Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11690
Peer-review started: June 30, 2022
First decision: August 21, 2022
Revised: August 30, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 16, 2022
Processing time: 131 Days and 5 Hours
Breast cancer is the most frequently diagnosed cancer in women, accounting for 30% of new diagnosing female cancers. Emerging evidence suggests that ubiquitin and ubiquitination played a role in a number of breast cancer etiology and progression processes. As the primary deubiquitinases in the family, ubiquitin-specific peptidases (USPs) are thought to represent potential therapeutic targets. The role of ubiquitin and ubiquitination in breast cancer, as well as the classification and involvement of USPs are discussed in this review, such as USP1, USP4, USP7, USP9X, USP14, USP18, USP20, USP22, USP25, USP37, and USP39. The reported USPs inhibitors investigated in breast cancer were also summarized, along with the signaling pathways involved in the investigation and its study phase. Despite no USP inhibitor has yet been approved for clinical use, the biological efficacy indicated their potential in breast cancer treatment. With the improvements in phenotypic discovery, we will know more about USPs and USPs inhibitors, developing more potent and selective clinical candidates for breast cancer.
Core Tip: Ubiquitin-specific proteases (USPs) are emerging as potential therapeutic targets in many diseases. In breast cancer, several USPs were overexpressed. In this study, we summarize the involvement of USPs in breast cancer and the development of USP inhibitors, providing more reference to discover potent and selective clinical candidates.
- Citation: Huang ML, Shen GT, Li NL. Emerging potential of ubiquitin-specific proteases and ubiquitin-specific proteases inhibitors in breast cancer treatment. World J Clin Cases 2022; 10(32): 11690-11701
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11690.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11690
Breast cancer is the most frequently diagnosed cancer in women, accounting for 30% of newly diagnosed cancers in females, an increase of 18% over lung cancer[1]. Emerging evidence suggests that dysregulation of the ubiquitin-proteasome system may play a critical role in the development and progression of breast cancer by affecting protein homeostasis, protein-protein interactions, and signal transduction[2]. Ubiquitination can regulate pathways involving tumor promotion and suppression in cancer[3]. Deubiquitinating enzymes (DUBs), mediating the ubiquitin removal and processing, might be functionally important but are less well understood. So far, about 100 human DUBs have been identified, over 90% of them are cysteine-proteases, containing conserved cysteine (C), histidine (H) in catalytic sites. DUBs are divided into the following super families: ovarian tumor protease, ubiquitin specific protease (USP), Machado-Josephin domain superfamily, ubiquitin C-terminal hydrolase (UCH), and zinc-containing metalloproteases. Similar to kinases, the ubiquitination system's components are frequently dysregulated, which results in a number of illnesses, including tumorigenesis[4].
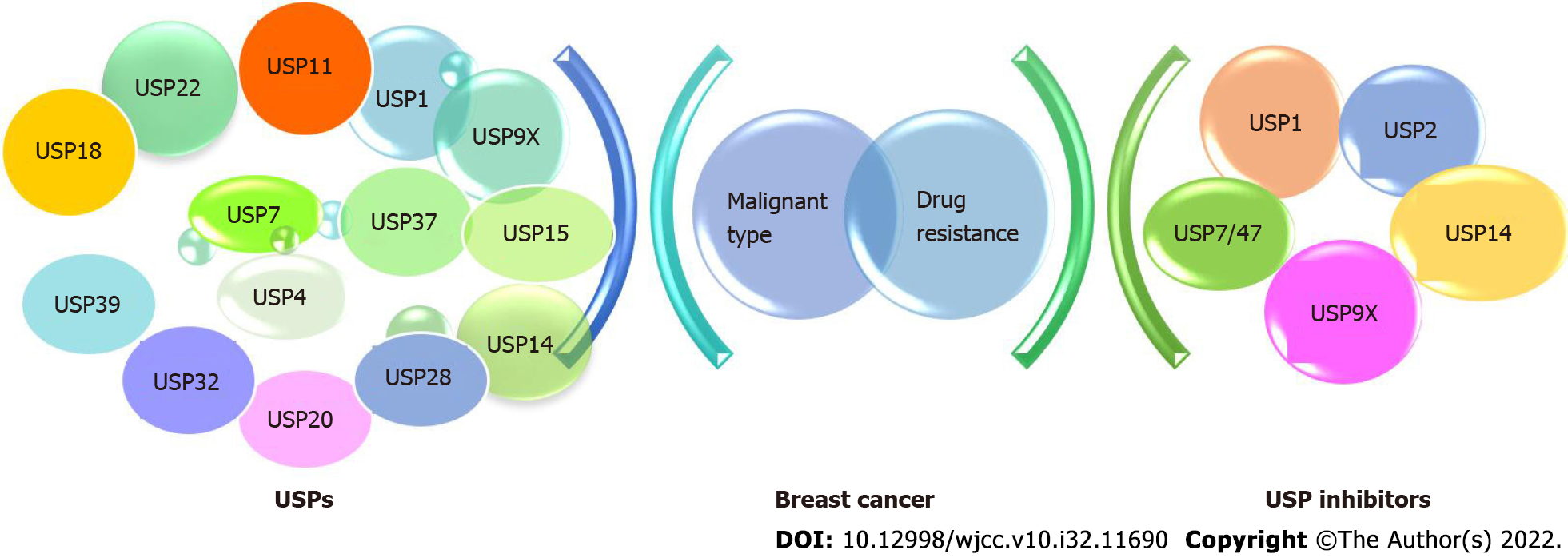
Ubiquitin-proteasome system, consisting of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), ubiquitin-ligase (E3), and the 26S proteasome, plays significant roles in various cellular proteins for breast cancer genesis[5]. Many well-studied proteins in the clinical breast cancer, like Skp2 (S-phase kinase-associated protein 2), BRCA1, BARD1, Efp etc., are major participants in the ubiquitination pathway[6]. Through PDCD4 ubiquitination, Skp2, the first F-box protein discovered, was deregulated to increase radiation tolerance and breast cancer carcinogenesis[7]. The BRCA1/BARD1 RING complex, functioning as an ubiquitin (Ub) ligase, abolished in familial breast cancer with deleterious missense mutations of BRCA1[8]. Ueki et al[9] proved that overexpression of the ubiquitin-conjugating enzyme E2T could result in the autoubiquitination and proteosomal destruction of BRCA1. Besides, ubiquitin-proteasome pathway may be crucial in the treatment of breast cancer patients who take anthracyclines[10]. One of the causes of advanced breast cancer's resistance to hormone therapy may be the estrogen-responsive E3 ubiquitin ligase Efp, which selectively targets 14-3-3 sigma for destruction[11]. Consequently, ubiquitin and ubiquitination played a role in a number of elements of the pathophysiology and development of breast cancer. In this report, we provide greater context for finding potent and targeted clinical candidates by summarizing the discovery of USP inhibitors and the role of USPs in breast cancer in this study (Figure 1).
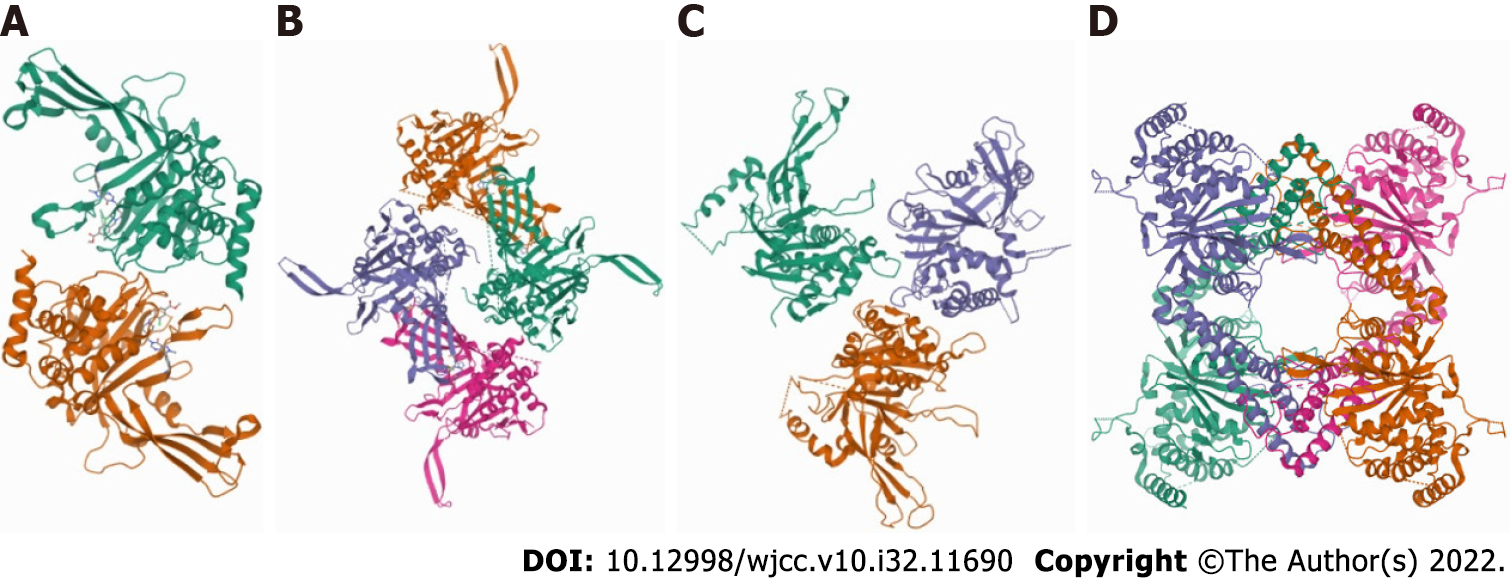
USPs, with more than 50 members, constitute the largest DUBs family. USPs can remove ubiquitin from specific protein substrates, allowing protein salvage and protein localization or activation regulation. All USPs feature highly conserved USP domains made up of three subdomains that resemble the right hand's palm, thumb, and fingers[12]. The finger domain is in charge of interactions with distal ubiquitin, and the catalytic site is situated between the palm and thumb domains[13]. Despite their relative structural diversity with additional domains and terminal extensions, most USPs shared the common feature of a typical conformational change. Upon ubiquitin binding, USPs drive the transition from an inactive form to a catalytically active state[14]. The first shown X-ray structure of USP protein was the catalytic core of HAUSP/USP7[15]. The crystal structure of the 45-kDa catalytic domain of USP14 was reported in 2005[16]. In 2018, Ward et al[17] reported that the structure of the deubiquitinase USP15 reveals a misaligned catalytic triad and an open ubiquitin-binding channel (Figure 2).
USPs belonging to cysteine proteases, are aberrantly expressed in tumors or their microenvironment, making them promising candidates as target for drug development[18]. The majority of USPs, including USP1, USP4, USP7, USP9X, USP14, USP18, USP20, USP22, USP25, USP37 and USP3, were overexpressed in breast cancer (Table 1)[19].
| USPs | Expression | Potential role in breast cancer | Signal pathway |
| USP1 | Upregulated | Tumor promoter | KPNA2, ERα signaling, Hippo signaling pathway, TGF-β signaling[20-23] |
| USP4 | Upregulated | Tumor suppressor | PDCD4, circBMPR2, PAK5-DNPEP pathway, Relaxin/TGF-β1/Smad2/MMP-9 signaling, TGF-β signaling[24-28] |
| USP7 | Upregulated | Tumor promoter | PHF8,DNA repair, Aurora-A kinase, ECT2[29-34] |
| USP9X | Upregulated | Tumor promoter, Tumor suppressor | CEP131, Hippo Pathway, Notch signaling, Cyclin D1,Wnt signaling, TRAIL, YAP1[35,37,38,40,41,45,46] |
| USP11 | Upregulated | Tumor promoter | TGFβ signaling, DNA damage, XIAP[47-49] |
| USP14 | Upregulated | Tumor promoter | CyclinB1, Wnt/β-catenin and PI3K/AKT pathways, cell cycle[53,54] |
| USP15 | Upregulated | Tumor promoter | DNA repair, ERα signaling[65,66] |
| USP18 | Upregulated | Tumor promoter | AKT/Skp2 pathway[68] |
| USP20 | Upregulated | Tumor promoter | SNAI2[69] |
| USP22 | Upregulated | Tumor promoter | c-Myc, Hh pathway[57,58] |
| USP28 | Upregulated | Tumor suppressor | HIF-independent pathway, LSD1[71,72] |
| USP32 | Upregulated | Tumor promoter | Unknown[73] |
| USP33 | Upregulated | Tumor suppressor | Slit-Robo signaling[74] |
| USP37 | Upregulated | Tumor promoter | Stemness, epithelial-mesenchymal transition[60] |
| USP39 | Upregulated | Tumor promoter | G0/G1-phase arrest, CHEK2[62,63] |
USP1, one of the best-characterized DUBs, is crucial in the control of DNA repair procedures. In breast cancer, USP1 inhibition was reported to suppress breast cancer metastasis via KPNA2[20]. Besides, USP1 was proved as a novel TAZ (WWTR1) regulator to increase breast cancer cell proliferation and migration[21]. USP1's non-genomic mechanism, which stabilizes the ER protein, can also hasten the development of breast cancer[22]. For triple negative breast cancer, a unique function of the USP1 was lighted in promoting TGF-β-induced EMT and migration via stabilization of TAK1[23].
Ubiquitin-specific protease 4 (USP4) is located in chromosome 3 (3p21,3) and identified as a tumor suppressor in breast cancer[24]. It was discovered that circBMPR2 acts as a miR-553 sponge and relieves USP4 repression to stop the spread of tamoxifen resistance of breast cancer[25]. Additionally discovered as a downstream target of the PAK5-DNPEP pathway, USP4 controls the growth and spread of breast cancer[26]. Besides, USP4 was an important determinant for the crosstalk between the TGF-β and AKT signalling pathways[27]. The signal from relaxin/TGF-1/Smad2/MMP-9 may be the mechanism via which USP4 encourages breast cancer invasion[28].
USP7, also known as Herpesvirus associated protease, is a 128 kDa cysteine protease and member of the USP DUB family. The grade of breast cancer's histology was strongly linked with USP7 overexpression[29]. USP7 strongly enhanced apoptotic gene expression and reduced metastasis of breast cancer cell lines[30]. USP7 can deubiquitinate and stabilize ECT2, ultimately maintaining oncogenic protein MDM2 levels in breast carcinogenesis[31]. Furthermore, ERα status is essential to the function of USP7 in breast carcinogenesis, ERα overexpression can rescue the USP7 silencing-induced cell cycle arrest and apoptosis[32]. Breast cancer was discovered to have a relationship between USP7 and the taxanes response, suggesting that the USP7 protein may be a potential predictor of outcome[33]. Stability of Aurora-A kinase affected by USP7 may be the possible mechanism in regulating mitosis progression and taxane sensitivity[34].
Numerous studies have shown that USP9x has a pro-carcinogenic influence on the development of breast cancer[35,36]. Hippo pathway[37], Notch signaling[38,39], cyclin-dependent pathway[40], and Wnt signaling were a few of the potential signaling pathways[41]. Additionally, USP9X contributed to the medication resistance in breast cancer. Tamoxifen, but not the ER downregulator fulvestrant, was able to stop proliferation due to the loss of activity in the deubiquitinase USP9X[42]. In breast cancer cells lacking the estrogen receptor, USP9X inhibition may improve cisplatin sensitivity[43]. Olaparib and methyl methanesulfonate are PARP inhibitors that are much more sensitive when USP9X is knocked down[44]. By interacting with β-catenin through deubiquitination in breast cancer cells, USP9x can be used as a therapeutic target for TRAIL-resistant breast cancers[45]. USP9X-YAP1 axis maybe an important regulatory mechanism to elevates cell sensitivity to chemotherapy[46].
USP11 takes involvement in a variety of cellular metabolic activities. In human breast cancer, USP11-mediated alteration of TGF-downstream signaling may increase EMT and metastasis[47]. USP11 also participates in DNA damage repair, involving in the BRCA2 pathway independently of BRCA2 deubiquitination[48]. Regulation of XIAP turnover reveals a role for USP11 inpromotion of breast tumorigenesis[49]. In addition, USP11 was discovered to be a novel ER transcriptional regulator in breast cancer and was linked to a poor prognosis in ER+ patients[50]. USP11 was also linked to outcome prediction in breast cancer patients after neoadjuvant therapy[51].
By eliminating ubiquitin chains from its substrates, USP14 prevents the breakdown of ubiquitinated proteins, but it can also speed up the process by enhancing proteasome activation. USP14 has a role in the spread of breast cancer by encouraging proliferation and metastasis while blocking apoptosis[52]. AR deubiquitination is critical for breast cancer growth and USP14 inhibition is a possible strategy to treat AR-positive breast cancer[53]. USP14 can regulate the cell cycle of breast cancer cells by regulating CyclinB1 ubiquitination[54]. Besides, USP14 inhibition could enhance the sensitivity of breast cancer to enzalutamide by AR-related signaling pathways, such as PI3K/AKT and Wnt/β-catenin pathways[55].
The expression level of USP22 protein, an independent prognostic factor for overall survival (OS) and disease-free survival of breast cancer, was significantly higher than that in breast fibroadenoma and normal breast tissues[56]. In murine and breast cancer cells, USP22 favorably controlled c-Myc stability and tumorigenic activity[57]. Additionally, USP22's deubiquitination activity was necessary for it to maintain ER stability, which improved ER action and conferred endocrine resistance in breast cancer[58].
Ubiquitin specific peptidase 37 (USP37), composed of 979 amino acids harboring three ubiquitin-interacting motifs between the Cys box and His box of the primary sequence, is a member of ubiquitin-specific processing proteases family localized mainly in the cytoplasm. USP37 was an independent poor prognostic biomarker for OS, recurrence-free survival and metastasis-free survival, dividing the luminal and triple negative breast cancer into subgroups with different prognosis[59]. In addition, USP37 can regulate the stemness, cell invasion, EMT and sensitivity to cisplatin in breast cancer cells[60]. USP37 knockdown could reverse the resistance of breast cancer cells to Adriamycin. USP37 down-regulation might be a potential strategy against ADR resistance in breast cancer treatment[61].
Ubiquitinspecific protease 39 (USP39) encodes a 65 kDa SR-associated protein, exhibits aberrant an expression and has oncogenic functions in several types of cancer. The identification of USP39 as a potential molecular target for breast cancer gene therapy was generated following the study of Wang and colleagues[62]. USP39 c.*208G>C was strongly associated with triple-negative breast tumors, regulating cancer-relevant tumor suppressor[63]. USP39 downregulation obviously reduced the proliferation and colony-forming ability of triple-negative breast cancer cells[64].
Limited exploration about USP15, USP18, USP20, USP28, USP32 and USP51 in breast cancer were published. As novel protector for preventing ERα degradation, USP15 is critical driver for breast cancer progression[65]. In addition, cancer-associated USP15 mutations could decrease USP15-BARD1 interaction and increases PARP inhibitor sensitivity in cancer cells[66]. USP18 mRNA levels in human breast tumor tissues were substantially greater in ER+-than in ER—breast cancer tissues. USP18 mRNA levels in ER+-tumor tissues were substantially greater than in their equivalent tumor-adjacent tissues[67]. USP18 may accelerate breast cancer growth by upregulating EGFR and activating the AKT/Skp2 pathway[68]. Higher USP20 expression was linked to a worse prognosis in patients with ER- breast cancer, suggesting that USP20 may facilitate the spread of breast cancer[69]. USP28 participated in various cancers including breast cancer, intestinal cancers, gliomas, non-small cell lung cancer, and bladder cancer[70]. Overexpression of USP28 correlated with a better survival in patients with invasive ductal breast carcinoma[71]. USP28 stabilized LSD1 and conferred stem-cell-like traits to breast cancer cells[72]. USP32 was overexpressed in 50% of breast cancer cell lines and 22% of primary breast tumors compared to mammary epithelial cells[73]. USP33 was also found overexpressed and inhibit breast metastasis[74]. USP51 was found to be a bona fide target of CDK4/6, and could be a viable therapeutic target for advanced human cancers[75]. There have been some new research on the relationship between USPs and breast cancer development in recent years, but more proof is still required.
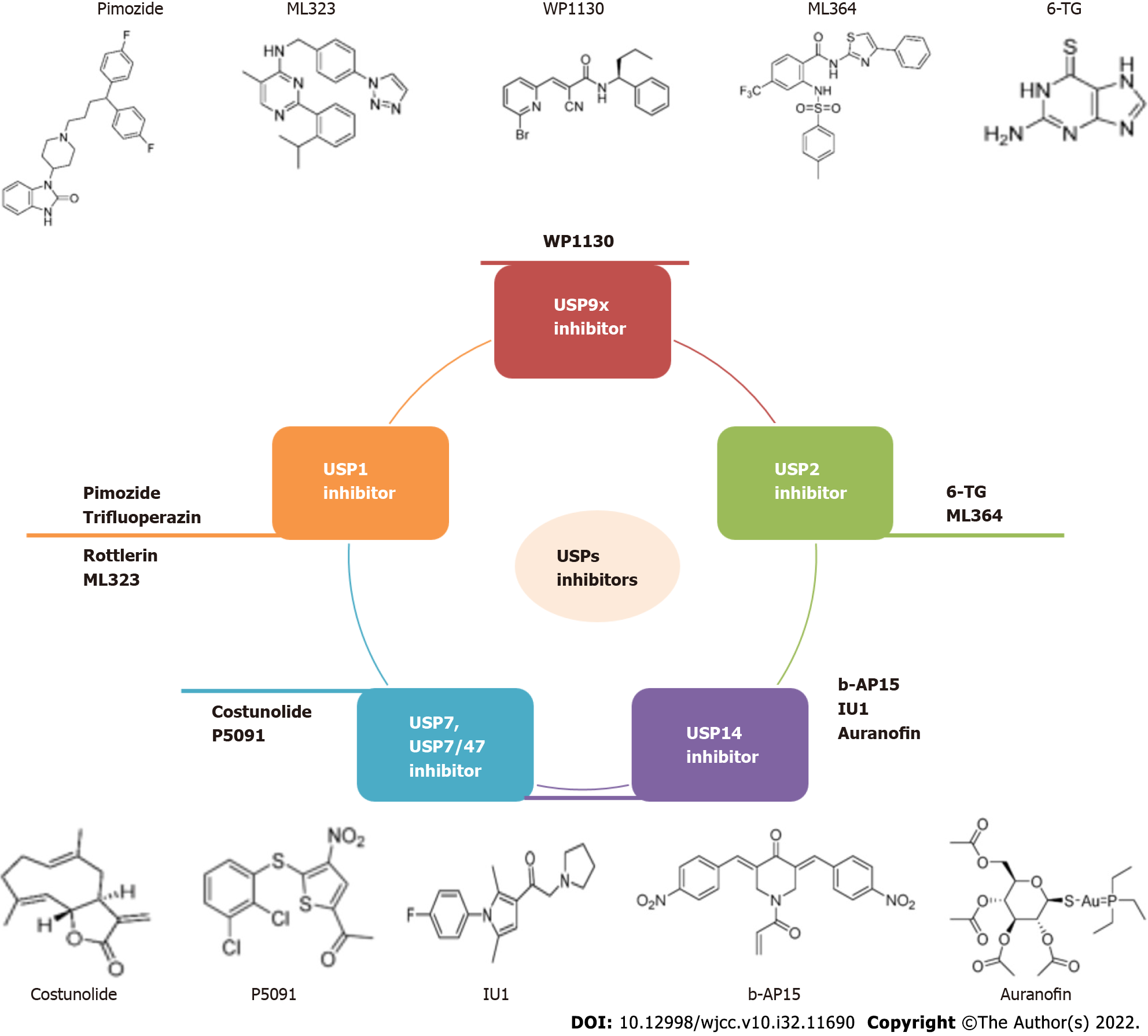
Since USPs and molecular signaling pathways are tightly connected, several efforts have been made to develop USPs inhibitors. Prior to 2014, the discovery of USP inhibitors reported mainly relied on high-throughput screening. Recently, based on the co-crystal structures of USP-inhibitor complexes, structure-guided drug design was conducted. In past ten years, USPs inhibitors have started to gradually emerge. More than 60 USPs inhibitors were reported and two of them (b-AP15 and VLX1570) was under clinical trial for multiple myeloma treatment[14]. For breast cancer, several USPs inhibitors were studied (Table 2), but none have been authorized for clinical use (Figure 3).
| Target | Breast cancer subtype | Experiment | Pathways | |
| USP1 | Pimozide | ER negative BC, TNBC | In vitro; In vivo | Cell cycle, AKT signaling pathway, EMT, MMP-9, vimentin, STAT3[76,78,79] |
| Trifluoperazin | TNBC | In vitro; In vivo | G0/G1 arrest, cyclinD1/CDK4, cyclinE/CDK2[80] | |
| Rottlerin | ER positive BC, TNBC, CSCs | In vitro | NFκB, cyclin D-1, p38 MAPK, AMPK, proteasome inhibition, Skp2[81-84] | |
| ML323 | BC | In vitro; In vivo | KPNA2[20] | |
| USP2 | 6-TG | BRCA2-defective PARP inhibitor-resistant BC, BRCA1-mutant BC, TNBC | In vitro; In vivo | DNA repair, PI3K-AKT, apoptosis pathway, lncRNA-miRNA-mRNA ceRNA network, DNMT1[85,87,88,90] |
| ML364 | ER-positive BC | In vitro | Endocytic degradation[91] | |
| USP7 | Costunolide | metastatic TNBC, BC | In vitro | NF-κB signaling, cell cycle regulation, c-Myc/p53, AKT/14-3-3 pathway, p38MAPK pathways[92-95] |
| USP7/47 | P5091 | BC | In vitro | EMT[96] |
| USP14 | b-AP15 | ER positive BC, TNBC | In vitro; In vivo | Autophagy, ERα signaling[98,99] |
| IU1 | AR-positive BC | In vitro; In vivo | Wnt/β-catenin, PI3K/AKT pathways[55] | |
| Auranofin | ER positive BC, TNBC | In vitro; In vivo | PTGR1 expression, ERK1/2-MYC, p38 MAPK signaling pathway, mitochondrial apoptosis[100-102] | |
| USP9x | WP1130 | ER-negative BC | In vitro | Mcl-1[43] |
Pimozide has been widely studied as a potential anticancer treatment in various cancers, including breast, lung, central nervous system tumours, prostate, melanoma, osteosarcoma, neuroblastoma, ovarian, colorectal, myeloproliferative neoplasms, pancreatic, and hepatocellular carcinoma[76]. Back to 1992, pimozide was regarded as potential noncytotoxic alternatives to tamoxifen for the treatment of tamoxifen-resistant human breast cancer[77]. Antitumor activity of pimozide against breast cancer development was demonstrated by suppressing angiogenesis and by paracrine stimulation[78]. In triple-negative breast cancer, Pimozide could dramatically lessen invasion and migration via phos
Trifluoperazin, Rottlerin and ML323 were all USP1 inhibitors. By causing G0/G1 arrest and apoptosis, trifluoperazine hydrochloride was discovered to inhibit the growth of triple-negative breast cancer tumors and brain metastasis[80]. Rottlerin could exhibit antiangiogenic effects in breast cancer cells[81,82]. The fact that rottlerin induces autophagy, which results in apoptosis for breast cancer stem cells, suggests that rottlerin may be a safe therapy option for breast cancer[83,84]. Limited study was reported about ML323 in breast cancer, KPNA2 maybe the targets of ML323 in suppressesing breast cancer metastasis[20].
Only two USP2 inhibitors were reported in breast cancer application. 6-thioguanine (6-TG) was reported to selectively kill BRCA2-defective tumors and overcomes PARP inhibitor resistance[85]. BRCA1-deficient breast cancer cell lines are distinct sensitivities to 6-TG[86]. The function of 6-TG in triple-negative breast cancer was involved with lncRNA[87,88].
Differentially expressed genes and competitive endogenous (ce)RNA molecules may have contributed to the mechanism by which 6-TG inhibits the development of MCF-7 cells[89,90]. Another USP2 inhibitor, ML364, may make breast cancer cells that are HER2-positive more susceptible to HSP90 inhibition[91].
USP 7 inhibitor costunolide suppress breast cancer growth and metastases and may be promising anticancer drugs, especially for metastatic breast cancer[92]. By targeting cell cycle regulation, costu
The USP7/47 inhibitor P5091 was able to reverse morphological alterations in MCF-10A cells and lower the expression of EMT markers[96]. Blockage of deubiquitination by P5091 could reduce cell proliferation, colony formation, migration, and sphere dissemination for breast cancer cell lines[30].
Proteasome-associated deubiquitinases (USP14 and UCHL5) inhibitors b-AP15 can inhibit tumor progression of MCF-7 breast cancer cell line[97]. In 2015, the effect of b-AP15 and RA-9 on triple negative breast cancer cell lines was proved[98]. Moreover, b-AP15 and PtPT may have the potential for the treatment of estrogen receptor-positive breast cancer[99].
Auranofin, a USP14 inhibitor, demonstrated synergistic breast cancer inhibition. The combination of Auranofin and Vitamin C was efficient against triple-negative breast cancer[100]. Cooperation was found between auranofin and anti-PD-L1 antibody for treatment of triple-negative breast cancer[101]. A unique therapeutic approach for breast cancer may be used to take advantage of the synergistic effects of auranofin and trametinib[102]. In addition, IU1, another USP14 inhibitor, had the capacity to improve enzalutamide's ability to suppress cell proliferation and induce apoptosis in breast cancer cell lines both in vitro and in vivo[55].
USP9x inhibitor was rarely reported. It was discovered that WP1130 increased the cytotoxicity of cisplatin in ER-negative breast cancer cells. In the meantime, simultaneous therapy with WP1130 may improve cisplatin sensitivity in estrogen receptor-negative breast cancer cells in a USP9x-dependent manner[43].
USPs are a highly specialized class of DUBs with emerging potential in breast cancer. USPs involved into many important signaling pathways, including ERα signaling, Hippo signaling pathway, TGF-βsignaling, PI3K/AKT pathways, Notch signaling, etc. USPs have garnered more attention as possible targets, and USPs inhibitors have begun to progressively appear. Although no USP inhibitor has been authorized for clinical use to far, biological efficacy suggested they may be useful in the treatment of breast cancer. We will learn more about USPs and USPs inhibitors as phenotypic discovery advances, leading to the identification of more effective and targeted therapeutic candidates for breast cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Agarwal P, India; Oura S, Japan; Singh R, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15229] [Article Influence: 3045.8] [Reference Citation Analysis (4)] |
| 2. | Xiao Z, Zhang P, Ma L. The role of deubiquitinases in breast cancer. Cancer Metastasis Rev. 2016;35:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Fraile JM, Quesada V, Rodríguez D, Freije JM, López-Otín C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene. 2012;31:2373-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Shi D, Grossman SR. Ubiquitin becomes ubiquitous in cancer: emerging roles of ubiquitin ligases and deubiquitinases in tumorigenesis and as therapeutic targets. Cancer Biol Ther. 2010;10:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Park J, Cho J, Song EJ. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res. 2020;43:1144-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 6. | Ohta T, Fukuda M. Ubiquitin and breast cancer. Oncogene. 2004;23:2079-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Li C, Du L, Ren Y, Liu X, Jiao Q, Cui D, Wen M, Wang C, Wei G, Wang Y, Ji A, Wang Q. SKP2 promotes breast cancer tumorigenesis and radiation tolerance through PDCD4 ubiquitination. J Exp Clin Cancer Res. 2019;38:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Brzovic PS, Keeffe JR, Nishikawa H, Miyamoto K, Fox D 3rd, Fukuda M, Ohta T, Klevit R. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc Natl Acad Sci U S A. 2003;100:5646-5651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Ueki T, Park JH, Nishidate T, Kijima K, Hirata K, Nakamura Y, Katagiri T. Ubiquitination and downregulation of BRCA1 by ubiquitin-conjugating enzyme E2T overexpression in human breast cancer cells. Cancer Res. 2009;69:8752-8760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Orlowski RZ, Dees EC. The role of the ubiquitination-proteasome pathway in breast cancer: applying drugs that affect the ubiquitin-proteasome pathway to the therapy of breast cancer. Breast Cancer Res. 2003;5:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Horie-Inoue K, Inoue S. Epigenetic and proteolytic inactivation of 14-3-3sigma in breast and prostate cancers. Semin Cancer Biol. 2006;16:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Suresh B, Lee J, Kim KS, Ramakrishna S. The Importance of Ubiquitination and Deubiquitination in Cellular Reprogramming. Stem Cells Int. 2016;2016:6705927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Young MJ, Hsu KC, Lin TE, Chang WC, Hung JJ. The role of ubiquitin-specific peptidases in cancer progression. J Biomed Sci. 2019;26:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Chen S, Liu Y, Zhou H. Advances in the Development Ubiquitin-Specific Peptidase (USP) Inhibitors. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE, Shi Y. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell. 2002;111:1041-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 519] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 16. | Hu M, Li P, Song L, Jeffrey PD, Chenova TA, Wilkinson KD, Cohen RE, Shi Y. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005;24:3747-3756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 352] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Ward SJ, Gratton HE, Indrayudha P, Michavila C, Mukhopadhyay R, Maurer SK, Caulton SG, Emsley J, Dreveny I. The structure of the deubiquitinase USP15 reveals a misaligned catalytic triad and an open ubiquitin-binding channel. J Biol Chem. 2018;293:17362-17374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Pal A, Young MA, Donato NJ. Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res. 2014;74:4955-4966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 19. | Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1474] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 20. | Ma A, Tang M, Zhang L, Wang B, Yang Z, Liu Y, Xu G, Wu L, Jing T, Xu X, Yang S. USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis. Oncogene. 2019;38:2405-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Mussell A, Shen H, Chen Y, Mastri M, Eng KH, Bshara W, Frangou C, Zhang J. USP1 Regulates TAZ Protein Stability Through Ubiquitin Modifications in Breast Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Niu Z, Li X, Feng S, Huang Q, Zhuang T, Yan C, Qian H, Ding Y, Zhu J, Xu W. The deubiquitinating enzyme USP1 modulates ERα and modulates breast cancer progression. J Cancer. 2020;11:6992-7000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Han D, Wang L, Chen B, Zhao W, Liang Y, Li Y, Zhang H, Liu Y, Wang X, Chen T, Li C, Song X, Luo D, Li Z, Yang Q. USP1-WDR48 deubiquitinase complex enhances TGF-β induced epithelial-mesenchymal transition of TNBC cells via stabilizing TAK1. Cell Cycle. 2021;20:320-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Li Y, Jiang D, Zhang Q, Liu X, Cai Z. Ubiquitin-specific protease 4 inhibits breast cancer cell growth through the upregulation of PDCD4. Int J Mol Med. 2016;38:803-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Liang Y, Song X, Li Y, Ma T, Su P, Guo R, Chen B, Zhang H, Sang Y, Liu Y, Duan Y, Zhang N, Li X, Zhao W, Wang L, Yang Q. Targeting the circBMPR2/miR-553/USP4 Axis as a Potent Therapeutic Approach for Breast Cancer. Mol Ther Nucleic Acids. 2019;17:347-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Geng N, Li Y, Zhang W, Wang F, Wang X, Jin Z, Xing Y, Li D, Zhang H, Li X, Cheng M, Jin F, Li F. A PAK5-DNPEP-USP4 axis dictates breast cancer growth and metastasis. Int J Cancer. 2020;146:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, ten Dijke P. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat Cell Biol. 2012;14:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 28. | Cao WH, Liu XP, Meng SL, Gao YW, Wang Y, Ma ZL, Wang XG, Wang HB. USP4 promotes invasion of breast cancer cells via Relaxin/TGF-β1/Smad2/MMP-9 signal. Eur Rev Med Pharmacol Sci. 2016;20:1115-1122. [PubMed] |
| 29. | Wang Q, Ma S, Song N, Li X, Liu L, Yang S, Ding X, Shan L, Zhou X, Su D, Wang Y, Zhang Q, Liu X, Yu N, Zhang K, Shang Y, Yao Z, Shi L. Stabilization of histone demethylase PHF8 by USP7 promotes breast carcinogenesis. J Clin Invest. 2016;126:2205-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 30. | Hayal TB, DoĞan A, ŞİŞlİ HB, Kiratli B, Şahİn F. Ubiquitin-specific protease 7 downregulation suppresses breast cancer in vitro. Turk J Biol. 2020;44:145-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Zhang Q, Cao C, Gong W, Bao K, Wang Q, Wang Y, Bi L, Ma S, Zhao J, Liu L, Tian S, Zhang K, Yang J, Yao Z, Song N, Shi L. A feedforward circuit shaped by ECT2 and USP7 contributes to breast carcinogenesis. Theranostics. 2020;10:10769-10790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Xia X, Liao Y, Huang C, Liu Y, He J, Shao Z, Jiang L, Dou QP, Liu J, Huang H. Deubiquitination and stabilization of estrogen receptor α by ubiquitin-specific protease 7 promotes breast tumorigenesis. Cancer Lett. 2019;465:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Hernández-Pérez S, Cabrera E, Salido E, Lim M, Reid L, Lakhani SR, Khanna KK, Saunus JM, Freire R. DUB3 and USP7 de-ubiquitinating enzymes control replication inhibitor Geminin: molecular characterization and associations with breast cancer. Oncogene. 2017;36:4802-4809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Giovinazzi S, Morozov VM, Summers MK, Reinhold WC, Ishov AM. USP7 and Daxx regulate mitosis progression and taxane sensitivity by affecting stability of Aurora-A kinase. Cell Death Differ. 2013;20:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Li X, Song N, Liu L, Liu X, Ding X, Song X, Yang S, Shan L, Zhou X, Su D, Wang Y, Zhang Q, Cao C, Ma S, Yu N, Yang F, Yao Z, Shang Y, Shi L. USP9X regulates centrosome duplication and promotes breast carcinogenesis. Nat Commun. 2017;8:14866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Lu Q, Lu D, Shao ZM, Li DQ. Deubiquitinase ubiquitin-specific protease 9X regulates the stability and function of E3 ubiquitin ligase ring finger protein 115 in breast cancer cells. Cancer Sci. 2019;110:1268-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Toloczko A, Guo F, Yuen HF, Wen Q, Wood SA, Ong YS, Chan PY, Shaik AA, Gunaratne J, Dunne MJ, Hong W, Chan SW. Deubiquitinating Enzyme USP9X Suppresses Tumor Growth via LATS Kinase and Core Components of the Hippo Pathway. Cancer Res. 2017;77:4921-4933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Izrailit J, Jaiswal A, Zheng W, Moran MF, Reedijk M. Cellular stress induces TRB3/USP9x-dependent Notch activation in cancer. Oncogene. 2017;36:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Shen Q, Reedijk M. Notch Signaling and the Breast Cancer Microenvironment. Adv Exp Med Biol. 2021;1287:183-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Li H, Zheng B. Overexpression of the Ubiquitin-Specific Peptidase 9 X-Linked (USP9X) Gene is Associated with Upregulation of Cyclin D1 (CCND1) and Downregulation of Cyclin-Dependent Inhibitor Kinase 1A (CDKN1A) in Breast Cancer Tissue and Cell Lines. Med Sci Monit. 2019;25:4207-4216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Shang Z, Zhao J, Zhang Q, Cao C, Tian S, Zhang K, Liu L, Shi L, Yu N, Yang S. USP9X-mediated deubiquitination of B-cell CLL/lymphoma 9 potentiates Wnt signaling and promotes breast carcinogenesis. J Biol Chem. 2019;294:9844-9857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Oosterkamp HM, Hijmans EM, Brummelkamp TR, Canisius S, Wessels LF, Zwart W, Bernards R. USP9X downregulation renders breast cancer cells resistant to tamoxifen. Cancer Res. 2014;74:3810-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Fu P, Du F, Liu Y, Yao M, Zhang S, Zheng X, Zheng S. WP1130 increases cisplatin sensitivity through inhibition of usp9x in estrogen receptor-negative breast cancer cells. Am J Transl Res. 2017;9:1783-1791. [PubMed] |
| 44. | Lu Q, Zhang FL, Lu DY, Shao ZM, Li DQ. USP9X stabilizes BRCA1 and confers resistance to DNA-damaging agents in human cancer cells. Cancer Med. 2019;8:6730-6740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Ouyang W, Zhang S, Yang B, Yang C, Zhang J, Zhou F, Xie C. β-catenin is regulated by USP9x and mediates resistance to TRAIL-induced apoptosis in breast cancer. Oncol Rep. 2016;35:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Li L, Liu T, Li Y, Wu C, Luo K, Yin Y, Chen Y, Nowsheen S, Wu J, Lou Z, Yuan J. The deubiquitinase USP9X promotes tumor cell survival and confers chemoresistance through YAP1 stabilization. Oncogene. 2018;37:2422-2431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 47. | Garcia DA, Baek C, Estrada MV, Tysl T, Bennett EJ, Yang J, Chang JT. USP11 Enhances TGFβ-Induced Epithelial-Mesenchymal Plasticity and Human Breast Cancer Metastasis. Mol Cancer Res. 2018;16:1172-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Schoenfeld AR, Apgar S, Dolios G, Wang R, Aaronson SA. BRCA2 is ubiquitinated in vivo and interacts with USP11, a deubiquitinating enzyme that exhibits prosurvival function in the cellular response to DNA damage. Mol Cell Biol. 2004;24:7444-7455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Zhou Z, Luo A, Shrivastava I, He M, Huang Y, Bahar I, Liu Z, Wan Y. Regulation of XIAP Turnover Reveals a Role for USP11 in Promotion of Tumorigenesis. EBioMedicine. 2017;15:48-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Dwane L, O'Connor AE, Das S, Moran B, Mulrane L, Pinto-Fernandez A, Ward E, Blümel AM, Cavanagh BL, Mooney B, Dirac AM, Jirström K, Kessler BM, Ní Chonghaile T, Bernards R, Gallagher WM, O'Connor DP. A Functional Genomic Screen Identifies the Deubiquitinase USP11 as a Novel Transcriptional Regulator of ERα in Breast Cancer. Cancer Res. 2020;80:5076-5088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Bayraktar S, Gutierrez Barrera AM, Liu D, Pusztai L, Litton J, Valero V, Hunt K, Hortobagyi GN, Wu Y, Symmans F, Arun B. USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J. 2013;19:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Zhu L, Yang S, He S, Qiang F, Cai J, Liu R, Gu C, Guo Z, Wang C, Zhang W, Zhang C, Wang Y. Downregulation of ubiquitin-specific protease 14 (USP14) inhibits breast cancer cell proliferation and metastasis, but promotes apoptosis. J Mol Histol. 2016;47:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Liao Y, Xia X, Liu N, Cai J, Guo Z, Li Y, Jiang L, Dou QP, Tang D, Huang H, Liu J. Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination. Oncogene. 2018;37:1896-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 54. | Liu B, Liu Y, Wang Y, Xie C, Gan M, Han T, Cao J, Wang J. CyclinB1 deubiquitination by USP14 regulates cell cycle progression in breast cancer. Pathol Res Pract. 2019;215:152592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Xia X, Huang C, Liao Y, Liu Y, He J, Guo Z, Jiang L, Wang X, Liu J, Huang H. Inhibition of USP14 enhances the sensitivity of breast cancer to enzalutamide. J Exp Clin Cancer Res. 2019;38:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 56. | Zhang Y, Yao L, Zhang X, Ji H, Wang L, Sun S, Pang D. Elevated expression of USP22 in correlation with poor prognosis in patients with invasive breast cancer. J Cancer Res Clin Oncol. 2011;137:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 57. | Kim D, Hong A, Park HI, Shin WH, Yoo L, Jeon SJ, Chung KC. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 2017;232:3664-3676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 58. | Wang S, Zhong X, Wang C, Luo H, Lin L, Sun H, Sun G, Zeng K, Zou R, Liu W, Sun N, Song H, Zhang Q, Liao Z, Teng X, Zhou T, Sun X, Zhao Y. USP22 positively modulates ERα action via its deubiquitinase activity in breast cancer. Cell Death Differ. 2020;27:3131-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Zhang QX, Wang XC, Chen SP, Qin XT. [Predictive value of deubiquitination enzymes USP37 in the prognosis of breast cancer]. Zhonghua Yi Xue Za Zhi. 2016;96:944-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 60. | Qin T, Li B, Feng X, Fan S, Liu L, Liu D, Mao J, Lu Y, Yang J, Yu X, Zhang Q, Zhang J, Song B, Li M, Li L. Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity. J Exp Clin Cancer Res. 2018;37:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 61. | Qin T, Cui XY, Xiu H, Huang C, Sun ZN, Xu XM, Li LH, Yue L. USP37 downregulation elevates the Chemical Sensitivity of Human Breast Cancer Cells to Adriamycin. Int J Med Sci. 2021;18:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Wang H, Ji X, Liu X, Yao R, Chi J, Liu S, Wang Y, Cao W, Zhou Q. Lentivirus-mediated inhibition of USP39 suppresses the growth of breast cancer cells in vitro. Oncol Rep. 2013;30:2871-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Kuligina ES, Sokolenko AP, Bizin IV, Romanko AA, Zagorodnev KA, Anisimova MO, Krylova DD, Anisimova EI, Mantseva MA, Varma AK, Hasan SK, Ni VI, Koloskov AV, Suspitsin EN, Venina AR, Aleksakhina SN, Sokolova TN, Milanović AM, Schürmann P, Prokofyeva DS, Bermisheva MA, Khusnutdinova EK, Bogdanova N, Dörk T, Imyanitov EN. Exome sequencing study of Russian breast cancer patients suggests a predisposing role for USP39. Breast Cancer Res Treat. 2020;179:731-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Liu S, Liu X, Wang H, Zhou Q, Liang Y, Sui A, Yao R, Zhao B, Sun M. Lentiviral vector-mediated doxycycline-inducible USP39 shRNA or cDNA expression in triple-negative breast cancer cells. Oncol Rep. 2015;33:2477-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Xia X, Huang C, Liao Y, Liu Y, He J, Shao Z, Hu T, Yu C, Jiang L, Liu J, Huang H. The deubiquitinating enzyme USP15 stabilizes ERα and promotes breast cancer progression. Cell Death Dis. 2021;12:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Peng Y, Liao Q, Tan W, Peng C, Hu Z, Chen Y, Li Z, Li J, Zhen B, Zhu W, Li X, Yao Y, Song Q, Liu C, Qi X, He F, Pei H. The deubiquitylating enzyme USP15 regulates homologous recombination repair and cancer cell response to PARP inhibitors. Nat Commun. 2019;10:1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 67. | Fang Q, Yao S, Luo G, Zhang X. Identification of differentially expressed genes in human breast cancer cells induced by 4-hydroxyltamoxifen and elucidation of their pathophysiological relevance and mechanisms. Oncotarget. 2018;9:2475-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Tan Y, Zhou G, Wang X, Chen W, Gao H. USP18 promotes breast cancer growth by upregulating EGFR and activating the AKT/Skp2 pathway. Int J Oncol. 2018;53:371-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Li W, Shen M, Jiang YZ, Zhang R, Zheng H, Wei Y, Shao ZM, Kang Y. Deubiquitinase USP20 promotes breast cancer metastasis by stabilizing SNAI2. Genes Dev. 2020;34:1310-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 70. | Wang X, Liu Z, Zhang L, Yang Z, Chen X, Luo J, Zhou Z, Mei X, Yu X, Shao Z, Feng Y, Fu S, Zhang Z, Wei D, Jia L, Ma J, Guo X. Targeting deubiquitinase USP28 for cancer therapy. Cell Death Dis. 2018;9:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 71. | Richter K, Paakkola T, Mennerich D, Kubaichuk K, Konzack A, Ali-Kippari H, Kozlova N, Koivunen P, Haapasaari KM, Jukkola-Vuorinen A, Teppo HR, Dimova EY, Bloigu R, Szabo Z, Kerkelä R, Kietzmann T. USP28 Deficiency Promotes Breast and Liver Carcinogenesis as well as Tumor Angiogenesis in a HIF-independent Manner. Mol Cancer Res. 2018;16:1000-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Wu Y, Wang Y, Yang XH, Kang T, Zhao Y, Wang C, Evers BM, Zhou BP. The deubiquitinase USP28 stabilizes LSD1 and confers stem-cell-like traits to breast cancer cells. Cell Rep. 2013;5:224-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 73. | Akhavantabasi S, Akman HB, Sapmaz A, Keller J, Petty EM, Erson AE. USP32 is an active, membrane-bound ubiquitin protease overexpressed in breast cancers. Mamm Genome. 2010;21:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci U S A. 2009;106:14530-14535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Zhang Z, Li J, Ou Y, Yang G, Deng K, Wang Q, Wang Z, Wang W, Zhang Q, Wang H, Sun W, Sun P, Yang S. CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther. 2020;5:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 76. | Gonçalves JM, Silva CAB, Rivero ERC, Cordeiro MMR. Inhibition of cancer stem cells promoted by Pimozide. Clin Exp Pharmacol Physiol. 2019;46:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 77. | Strobl JS, Peterson VA. Tamoxifen-resistant human breast cancer cell growth: inhibition by thioridazine, pimozide and the calmodulin antagonist, W-13. J Pharmacol Exp Ther. 1992;263:186-193. [PubMed] |
| 78. | Dakir el-H, Pickard A, Srivastava K, McCrudden CM, Gross SR, Lloyd S, Zhang SD, Margariti A, Morgan R, Rudland PS, El-Tanani M. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget. 2018;9:34889-34910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Dees S, Pontiggia L, Jasmin JF, Mercier I. Phosphorylated STAT3 (Tyr705) as a biomarker of response to pimozide treatment in triple-negative breast cancer. Cancer Biol Ther. 2020;21:506-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Feng Z, Xia Y, Gao T, Xu F, Lei Q, Peng C, Yang Y, Xue Q, Hu X, Wang Q, Wang R, Ran Z, Zeng Z, Yang N, Xie Z, Yu L. The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Dis. 2018;9:1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Valacchi G, Pecorelli A, Sticozzi C, Torricelli C, Muscettola M, Aldinucci C, Maioli E. Rottlerin exhibits antiangiogenic effects in vitro. Chem Biol Drug Des. 2011;77:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Park EJ, Kwon TK. Rottlerin enhances IL-1β-induced COX-2 expression through sustained p38 MAPK activation in MDA-MB-231 human breast cancer cells. Exp Mol Med. 2011;43:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer. 2013;12:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Yin X, Zhang Y, Su J, Hou Y, Wang L, Ye X, Zhao Z, Zhou X, Li Y, Wang Z. Rottlerin exerts its anti-tumor activity through inhibition of Skp2 in breast cancer cells. Oncotarget. 2016;7:66512-66524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Issaeva N, Thomas HD, Djureinovic T, Jaspers JE, Stoimenov I, Kyle S, Pedley N, Gottipati P, Zur R, Sleeth K, Chatzakos V, Mulligan EA, Lundin C, Gubanova E, Kersbergen A, Harris AL, Sharma RA, Rottenberg S, Curtin NJ, Helleday T. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res. 2010;70:6268-6276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Gu Y, Helenius M, Väänänen K, Bulanova D, Saarela J, Sokolenko A, Martens J, Imyanitov E, Kuznetsov S. BRCA1-deficient breast cancer cell lines are resistant to MEK inhibitors and show distinct sensitivities to 6-thioguanine. Sci Rep. 2016;6:28217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Zhang D, An X, Li Q, Man X, Chu M, Li H, Zhang N, Dai X, Yu H, Li Z. Thioguanine Induces Apoptosis in Triple-Negative Breast Cancer by Regulating PI3K-AKT Pathway. Front Oncol. 2020;10:524922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Zhang D, An X, Yu H, Li Z. The regulatory effect of 6-TG on lncRNA-miRNA-mRNA ceRNA network in triple-negative breast cancer cell line. Biosci Rep. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Li H, An X, Li Q, Yu H, Li Z. Construction and analysis of competing endogenous RNA network of MCF-7 breast cancer cells based on the inhibitory effect of 6-thioguanine on cell proliferation. Oncol Lett. 2021;21:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Li H, An X, Zhang D, Li Q, Zhang N, Yu H, Li Z. Transcriptomics Analysis of the Tumor-Inhibitory Pathways of 6-Thioguanine in MCF-7 Cells via Silencing DNMT1 Activity. Onco Targets Ther. 2020;13:1211-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Zhang J, Liu S, Li Q, Shi Y, Wu Y, Liu F, Wang S, Zaky MY, Yousuf W, Sun Q, Guo D, Wang T, Zhang Y, Wang Y, Li M, Liu H. The deubiquitylase USP2 maintains ErbB2 abundance via counteracting endocytic degradation and represents a therapeutic target in ErbB2-positive breast cancer. Cell Death Differ. 2020;27:2710-2725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 92. | Choi YK, Cho SG, Woo SM, Yun YJ, Jo J, Kim W, Shin YC, Ko SG. Saussurea lappa Clarke-Derived Costunolide Prevents TNF α -Induced Breast Cancer Cell Migration and Invasion by Inhibiting NF- κ B Activity. Evid Based Complement Alternat Med. 2013;2013:936257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Roy A, Manikkam R. Cytotoxic Impact of Costunolide Isolated from Costus speciosus on Breast Cancer via Differential Regulation of Cell Cycle-An In-vitro and In-silico Approach. Phytother Res. 2015;29:1532-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Peng Z, Wang Y, Fan J, Lin X, Liu C, Xu Y, Ji W, Yan C, Su C. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci Rep. 2017;7:41254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 95. | Liu D, Zeng M, Pi JW, Liu MJ, Ding WZ, Mei XY, Liu JL, Cao XY. Exploring the Potential Mechanism of Costunolide-Induced MCF-7 Cells Apoptosis by Multi-Spectroscopy, Molecular Docking and Cell Experiments. Chem Biodivers. 2021;18:e2001069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Silvestrini VC, Thomé CH, Albuquerque D, de Souza Palma C, Ferreira GA, Lanfredi GP, Masson AP, Delsin LEA, Ferreira FU, de Souza FC, de Godoy LMF, Aquino A, Carrilho E, Panepucci RA, Covas DT, Faça VM. Proteomics analysis reveals the role of ubiquitin specific protease (USP47) in Epithelial to Mesenchymal Transition (EMT) induced by TGFβ2 in breast cells. J Proteomics. 2020;219:103734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | D'Arcy P, Brnjic S, Olofsson MH, Fryknäs M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson R, Linder S. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med. 2011;17:1636-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 98. | Vogel RI, Coughlin K, Scotti A, Iizuka Y, Anchoori R, Roden RB, Marastoni M, Bazzaro M. Simultaneous inhibition of deubiquitinating enzymes (DUBs) and autophagy synergistically kills breast cancer cells. Oncotarget. 2015;6:4159-4170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Xia X, Liao Y, Guo Z, Li Y, Jiang L, Zhang F, Huang C, Liu Y, Wang X, Liu N, Liu J, Huang H. Targeting proteasome-associated deubiquitinases as a novel strategy for the treatment of estrogen receptor-positive breast cancer. Oncogenesis. 2018;7:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 100. | Hatem E, Azzi S, El Banna N, He T, Heneman-Masurel A, Vernis L, Baïlle D, Masson V, Dingli F, Loew D, Azzarone B, Eid P, Baldacci G, Huang ME. Auranofin/Vitamin C: A Novel Drug Combination Targeting Triple-Negative Breast Cancer. J Natl Cancer Inst. 2019;111:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 101. | Raninga PV, Lee AC, Sinha D, Shih YY, Mittal D, Makhale A, Bain AL, Nanayakarra D, Tonissen KF, Kalimutho M, Khanna KK. Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti-PD-L1 antibody for treatment of triple-negative breast cancer. Int J Cancer. 2020;146:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 102. | Joo MK, Shin S, Ye DJ, An HG, Kwon TU, Baek HS, Kwon YJ, Chun YJ. Combined treatment with auranofin and trametinib induces synergistic apoptosis in breast cancer cells. J Toxicol Environ Health A. 2021;84:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |