Wang R, Ren W, Zhao S, Niu X, Tan P, Du H, Nan Y. [Clinical study on FibroTouch and multi-parameter model for diagnosis of hepatic fibrosis in patients with chronic liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Feld J, Janssen HL, Abbas Z, Elewaut A, Ferenci P, Isakov V, Khan AG, Lim SG, Locarnini SA, Ono SK, Sollano J, Spearman CW, Yeh CT, Yuen MF, LeMair A; Review Team:. World Gastroenterology Organisation Global Guideline Hepatitis B: September 2015. J Clin Gastroenterol. 2016;50:691-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13773] [Cited by in RCA: 12299] [Article Influence: 286.0] [Reference Citation Analysis (0)] |
| 13. | Cai QC, Yu ED, Xiao Y, Bai WY, Chen X, He LP, Yang YX, Zhou PH, Jiang XL, Xu HM, Fan H, Ge ZZ, Lv NH, Huang ZG, Li YM, Ma SR, Chen J, Li YQ, Xu JM, Xiang P, Yang L, Lin FL, Li ZS. Derivation and validation of a prediction rule for estimating advanced colorectal neoplasm risk in average-risk Chinese. Am J Epidemiol. 2012;175:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Schroy PC 3rd, Wong JB, O'Brien MJ, Chen CA, Griffith JL. A Risk Prediction Index for Advanced Colorectal Neoplasia at Screening Colonoscopy. Am J Gastroenterol. 2015;110:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 993] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 16. | Kerr KF, Brown MD, Zhu K, Janes H. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J Clin Oncol. 2016;34:2534-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 17. | Chinese Society of Hepatology; Chinese Medical Association. [Chinese guidelines on the management of liver cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:846-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 18. | Liang Z, Liu Y, Nie Y. Efficacy Analysis of Double-Low Dynamic Contrast-Enhanced CT and Hepatic Extracellular Volume Fraction in the Diagnosis of Liver Fibrosis. Contrast Media Mol Imaging. 2022;2022: 8089914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |