Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11726
Peer-review started: August 8, 2022
First decision: September 25, 2022
Revised: October 2, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 16, 2022
Processing time: 91 Days and 22.9 Hours
There is no unified standard to predict postoperative survival in patients with tongue squamous cell carcinoma (TSCC), hence the urgency to develop a model to accurately predict the prognosis of these patients.
To develop and validate nomograms for predicting overall survival (OS) and cancer-specific survival (CSS) of patients with TSCC.
A cohort of 3454 patients with TSCC from the Surveillance, Epidemiology, and End Results (SEER) database was used to develop nomograms; another inde
Eight variables were selected and used to develop nomograms for patients with TSCC. The C-index (0.741 and 0.757 for OS and CSS in the training cohort and 0.800 and 0.830 in the validation cohort, respectively) and AUC indicated that the discrimination abilities of these nomograms were acceptable. The calibration curves of OS and CSS indicated that the predicted and actual values were consistent in both the training and validation cohorts. The NRI values (training cohort: 0.493 and 0.482 for 3- and 5-year OS and 0.424 and 0.402 for 3- and 5-year CSS; validation cohort: 0.635 and 0.750 for 3- and 5-year OS and 0.354 and 0.608 for 3- and 5-year CSS, respectively) and DCA results indicated that the nomograms were significantly better than the tumor-node-metastasis staging system in predicting the prognosis of patients with TSCC.
Our nomograms can accurately predict patient prognoses and assist clinicians in improving decision-making concerning patients with TSCC in clinical practice.
Core Tip: In order to predict prognosis more accurately and precisely, we used two cohorts to develop nomograms in predicting overall survival and cancer-specific survival of patients with tongue squamous cell carcinoma. We adhered to the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis statement-not only evaluated these nomograms in discrimination, calibration, but also their clinical utility. Additionally, the net reclassification index was also used to assess the accuracy of them. These nomograms provide patients and clinicians with an accurate prognosis, so as to facilitate patient-clinician communications and assist clinicians in improving decision-making.
- Citation: Luo XY, Zhang YM, Zhu RQ, Yang SS, Zhou LF, Zhu HY. Development and validation of novel nomograms to predict survival of patients with tongue squamous cell carcinoma. World J Clin Cases 2022; 10(32): 11726-11742
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11726.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11726
Tongue squamous cell carcinoma (TSCC) is the most common malignancy of the oral cavity and pharynx and has a high risk of local invasion and lymph node metastasis[1-3]. Surgical resection is the first-line treatment, followed by adjuvant radiotherapy, chemotherapy, or chemoradiation therapy. Despite substantial improvements in diagnostic techniques and multimodal treatment in recent years, the survival rate of TSCC remains low[4,5].
Treatment strategies for TSCC and its prognosis are based principally on the tumor-node-metastasis (TNM) cancer staging system established by the American Joint Committee on Cancer (AJCC)[6]. However, the prognoses can vary among patients with the same TNM stage who are receiving similar treatments[7-9]. Such variation suggests that the TNM staging system does not adequately predict prognosis because it does not consider patient characteristics (e.g., age and marital status) or treatment (e.g., type of surgery)[10,11]. Therefore, a new model that incorporates these variables is required to supplement the TNM staging system and accurately predict patient prognoses.
A nomogram is a graphical model that estimates the probability of a clinical event for an individual patient based on specific biological and clinical factors[12]. Nomograms are more accurate than the TNM staging system in predicting prognoses; they have been widely used to evaluate gastric[13-15], hepatocellular[16-19], and head and neck[20-23] carcinomas. However, there are few studies regarding the prediction of the prognosis of TSCC. Although Mair et al[24] predicted the prognosis of TSCC, the clinical utility of the prediction model (i.e., whether they facilitate decision-making and thus improve patient outcomes[12]) was not evaluated; thus, the model would be difficult to apply in clinical practice. Currently, individually predicting the prognosis of patients with TSCC remains insufficient.
Therefore, this study aimed to develop nomograms for predicting overall survival (OS) and cancer-specific survival (CSS) in patients with TSCC to externally validate the established nomograms (discrimination, calibration, and clinical utility) and to assist clinicians in improving therapeutic decision-making.
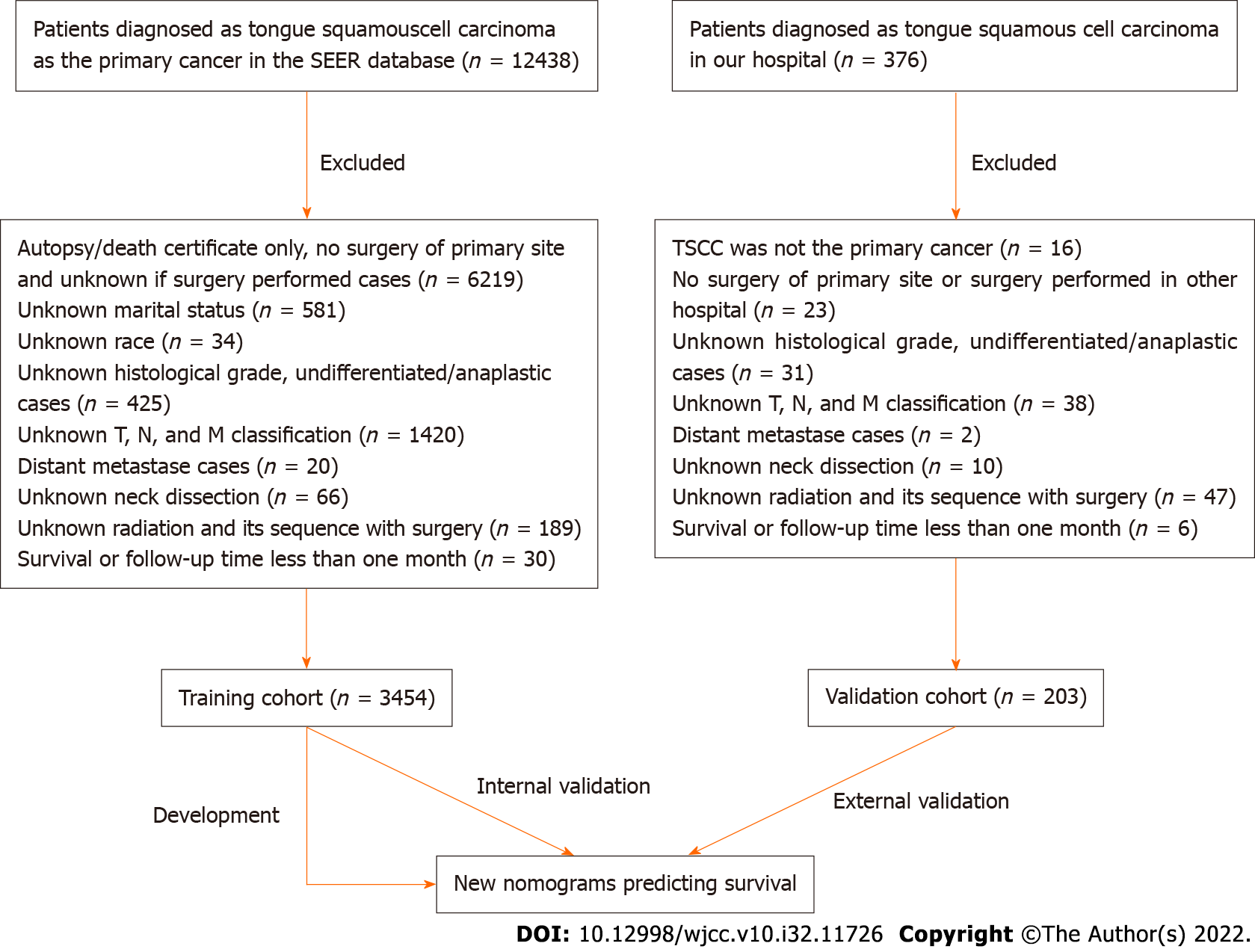
Patients diagnosed with TSCC between 2010 and 2015 were selected from the Surveillance, Epide
We retrospectively retrieved data regarding age, sex, marital status, ethnicity, tumor site, T stage, N stage, TNM stage, pathology grade, neck dissection status, and radiation treatment status. The tumor grading system of the 7th edition of the AJCC Cancer Staging Manual was used. The subclassifications of each variable are shown in Table 1. The study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
| Variables | Training cohort (n = 3454) | Validation cohort (n = 203) |
| Cases (%) | Cases (%) | |
| Age1(yr) | ||
| < 50 | 685 (19.8) | 42 (20.7) |
| ≥ 50, < 60 | 1049 (30.4) | 65 (32.0) |
| ≥ 60, < 70 | 976 (28.3) | 54 (26.6) |
| ≥ 70 | 744 (21.5) | 42 (20.7) |
| Sex | ||
| Male | 2218 (64.2) | 121(59.6) |
| Female | 1236 (35.8) | 82 (40.4) |
| Marital status | ||
| Married | 2098 (60.7) | 179 (88.2) |
| Unmarried2 | 1356 (39.3) | 24 (11.8) |
| Race | ||
| White | 2978 (86.2) | 0 |
| Black | 175 (5.1) | 0 |
| Other3 | 301 (8.7) | 203 (100) |
| Site | ||
| Anterior 2/3 of tongue4 | 2577 (74.6) | 167 (82.3) |
| Base of tongue | 877 (25.4) | 36 (17.7) |
| T stage | ||
| T1 | 1720 (49.8) | 88 (43.3) |
| T2 | 1095 (31.7) | 98 (48.3) |
| T3 | 348 (10.1) | 12 (5.9) |
| T4 | 291 (8.4) | 5 (2.5) |
| N stage | ||
| N0 | 1920 (55.6) | 133 (65.5) |
| N1 | 513 (14.9) | 24 (11.8) |
| N2 | 973 (28.2) | 45 (22.2) |
| N3 | 48 (1.4) | 1 (0.5) |
| TNM stage | ||
| I | 1237 (35.8) | 65 (32.0) |
| II | 516 (14.9) | 59 (29.1) |
| III | 565 (16.4) | 31 (15.3) |
| IV | 1136 (32.9) | 48 (23.6) |
| Pathology grade | ||
| Well differentiated | 722 (20.9) | 141 (69.5) |
| Moderately differentiated | 1787 (51.7) | 56 (27.6) |
| Poorly differentiated | 945 (27.4) | 6 (3.0) |
| Neck dissection | ||
| No | 963 (27.9) | 9 (4.4) |
| Yes | 2491 (72.1) | 194 (95.6) |
| Radiation | ||
| No radiation | 1701 (49.2) | 107 (52.7) |
| Radiation prior to surgery | 43 (1.2) | 4 (2.0) |
| Radiation after surgery | 1698 (49.2) | 73 (36.0) |
| Radiation before and after surgery | 12 (0.3) | 19 (9.4) |
First, descriptive statistics were generated for the demographic and tumor clinicopathological characteristics. Then, univariate and multivariate Cox proportional hazards models were constructed. Coefficients, hazard ratios, and 95% confidence intervals (CIs) were obtained for prognostic factors in the training cohort. Finally, nomograms that integrated significant independent risk factors were constructed based on the predicted 3- and 5-year OS and CSS in the training cohort. OS was defined as the time from surgery until death from any cause or the last follow-up. CSS was defined as the time from surgery until death from TSCC or the last follow-up.
Internal and external validation analyses were performed to assess the predictive accuracies of the nomograms for the training and validation cohorts. Discriminative ability was evaluated based on the concordance index (C-index) and area under the receiver operating characteristic curve (AUC). The C-index and AUC values are often used interchangeably and range from 0.5 to 1 (no discrimination ability and perfect discrimination, respectively)[12]. Meanwhile, a C-index or AUC value of > 0.7 indicates satisfactory discrimination. The concordance between predicted and actual survival was assessed using calibration curves. The reference line is a 45° diagonal line that ideally includes both predicted and actual survival rates.
The clinical benefits and utility of the nomograms were compared with those of the TNM staging system using the net reclassification index (NRI) and decision curve analysis (DCA). The NRI is used to assess the predictive accuracies and utility of nomograms[25,26]. The DCA is used to estimate the clinical and net benefits of nomograms based on threshold probabilities[27,28]. A horizontal reference line indicates that no intervention was performed (i.e., there was no clinical benefit), while an oblique line indicates that all patients underwent the intervention (i.e., the clinical benefit was maximized).
R statistical software (ver. 4.0.5; R Development Core Team, Vienna, Austria) was used to perform all analyses. P values < 0.05 were considered statistically significant.
The clinicopathological characteristics of the SEER cohort and our cohort are described in Table 1. Most of the patients [training cohort, n = 1049 (30.4%); validation cohort, n = 65 (32.0%)] were aged 50-59 years, and approximately 60% patients were men. Overall, the proportion of married patients was significantly greater than that of unmarried patients; the proportion of married patients was greater in the validation cohort [n = 179 (88.2%)] than in the training cohort [n = 2098 (60.7%)]. Approximately 90% of patients in the training cohort were White, whereas all patients in the validation cohort were Asian. In both cohorts, the proportion of TSCCs located on the anterior 2/3 of the tongue was greater than that located on the base of the tongue (training cohort, 74.6% vs 25.4%; validation cohort, 82.3% vs 17.7%, respectively). In both cohorts, most TSCCs were stage T1 and T2 [training cohort, n = 2815 (81.5%); validation cohort, n = 186 (91.6%)]. Meanwhile, more than half of all TSCCs were stage N0 [training cohort, n = 1920 (55.6%); validation cohort, n = 133 (65.5%)], while a few TSCCs were stage N3 [training cohort, n = 48 (1.4%); validation cohort, n = 1 (0.5%)]. The proportion of TSCCs was evenly distributed across subclassifications of TNM stages. Approximately half of the TSCCs in the training cohort was moderately differentiated, whereas 69.5% of TSCCs in the validation cohort was well-differentiated. Most of the patients in both cohorts underwent neck dissection [training cohort, n = 2491 (72.1%); validation cohort, n = 194 (95.6%)]. The proportion of patients who did and did not undergo radiation after surgery was 49.2% and 49.2% in the training cohort, and 52.7% and 36.0% in the validation cohort, respectively.
Eleven candidate variables associated with OS and CSS were evaluated by univariate and multivariate Cox analyses of the SEER cohort. Univariate analysis showed that age, marital status, ethnicity, tumor site, T stage, N stage, TNM stage, pathology grade, neck dissection status, and radiation treatment status were significantly associated with OS and CSS in (P < 0.05 for all; Tables 2 and 3). Multivariate analysis showed that age, marital status, tumor site, T stage, N stage, pathology grade, neck dissection status, and radiation treatment status were independently associated with OS and CSS (P < 0.05 for all; Tables 2 and 3).
| Variables | OS | |||
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| < 50 | 1.0 | 1.0 | ||
| ≥ 50, < 60 | 1.002 (0.821, 1.223) | 0.984 | 0.982 (0.803, 1.201) | 0.858 |
| ≥ 60, < 70 | 1.181 (0.971, 1.438) | 0.097 | 1.276 (1.045, 1.559) | 0.017 |
| ≥ 70 | 1.800 (1.482, 2.187) | < 0.001 | 2.217 (1.815, 2.710) | < 0.001 |
| Sex | ||||
| Male | 1.0 | 1.0 | ||
| Female | 0.983 (0.859, 1.124) | 0.801 | 0.901 (0.783, 1.037) | 0.147 |
| Marital status | ||||
| Married | 1.0 | 1.0 | ||
| Unmarried2 | 1.606 (1.413, 1.827) | < 0.001 | 1.388 (1.216, 1.585) | < 0.001 |
| Race | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.649 (1.296, 2.100) | < 0.001 | 1.199 (0.935, 1.538) | 0.153 |
| Other3 | 1.077 (0.857, 1.354) | 0.526 | 1.102 (0.874, 1.390) | 0.411 |
| Site | ||||
| Anterior 2/3 of tongue4 | 1.0 | 1.0 | ||
| Base of tongue | 0.757 (0.647, 0.886) | < 0.001 | 0.413 (0.342, 0.497) | < 0.001 |
| T stage | ||||
| T1 | 1.0 | 1.0 | ||
| T2 | 2.168 (1.847, 2.544) | < 0.001 | 1.969 (1.540, 2.518) | < 0.001 |
| T3 | 3.997 (3.293, 4.852) | < 0.001 | 3.142 (2.411, 4.095) | < 0.001 |
| T4 | 5.070 (4.171, 6.163) | < 0.001 | 4.682 (3.498, 6.268) | < 0.001 |
| N stage | ||||
| N0 | 1.0 | 1.0 | ||
| N1 | 2.066 (1.725, 2.475) | < 0.001 | 2.080 (1.497, 2.889) | < 0.001 |
| N2 | 2.489 (2.154, 2.878) | < 0.001 | 3.749 (2.554, 5.503) | < 0.001 |
| N3 | 3.040 (1.995, 4.634) | < 0.001 | 5.641 (3.223, 9.873) | < 0.001 |
| TNM stage | ||||
| I | 1.0 | 1.0 | ||
| II | 1.831 (1.459, 2.299) | < 0.001 | 1.011 (0.719, 1.420) | 0.952 |
| III | 2.617 (2.129, 3.216) | < 0.001 | 1.071 (0.709, 1.619) | 0.745 |
| IV | 3.439 (2.890, 4.092) | < 0.001 | 0.774 (0.475, 1.262) | 0.305 |
| Pathology grade | ||||
| Well differentiated | 1.0 | 1.0 | ||
| Moderately differentiated | 2.141 (1.748, 2.622) | < 0.001 | 1.781 (1.442, 2.200) | < 0.001 |
| Poorly differentiated | 2.045 (1.642, 2.546) | < 0.001 | 1.733 (1.360, 2.209) | < 0.001 |
| Neck dissection | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.407 (1.209, 1.638) | < 0.001 | 0.766 (0.640, 0.916) | 0.004 |
| Radiation | ||||
| No radiation | 1.0 | 1.0 | ||
| Radiation prior to surgery | 3.235 (2.156, 4.854) | < 0.001 | 1.345 (0.870, 2.080) | 0.182 |
| Radiation after surgery | 1.629 (1.425, 1.861) | < 0.001 | 0.716 (0.5994, 0.856) | < 0.001 |
| Radiation before and after surgery | 3.111 (1.472, 6.575) | 0.003 | 1.025 (0.475, 2.212) | 0.0.949 |
| Variables | CSS | |||
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| < 50 | 1.0 | 1.0 | ||
| ≥ 50, < 60 | 0.940 (0.760, 1.164) | 0.571 | 0.910 (0.734, 1.129) | 0.390 |
| ≥ 60, < 70 | 1.093 (0.885, 1.350) | 0.408 | 1.180 (0.952, 1.463) | 0.131 |
| ≥ 70 | 1.359 (1.094, 1.689) | 0.006 | 1.750 (1.399, 2.189) | < 0.001 |
| Sex | ||||
| Male | 1.0 | 1.0 | ||
| Female | 1.021 (0.879, 1.185) | 0.786 | 0.999 (0.854, 1.167) | 0.986 |
| Marital status | ||||
| Married | 1.0 | 1.0 | ||
| Unmarried2 | 1.515 (1.312, 1.749) | < 0.001 | 1.291 (1.114, 1.497) | < 0.001 |
| Race | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.739 (1.337, 2.262) | < 0.001 | 1.213 (0.925, 1.590) | 0.163 |
| Other3 | 1.079 (0.836, 1.394) | 0.558 | 1.113 (0.0.859, 1.442) | 0.420 |
| Site | ||||
| Anterior 2/3 of tongue4 | 1.0 | 1.0 | ||
| Base of tongue | 0.795 (0.669, 0.945) | 0.009 | 0.393 (0.320, 0.482) | < 0.001 |
| T stage | ||||
| T1 | 1.0 | 1.0 | ||
| T2 | 2.397 (1.994, 2.880) | < 0.001 | 1.973 (1.520, 2.561) | < 0.001 |
| T3 | 4.832 (3.898, 5.991) | < 0.001 | 3.220 (2.429, 4.268) | < 0.001 |
| T4 | 5.933 (4.771, 7.377) | < 0.001 | 4.786 (3.519, 6.510) | < 0.001 |
| N stage | ||||
| N0 | 1.0 | 1.0 | ||
| N1 | 2.756 (2.250, 3.375) | < 0.001 | 2.376 (1.635, 3.454) | < 0.001 |
| N2 | 3.401 (2.880, 4.016) | < 0.001 | 5.216 (3.337, 8.154) | < 0.001 |
| N3 | 4.400 (2.843, 6.810) | < 0.001 | 8.289 (4.498, 15.275) | < 0.001 |
| TNM stage | ||||
| I | 1.0 | 1.0 | ||
| II | 1.865 (1.405, 2.475) | < 0.001 | 1.019 (0.689, 1.507) | 0.926 |
| III | 3.597 (2.829, 4.572) | < 0.001 | 1.226 (0.766, 1.964) | 0.396 |
| IV | 4.720 (3.830, 5.816) | < 0.001 | 0.710 (0.404, 1.247) | 0.233 |
| Pathology grade | ||||
| Well differentiated | 1.0 | 1.0 | ||
| Moderately differentiated | 2.586 (2.024, 3.304) | < 0.001 | 2.895 (1.469, 2.444) | < 0.001 |
| Poorly differentiated | 2.632 (2.030, 3.414) | < 0.001 | 1.911 (1.438, 2.540) | < 0.001 |
| Neck dissection | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.710 (1.429, 2.047) | < 0.001 | 0.775 (0.628, 0.957) | 0.018 |
| Radiation | ||||
| No radiation | 1.0 | 1.0 | ||
| Radiation prior to surgery | 4.294 (2.800, 6.586) | < 0.001 | 1.511 (0.952, 2.399) | 0.080 |
| Radiation after surgery | 2.079 (1.782, 2.426) | < 0.001 | 0.769 (0.628, 0.943) | 0.012 |
| Radiation before and after surgery | 4.478 (2.112, 9.495) | < 0.001 | 1.271 (0.585, 2.760) | 0.545 |
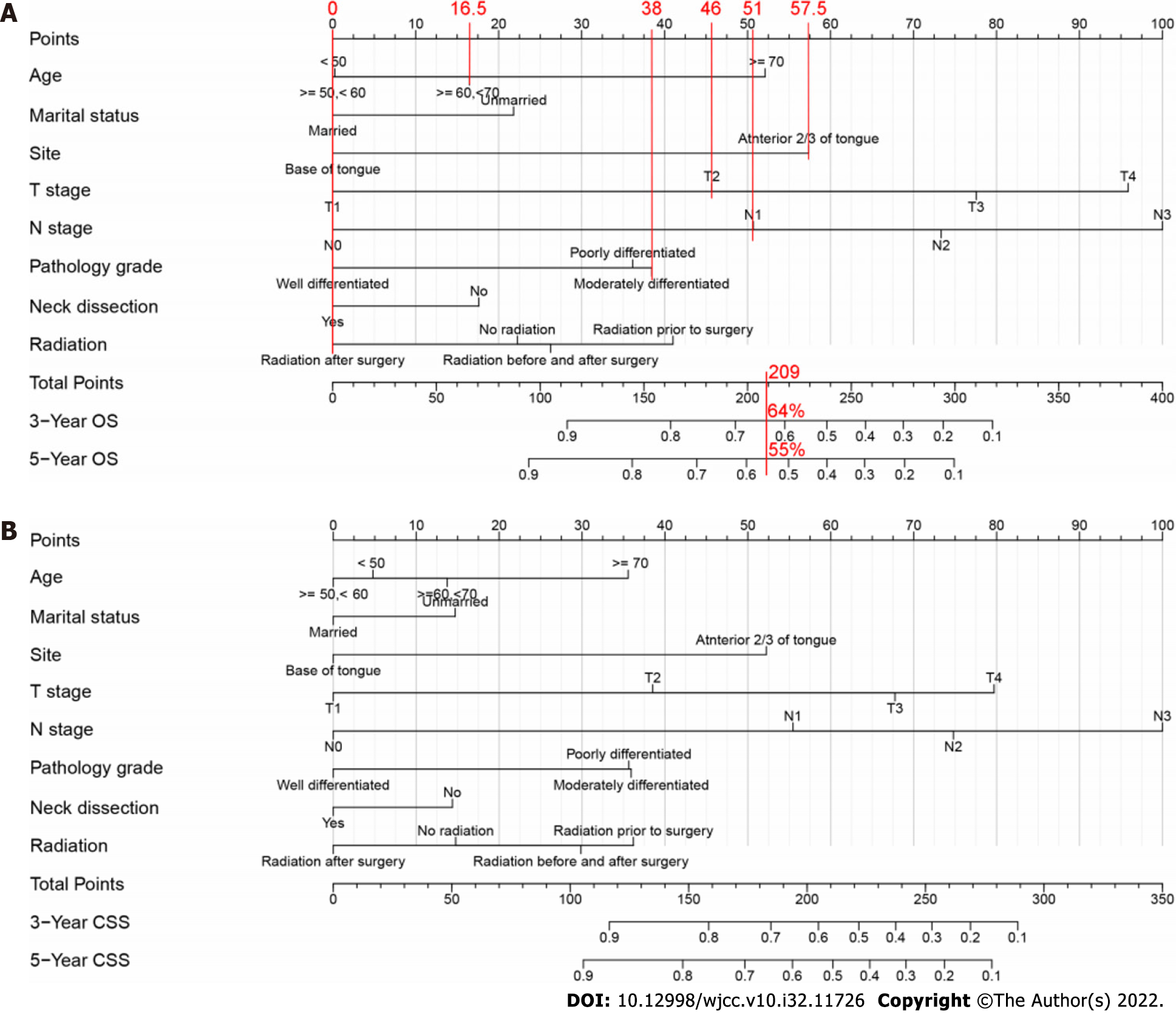
Based on the results of the multivariate analysis, eight prognostic variables (age, marital status, tumor site, T stage, N stage, pathology grade, neck dissection status, and radiation treatment status) were used to develop the nomograms. Figure 2 shows the OS and CSS predictions from the nomograms. N and T stages had the greatest effects on OS followed by tumor site and age. N stage had the greatest effect on CSS followed by T stage and tumor site. Generally, OS and CSS were better in younger patients with lower T and N stages. The predicted 3- and 5-year OS and CSS for individual patients are shown at the bottom of the nomograms based on the sum of scores across variables.
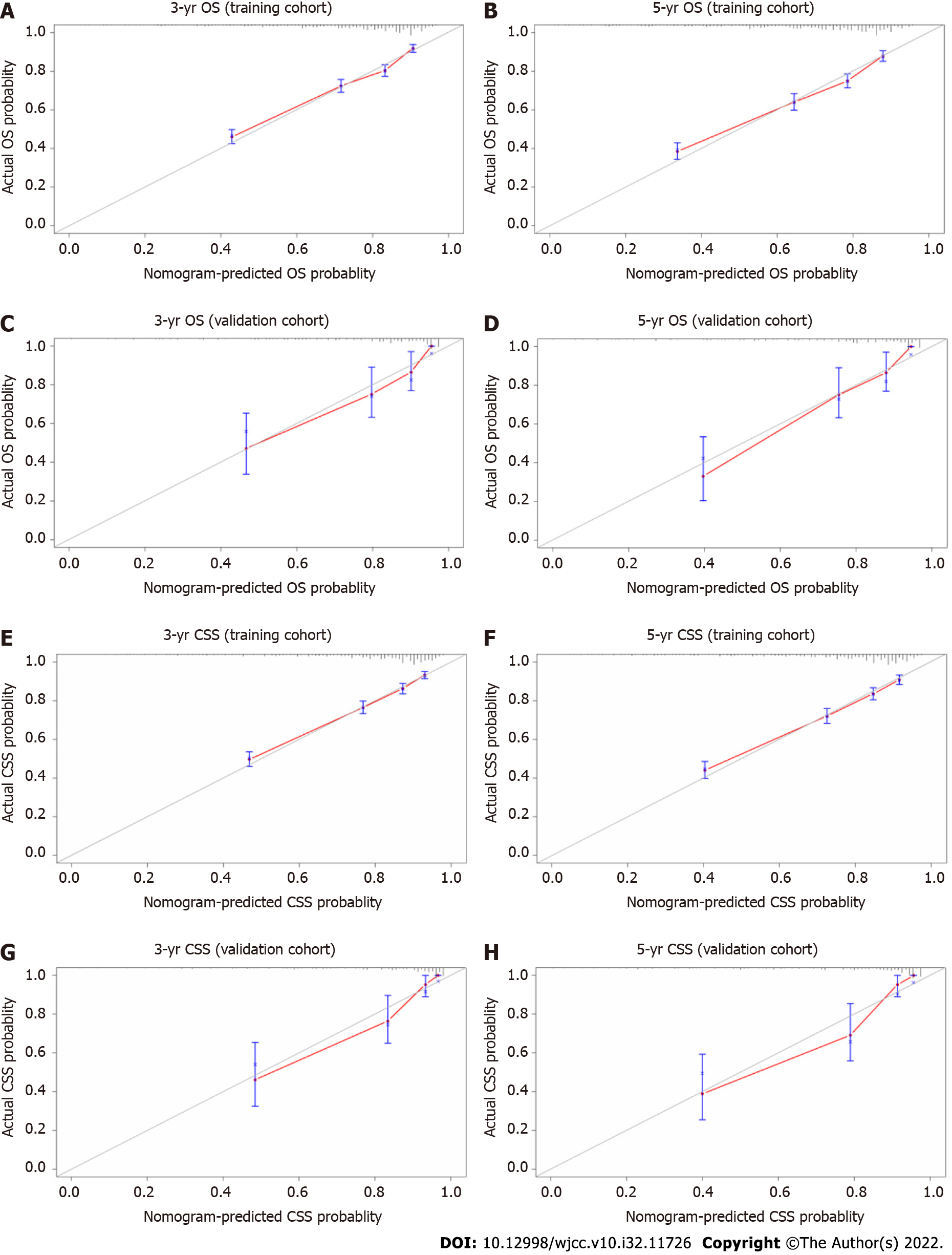
The results of the internal and external validation analyses are shown in Figure 3. In the training cohort, the internal calibration curves indicated excellent consistency between the predicted and actual 3- and 5-year OS and CSS (Figures 3A, B, E, and F), which was also observed in the validation cohort (Figures 3C, D, G, and H). The C-index values were 0.741 (95%CI: 0.725, 0.756) and 0.757 (95%CI: 0.739, 0.775) for OS and CSS in the internal validation analysis; these respective values were 0.800 (95%CI: 0.747, 0.853) and 0.830 (95%CI: 0.779, 0.881) in the external validation analysis, respectively (Table 4). Overall, the nomograms exhibited satisfactory discrimination and calibration.
| Index | Training cohort | Validation cohort | ||
| Estimate | 95%CI | Estimate | 95%CI | |
| NRI (vs TNM stage) | ||||
| For 3-year OS | 0.493 | (0.418, 0.589) | 0.635 | (0.228, 1.096) |
| For 5-year OS | 0.482 | (0.413, 0.613) | 0.750 | (0.397, 1.240) |
| For 3-year CSS | 0.424 | (0.354, 0.523) | 0.354 | (0.145, 1.037) |
| For 5-year CSS | 0.402 | (0.345, 0.536) | 0.608 | (0.180, 1.186) |
| C-index | ||||
| The nomogram OS | 0.741 | (0.725, 0.756) | 0.800 | (0.747, 0.853) |
| The nomogram CSS | 0.757 | (0.739, 0.775) | 0.830 | (0.779, 0.881) |
| TNM stage OS | 0.643 | (0.636, 0.668) | 0.695 | (0.617, 0.750) |
| TNM stage CSS | 0.678 | (0.660, 0.696) | 0.749 | (0.673, 0.825) |
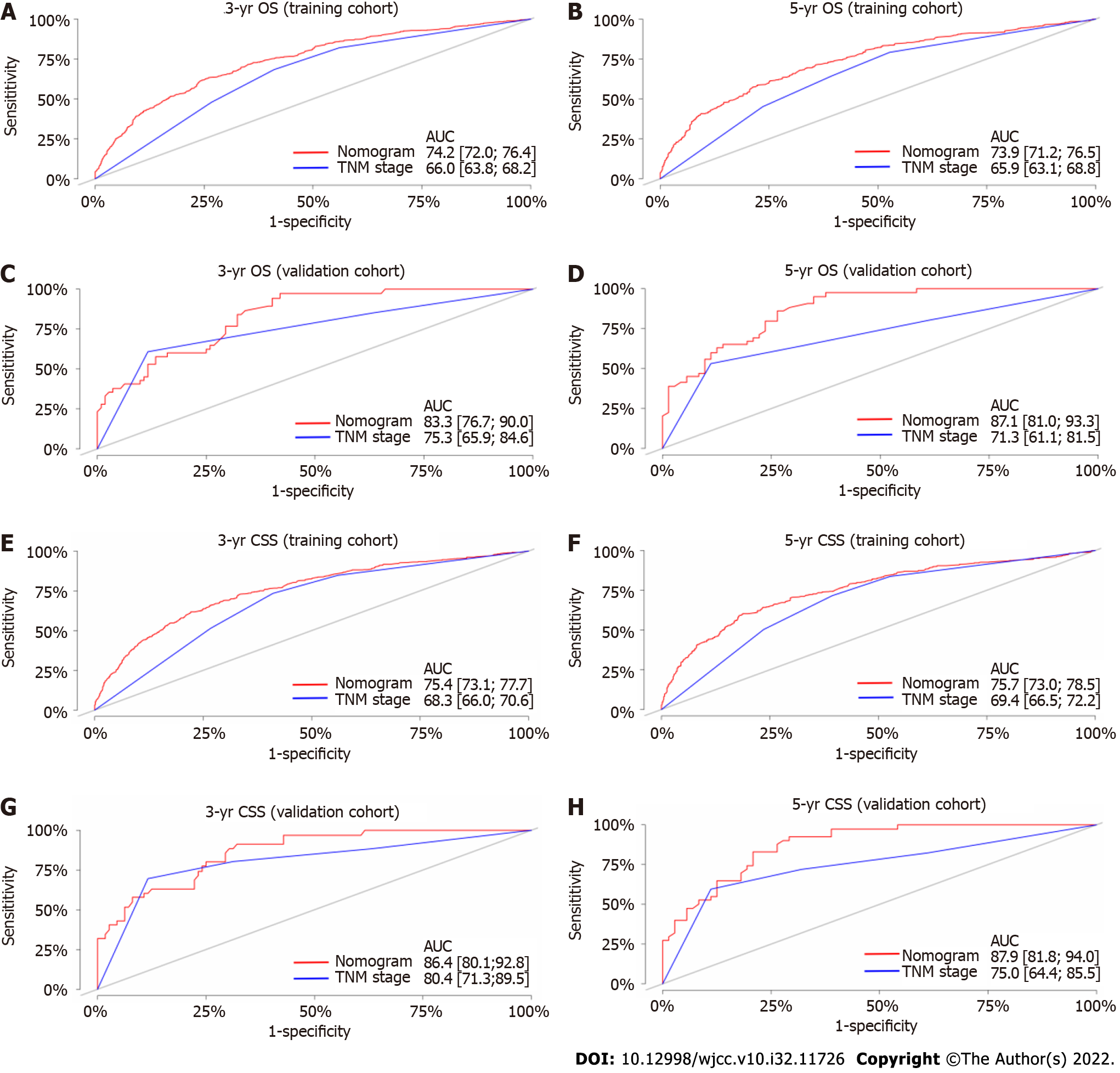
The C-index values of the TNM staging system for OS and CSS were also estimated in both the internal and external validation analyses (Table 4). The C-index values of the nomograms were higher than those of the TNM staging system (Table 4). In terms of predictive accuracy, the AUC values for the nomograms were higher than those of the TNM staging system (3-year OS, 74.2 vs 66.0; 5-year OS, 73.9 vs 65.9; 3-year CSS, 75.4 vs 68.3; 5-year CSS, 75.7 vs 69.4) in the training cohort (Figures 4A, B, E, and F) as well as in the validation cohort (3-year OS, 83.3 vs 75.3; 5-year OS, 87.1 vs 71.3; 3-year CSS, 86.4 vs 80.4; 5-year CSS, 87.9 vs 75.0) (Figures 4C, D, G, and H).
As shown in Table 4, the NRI values for the 3- and 5-year OS and CSS in the training cohort were 0.493 (95%CI: 0.418, 0.589) and 0.482 (95%CI: 0.413, 0.613), and 0.424 (95%CI: 0.354, 0.523) and 0.402 (95%CI: 0.345, 0.536), respectively, which were confirmed in the validation cohort (Table 4). Notably, the nomograms performed significantly better than the TNM staging system in both the training and validation cohorts.
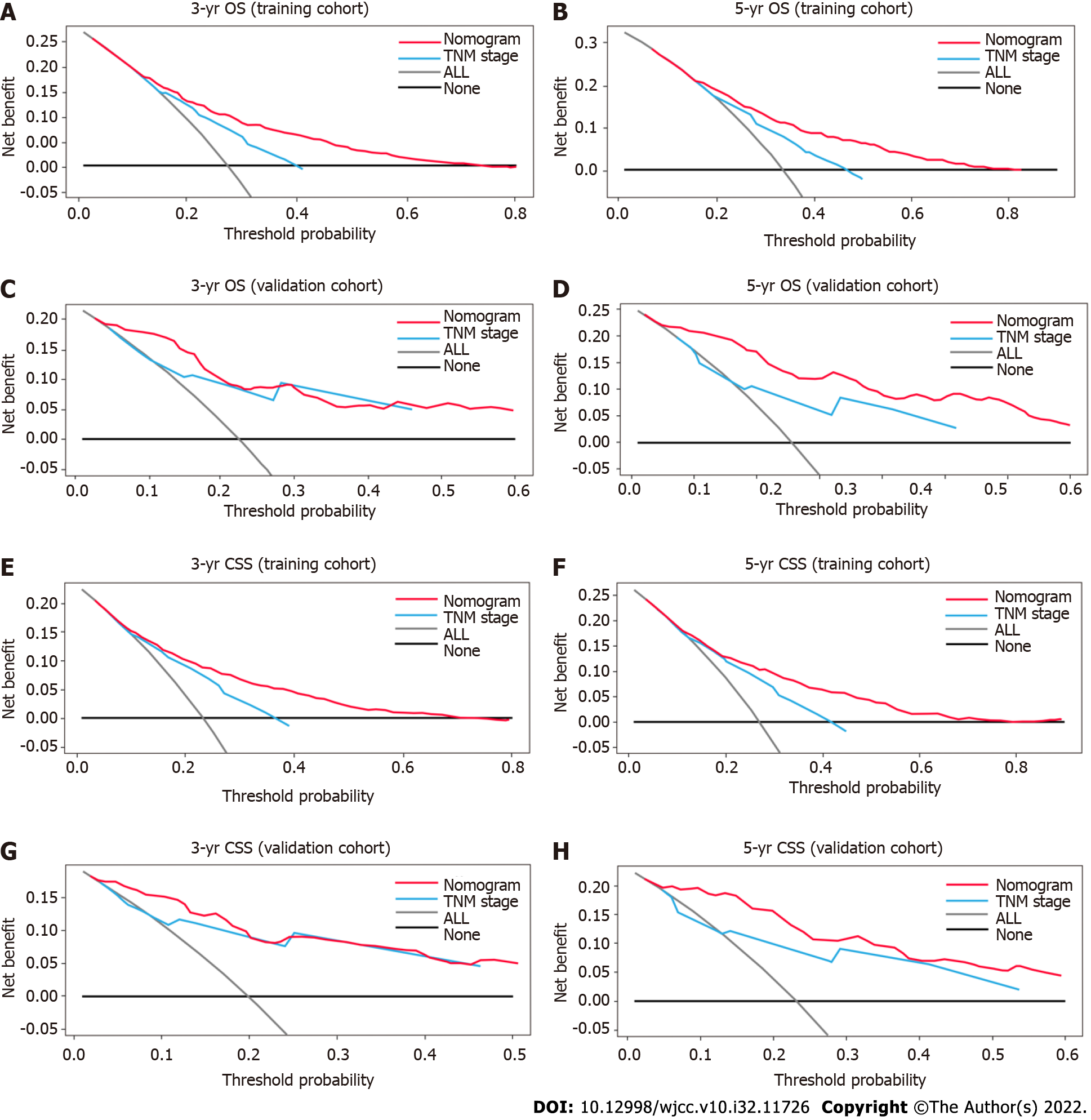
The DCA was used to compare clinical benefits between the nomograms and the TNM staging system. As shown in Figure 5, the nomograms exhibited greater net benefits than the TNM staging system at all threshold probabilities in the training cohort (i.e., they were better able to predict both 3- and 5-year OS and CSS). For the 3-year OS and CSS in the validation cohort, the net benefits of the TNM staging system were generally equivalent to the nomograms, whereas the nomograms showed greater net benefits than the TNM staging system at almost all threshold probabilities for the 5-year OS and CSS.
We developed new nomograms to predict the 3- and 5-year OS and CSS in patients with TSCC, evaluated their discrimination and calibration abilities, and compared their clinical utilities with those of the TNM staging system. Our results showed that our nomograms accurately predicted both the OS and CSS of patients with TSCC. Additionally, the C-index and AUC values along with the calibration curves showed that the nomograms had satisfactory discrimination and calibration. Moreover, compared with the TNM staging system, the predictive accuracies of OS and CSS were higher for the nomograms, as revealed by the NRI values and DCA curves. Thus, the aforementioned results indicate that our nomograms exhibited satisfactory discrimination, calibration, and clinical utility.
In this study, age, marital status, tumor site, T stage, N stage, pathology grade, neck dissection status, and radiation treatment status were selected to develop nomograms to predict the 3- and 5-year OS and CSS of patients with TSCC. As an example, Figure 2 compares two patients with similar staging results but different treatments. The first patient was 60 years old, married, and with T2 and N1 stage cancer on the anterior 2/3 of the tongue that exhibited moderate differentiation; that patient underwent neck dissection and received postoperative chemotherapy. The second patient was 70 years old, unmarried, and with T2 and N1 stage cancer on the anterior 2/3 of the tongue that exhibited high differentiation; that patient underwent neck dissection but did not receive radiation treatment. According to the conventional TNM staging system, both patients had the same TNM stage and therefore should have similar OS. However, our nomograms predicted that the respective 3- and 5-year OS were 64% and 55% for the first patient, whereas they were 43% and 33% for the second patient. The inclusion of additional information regarding clinicopathological characteristics and demographics provides our nomograms with a more accurate prognosis prediction ability; we expect these nomograms to serve as a powerful supplement to the TNM staging system for predicting prognoses.
The N stage had the greatest prognostic power followed by T stage, tumor site, and age (Figure 2). Advanced T and N stages were associated with poor OS and CSS, consistent with findings in previous studies[4,9]. These results indicate that the prognosis of patients with TSCC is greatly affected by the T and N stages; the more advanced the T and/or N stage, the worse the OS and CSS. Meanwhile, the inclusion of age and radiation treatment status in our nomograms may be considered controversial. Previous studies revealed that age was independently associated with both OS and CSS; younger patients had better survival, whereas older patients had a significantly greater mortality risk[29-31]. Moreover, compared with younger patients, older patients with advanced tumor stages (III, IV) had a nearly two-fold greater mortality risk. Similar to radiation treatment, surgery alone is generally associated with a high risk of relapse, particularly in patients with advanced TSCC; adjuvant therapies are thus necessary[32]. Radiation treatment has been shown to improve locoregional control and survival in patients with TSCC after surgery, particularly in advanced cases[33-36]. Here we found that the ability of radiation treatment status for predicting OS and CSS was not inferior to that of pathology grade (Figure 2). Additionally, as shown in Tables 2 and 3, age and radiation treatment status were independent predictors of OS and CSS in patients with TSCC. Taken together, our results indicate that age and radiation treatment status have prognostic significance. It has been demonstrated that marital status is an independent prognostic factor in patients with TSCC[9]. Married patients had better OS and CSS than unmarried patients[37], which is consistent with our findings in this study. We found the independent and significant role of marital status as a prognostic factor of patients with TSCC. In addition to the above variables, our study identified tumor site, pathology grade, and neck dissection status as independent prognostic factors of patients with TSCC. The OS and CSS of patients with TSCC are affected by these factors, which are shown in Tables 2 and 3, and Figure 2.
Our nomograms accurately and effectively predicted the prognosis of patients with TSCC and exhibited high clinical potential. The satisfactory discrimination and calibration abilities of these nomograms were confirmed by the calibration and receiver operating characteristic curves as well as the C-index and AUC values. The C-index values in external validation were higher than that in the training cohort, which is consistent with that constructed by Lu and Zhang for predicting tongue cancer and low-grade endometrial stromal sarcoma, respectively[7,38]. These results may indicate the extensionality and applicability of the constructed model. Moreover, we also compared the clinical utilities of the established nomograms with that of the TNM staging system, with the NRI values indicating that our nomograms had significantly better predictive accuracy. Similarly, DCA revealed that the nomograms had more clinical benefits and were better able to predict survival compared with the TNM staging system.
To reduce potential bias, we used multi-institution and multi-population data from the SEER database to develop our nomograms and to validate their discrimination and calibration abilities as well as their clinical utilities in both internal and external cohorts. Additionally, we adhered to the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis statement[39]. In summary, our nomograms were used to accurately determine the clinical prognosis of patients with TSCC.
Due to its retrospective nature, this study has some limitations. First, the depth of invasion (DOI) has been recognized as an independent predictor of survival[8,40]. Among the tumor parameters that were significant for prognosis, such as the tumor width, area, volume, and depth, the DOI was considered the most important[41]. Additionally, extranodal extension (ENE) has been widely recognized as a significant poor prognostic factor for patients with HNSCC[42,43]. Hence, the DOI and ENE were incorporated into the T and N classification, respectively, in the AJCC 8th edition of the cancer staging manual[44]. However, they were not available in the SEER database, thus not being included in our constructed model. Further improvements by incorporating these factors into the constructed nomogram should be undertaken in the future. Second, the current model only incorporates clinicopathological parameters to predict patient outcomes, which is nonsufficient for screening patients appropriate for adjuvant therapies, especially preoperative/postoperative adjuvant immunotherapy. More molecular markers should be incorporated into the constructed model to improve its clinical application value, such as PD-1[45-47], CD47[48], CXCL11[49], and CXCR3[50], which have been reported to engage in tumor immunity and included in some efficient predictive models. Third, this retrospective study had an unavoidable risk of selection bias. Thus, prospective validation studies are needed before these nomograms can be used in clinical practice.
We used two databases to develop and validate new nomograms for predicting the 3- and 5-year OS and CSS in patients with TSCC. Compared with the TNM staging system, these nomograms exhibit greater accuracy, effectiveness, and clinical utility for predicting the prognosis of patients with TSCC. Thus, they are a strong complement to the TNM staging system in the prediction of patient prognosis.
There is no unified standard to predict postoperative survival in patients with tongue squamous cell carcinoma (TSCC), hence the urgency to develop a model to accurately predict the prognosis of these patients.
Development of new models for predicting survival in patients with TSCC is important for facilitating patient-clinician communications and assisting clinicians in improving decision-making.
This study aimed to develop nomograms for predicting overall survival and cancer-specific survival in patients with TSCC based on demographic and histopathological variables, and to externally validate the established nomograms.
Two databases of patients with TSCC were used to develop nomograms and to perform external validation, respectively.
Eight variables were selected and used to develop nomograms for patients with TSCC. The C-index and area under the curve indicated that the discrimination abilities of these nomograms were acceptable. The calibration curves indicated that predicted and actual values were consistent. The NRI values and decision curve analysis results indicated that the nomograms were significantly better than the TNM staging system in predicting the prognosis of patients with TSCC.
The nomograms we developed exhibit great accuracy, effectiveness, and clinical utility for predicting the prognosis of patients with TSCC.
In addition to the demographic and histopathological characteristics, some molecular markers that have an impact on survival, such as PD-1, CD47, CXCL11, may be incorporated to predict the prognosis of patients with TSCC in future.
We thank Gui-Qi Zhu in the University of Fudan for his guidance in using R statistical software. He has no responsibility for the manuscript content.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eccher A, Italy; Tang XB, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64217] [Article Influence: 16054.3] [Reference Citation Analysis (174)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11375] [Article Influence: 3791.7] [Reference Citation Analysis (4)] |
| 3. | Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 230] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 4. | Li Y, Zhao Z, Liu X, Ju J, Chai J, Ni Q, Ma C, Gao T, Sun M. Nomograms to estimate long-term overall survival and tongue cancer-specific survival of patients with tongue squamous cell carcinoma. Cancer Med. 2017;6:1002-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | da Silva Souto AC, Vieira Heimlich F, Lima de Oliveira L, Bergmann A, Dias FL, Spíndola Antunes H, de Melo AC, Thuler LCS, Cohen Goldemberg D. Epidemiology of tongue squamous cell carcinoma: A retrospective cohort study. Oral Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6452] [Article Influence: 430.1] [Reference Citation Analysis (0)] |
| 7. | Lu Z, Yan W, Liang J, Yu M, Liu J, Hao J, Wan Q, Luo C, Chen Y. Nomogram Based on Systemic Immune-Inflammation Index to Predict Survival of Tongue Cancer Patients Who Underwent Cervical Dissection. Front Oncol. 2020;10:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Chang B, He W, Ouyang H, Peng J, Shen L, Wang A, Wu P. A Prognostic Nomogram Incorporating Depth of Tumor Invasion to Predict Long-term Overall Survival for Tongue Squamous Cell Carcinoma With R0 Resection. J Cancer. 2018;9:2107-2115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Sun W, Cheng M, Zhuang S, Chen H, Yang S, Qiu Z. Nomograms to predict survival of stage IV tongue squamous cell carcinoma after surgery. Medicine (Baltimore). 2019;98:e16206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kantola S, Parikka M, Jokinen K, Hyrynkangs K, Soini Y, Alho OP, Salo T. Prognostic factors in tongue cancer - relative importance of demographic, clinical and histopathological factors. Br J Cancer. 2000;83:614-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Aksu G, Karadeniz A, Saynak M, Fayda M, Kadehci Z, Kocaelli H. Treatment results and prognostic factors in oral tongue cancer: analysis of 80 patients. Int J Oral Maxillofac Surg. 2006;35:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2376] [Article Influence: 237.6] [Reference Citation Analysis (0)] |
| 13. | Lu J, Xu BB, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Truty MJ, Huang CM. Development and External Validation of a Nomogram to Predict Recurrence-Free Survival After R0 Resection for Stage II/III Gastric Cancer: An International Multicenter Study. Front Oncol. 2020;10:574611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Gao Z, Ni J, Ding H, Yan C, Ren C, Li G, Pan F, Jin G. A nomogram for prediction of stage III/IV gastric cancer outcome after surgery: A multicenter population-based study. Cancer Med. 2020;9:5490-5499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Chen D, Liu Z, Liu W, Fu M, Jiang W, Xu S, Wang G, Chen F, Lu J, Chen H, Dong X, Li G, Chen G, Zhuo S, Yan J. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun. 2021;12:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 16. | Fang Q, Chen H. Development of a Novel Autophagy-Related Prognostic Signature and Nomogram for Hepatocellular Carcinoma. Front Oncol. 2020;10:591356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Huang WY, Tsai CL, Que JY, Lo CH, Lin YJ, Dai YH, Yang JF, Shen PC, Lee MH, Cheng JC. Development and Validation of a Nomogram for Patients with Nonmetastatic BCLC Stage C Hepatocellular Carcinoma after Stereotactic Body Radiotherapy. Liver Cancer. 2020;9:326-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Wang YY, Xiang BD, Ma L, Zhong JH, Ye JZ, Wang K, Xing BC, Li LQ. Development and Validation of a Nomogram to Preoperatively Estimate Post-hepatectomy Liver Dysfunction Risk and Long-term Survival in Patients With Hepatocellular Carcinoma. Ann Surg. 2021;274:e1209-e1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Serenari M, Han KH, Ravaioli F, Kim SU, Cucchetti A, Han DH, Odaldi F, Ravaioli M, Festi D, Pinna AD, Cescon M. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 20. | Mell LK, Shen H, Nguyen-Tân PF, Rosenthal DI, Zakeri K, Vitzthum LK, Frank SJ, Schiff PB, Trotti AM 3rd, Bonner JA, Jones CU, Yom SS, Thorstad WL, Wong SJ, Shenouda G, Ridge JA, Zhang QE, Le QT. Nomogram to Predict the Benefit of Intensive Treatment for Locoregionally Advanced Head and Neck Cancer. Clin Cancer Res. 2019;25:7078-7088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Li X, Guo K, Feng Y, Guo Y. Analysis of chemotherapy effect on the second primary malignancy for head and neck cancer patients by a nomogram based on SEER database. Cancer Med. 2020;9:8029-8042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Huang Y, Liu Z, Zhong L, Wen Y, Ye Q, Cao D, Li P, Liu Y. Construction of an 11-microRNA-based signature and a prognostic nomogram to predict the overall survival of head and neck squamous cell carcinoma patients. BMC Genomics. 2020;21:691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Chen L, Wen Y, Zhang J, Sun W, Lui VWY, Wei Y, Chen F, Wen W. Prediction of radiotherapy response with a 5-microRNA signature-based nomogram in head and neck squamous cell carcinoma. Cancer Med. 2018;7:726-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Mair M, Nair D, Nair S, Malik A, Mishra A, Kannan S, Bobdey S, Singhvi H, Chaturvedi P. Comparison of tumor volume, thickness, and T classification as predictors of outcomes in surgically treated squamous cell carcinoma of the oral tongue. Head Neck. 2018;40:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | van Smeden M, Moons KGM. Event rate net reclassification index and the integrated discrimination improvement for studying incremental value of risk markers. Stat Med. 2017;36:4495-4497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Thomas LE, O'Brien EC, Piccini JP, D'Agostino RB, Pencina MJ. Application of net reclassification index to non-nested and point-based risk prediction models: a review. Eur Heart J. 2019;40:1880-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313:409-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 530] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 28. | Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3515] [Cited by in RCA: 3459] [Article Influence: 182.1] [Reference Citation Analysis (1)] |
| 29. | Mukdad L, Heineman TE, Alonso J, Badran KW, Kuan EC, St John MA. Oral tongue squamous cell carcinoma survival as stratified by age and sex: A surveillance, epidemiology, and end results analysis. Laryngoscope. 2019;129:2076-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Ansarin M, De Berardinis R, Corso F, Giugliano G, Bruschini R, De Benedetto L, Zorzi S, Maffini F, Sovardi F, Pigni C, Scaglione D, Alterio D, Cossu Rocca M, Chiocca S, Gandini S, Tagliabue M. Survival Outcomes in Oral Tongue Cancer: A Mono-Institutional Experience Focusing on Age. Front Oncol. 2021;11:616653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Tagliabue M, Belloni P, De Berardinis R, Gandini S, Chu F, Zorzi S, Fumagalli C, Santoro L, Chiocca S, Ansarin M. A systematic review and meta-analysis of the prognostic role of age in oral tongue cancer. Cancer Med. 2021;10:2566-2578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Langendijk JA, Ferlito A, Takes RP, Rodrigo JP, Suárez C, Strojan P, Haigentz M Jr, Rinaldo A. Postoperative strategies after primary surgery for squamous cell carcinoma of the head and neck. Oral Oncol. 2010;46:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Fuwa N, Kodaira T, Furutani K, Tachibana H, Nakamura T, Nakahara R, Tomoda T, Inokuti H, Daimon T. Arterial chemoradiotherapy for locally advanced tongue cancer: analysis of retrospective study of therapeutic results in 88 patients. Int J Radiat Oncol Biol Phys. 2008;72:1090-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Yokota T, Iida Y, Ogawa H, Kamijo T, Onozawa Y, Todaka A, Hamauchi S, Onoe T, Nakagawa M, Yurikusa T, Tanuma A, Yamashita A, Nishimura T, Yasui H, Onitsuka T. Prognostic Factors and Multidisciplinary Postoperative Chemoradiotherapy for Clinical T4a Tongue Cancer. Oncology. 2016;91:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Kim TH, Cha IH, Choi EC, Kim HR, Kim HJ, Kim SH, Keum KC, Lee CG. Postoperative Concurrent Chemoradiotherapy Versus Radiotherapy Alone for Advanced Oral Cavity Cancer in the Era of Modern Radiation Techniques. Front Oncol. 2021;11:619372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Silva PB, Lemos JV, Borges MM, do Rêgo TJ, Dantas TS, Leite CH, Lima MV, Cunha MP, Sousa FB. Prognostic factors on surgically and non-surgically treated oral squamous cell carcinoma: Advances in survival in fifteen years of follow up. J Clin Exp Dent. 2021;13:e240-e249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Sun W, Qiu Z, Tan W, Liu Z, Wang Z, Huang W, Cao M. The influence of marital status on survival in patients with oral tongue squamous cell carcinoma. Oncotarget. 2017;8:82092-82102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, Song Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun (Lond). 2020;40:301-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 39. | Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 2277] [Article Influence: 227.7] [Reference Citation Analysis (0)] |
| 40. | Tam S, Amit M, Zafereo M, Bell D, Weber RS. Depth of invasion as a predictor of nodal disease and survival in patients with oral tongue squamous cell carcinoma. Head Neck. 2019;41:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 41. | Yuen AP, Lam KY, Wei WI, Ho CM, Chow TL, Yuen WF. A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume, and clinicopathological features of oral tongue carcinoma. Am J Surg. 2000;180:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | de Juan J, García J, López M, Orús C, Esteller E, Quer M, León X. Inclusion of extracapsular spread in the pTNM classification system: a proposal for patients with head and neck carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Wreesmann VB, Katabi N, Palmer FL, Montero PH, Migliacci JC, Gönen M, Carlson D, Ganly I, Shah JP, Ghossein R, Patel SG. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1192-E1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 44. | Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 1049] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 45. | Girolami I, Pantanowitz L, Munari E, Martini M, Nocini R, Bisi N, Molteni G, Marchioni D, Ghimenton C, Brunelli M, Eccher A. Prevalence of PD-L1 expression in head and neck squamous precancerous lesions: a systematic review and meta-analysis. Head Neck. 2020;42:3018-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Paolino G, Pantanowitz L, Barresi V, Pagni F, Munari E, Moretta L, Brunelli M, Bariani E, Vigliar E, Pisapia P, Malapelle U, Troncone G, Girolami I, Eccher A. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol Res Pract. 2021;226:153605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 47. | Munari E, Mariotti FR, Quatrini L, Bertoglio P, Tumino N, Vacca P, Eccher A, Ciompi F, Brunelli M, Martignoni G, Bogina G, Moretta L. PD-1/PD-L1 in Cancer: Pathophysiological, Diagnostic and Therapeutic Aspects. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 48. | Pai S, Bamodu OA, Lin YK, Lin CS, Chu PY, Chien MH, Wang LS, Hsiao M, Yeh CT, Tsai JT. CD47-SIRPα Signaling Induces Epithelial-Mesenchymal Transition and Cancer Stemness and Links to a Poor Prognosis in Patients with Oral Squamous Cell Carcinoma. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Cao Y, Jiao N, Sun T, Ma Y, Zhang X, Chen H, Hong J, Zhang Y. CXCL11 Correlates With Antitumor Immunity and an Improved Prognosis in Colon Cancer. Front Cell Dev Biol. 2021;9:646252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 50. | Zhang Y, Luo X, Yu J, Qian K, Zhu H. An Immune Feature-Based, Three-Gene Scoring System for Prognostic Prediction of Head-and-Neck Squamous Cell Carcinoma. Front Oncol. 2021;11:739182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |