Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11338
Peer-review started: September 12, 2021
First decision: January 10, 2022
Revised: January 25, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: November 6, 2022
Processing time: 409 Days and 14.9 Hours
There are few nomograms for the prognosis of Chinese patients with triple-negative breast cancer (TNBC).
To construct and validate a nomogram for overall survival (OS) of Chinese TNBC patients after surgery.
This study used the data of SEER*stat 8.3.5 and selected Chinese patients with TNBC operated on between 2010 and 2015. Univariate and multivariate Cox proportional hazard regression models were used. The identified variables were integrated to form a predictive nomogram and risk stratification model; it was assessed with C-indexes and calibration curves.
The median and maximal OS of the 336 patients was 39 and 83 mo, respectively. The multivariate analysis showed that age (P = 0.043), marital status (P = 0.040), tumor localization (P = 0.030), grade (P = 0.035), T classification (P = 0.012), and N classification (P = 0.002) were independent prognostic factors. The six variables were combined to construct a 1-, 3- and 5-year OS nomogram. The C-indexes of the nomogram to predict OS were 0.766 and compared to the seventh edition staging system, which was higher (0.766 vs 0.707, P < 0.001). In order to categorize patients into different prognostic groups, a risk stratification model was created. There was a significant difference between the Kaplan–Meier curves of the entire cohort and each disease stage according to the nomogram.
The nomogram provided prognostic superiority over the traditional tumor, node and metastasis system. It could help clinicians make individual OS or risk predictions for Chinese TNBC patients after surgery.
Core tip: This study aimed to construct and validate a nomogram for overall survival (OS) of triple-negative breast cancer (TNBC) patients after surgery. The data from SEER*stat 8.3.5 of selected Chinese surgical patients with TNBC between 2010 and 2015 were used. The multivariate analysis showed that age (P = 0.043), marital status (P = 0.040), tumor localization (P = 0.030), grade (P = 0.035), T classification (P = 0.012), and N classification (P = 0.002) were independent prognostic factors. A risk stratification model was generated. The C-indexes of the nomogram to predict OS were 0.766 and compared to the seventh edition tumor, node, metastasis (TNM) staging system, which was higher (0.766 vs 0.707, P < 0.001). The nomogram provided a clear prognostic superiority over the traditional TNM system.
- Citation: Lin WX, Xie YN, Chen YK, Cai JH, Zou J, Zheng JH, Liu YY, Li ZY, Chen YX. Nomogram for predicting overall survival in Chinese triple-negative breast cancer patients after surgery. World J Clin Cases 2022; 10(31): 11338-11348
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11338.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11338
Breast cancer (BC) is the most common malignancy diagnosed in women worldwide, with 2 088 849 new cases and 626 679 deaths in 2018[1]. BC has several risk factors, including genetic causes, aging, reproductive history, hormone exposure, lifestyle factors, medical history, and radiation exposure[2-4]. The management of BC is multidisciplinary[2,5,6]. Endocrine therapy can be used to prevent the recurrence of hormone (estrogen and/or progesterone) receptor-positive BC, while anti-HER2 therapies can be used to improve the prognosis of HER2-positive BC[2]. In the USA, therefore, the 5-year survival rate for women with localized BC is 99%, 85% for women with regional spread, and 27% for women with distant metastases[2,4]. Factors affecting prognosis include tumor and disease characteristics, age, response to therapy, race, ethnicity, and body mass[2]. Triple-negative BC (TNBC) is characterized by the absence of hormone receptor expression and HER2 overexpression[7-9]. TNBC represents 15%–20% of all BCs and has more aggressive biology, earlier onset of metastatic disease, visceral metastases, rapidly progressive disease, inefficacy of endocrine therapy, short response duration to available therapies, and inferior survival outcomes[10-12]. The survival of patients with TNBC is influenced by age at diagnosis, tumor stage, positive lymph node ratio, and Ki-67[13]. Ovcaricek et al[14] showed that age and nodal status are prognostic factors in such patients, while Pistelli et al[15] showed that tumor size and lymphovascular invasion are prognostic factors for TNBC. Because of the malignant behavior of TNBC and to achieve a better prognostic assessment, stratifying patients with TNBC based on their individual characteristics is important. Nomograms are useful and convenient tools for cancer patients to quantify and predict risk and prognosis[16-18]. Many prognostication nomograms have been developed and validated for BC patients based on traditional clinicopathological features[19,20]. Hwang et al[19] limited their nomogram to pathological complete response after neoadjuvant chemotherapy, while Luo et al[20] included radiomics in their nomogram, which might not be readily available in all hospitals. Most models were developed using Caucasian and not Asian patients. A SEER-based study showed that Chinese women had better 10-year survival than Caucasians had[21]; similar results were observed in Japanese women[22]. A study suggested that the prognosis of TNBC was similar between Asians and Caucasians, but the sample size was small[23]. There are currently few nomograms for Chinese patients with TNBC. Yang et al[24] and Shi et al[25] developed such a nomogram, but their predictive value was fair at best. Wang et al[26] developed a nomogram specifically for TNBC patients with de novo metastasis.Thus, the present study aimed to establish and validate a nomogram for TNBC in Chinese patients after surgery. This tool could help improve the management of TNBC in Chinese women.
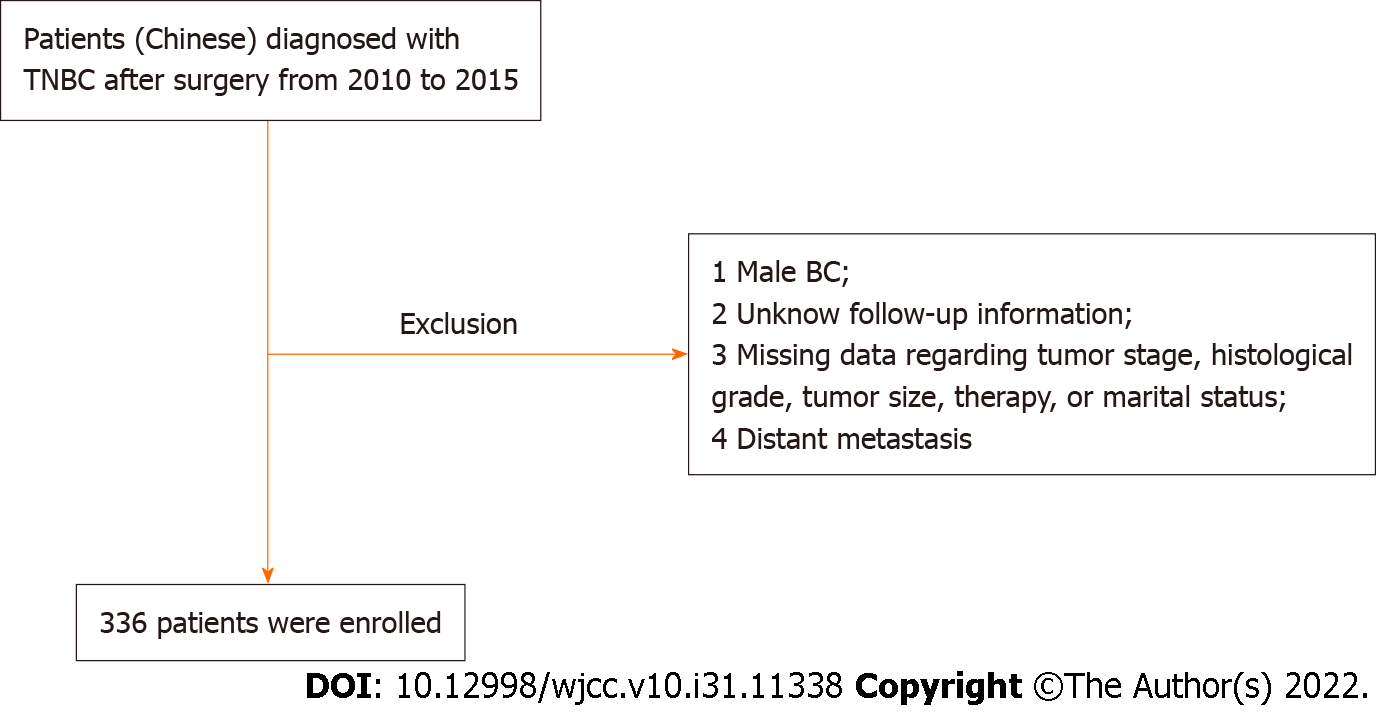
This study used the data from SEER*stat 8.3.5 and patients undergoing TNBC surgery between 2010 and 2015 were included. The inclusion conditions were (1) Chinese patients with histologically confirmed primary TNBC; (2) > 18 years of age at diagnosis; and (3) underwent primary tumor resection. The exclusion criteria were: (1) Male BC; (2) missing follow-up information; (3) missing data regarding tumor stage, histological grade, tumor size, therapy, or marital status; or (4) distant metastasis.
Categorical data were presented as n (%) and analyzed using the 2test. Univariate comparisons of survival data were performed using the Kaplan–Meier method and compared using the log-rank test and Cox analyses. Based on the univariable Cox analyses (P < 0.10) and considering clinically relevant factors, multivariate analysis using the Cox risk regression model with backward elimination was performed. Using the results of the multivariate analysis, variables with P = 0.05 were chosen for development of the nomograms.
To create the nomogram, we used 1-, 3- and 5-year OS. We calculated the C-indexes and generated calibration plots using 1000 bootstrap resamples to assess the nomogram’s predictive accuracy. Using the average score of each patient in the nomogram, the risk stratification model was developed to group the patients into two prognostic groups. The analyses were all performed with R (http://www.r-project.org) and Empower (R, v 3.6.3) (www.empowerstats.com, XY Solutions, Inc., Boston, MA, USA). Two-tailed P < 0.05 was considered statistically significant. The details of the nomogram and recursive partitioning analysis are presented as an appendix.
Finally, 336 patients were identified from the SEER database according to the screening (Figure 1). The median age was 57 years (range: 28–100 years). The median follow-up was 39 mo. The 1-, 3- and 5-year survival rates were 94.0%, 53.6% and 29.8%, respectively. Tumors ≥ 3 cm accounted for 16.4% of the cases. Among the 336 patients, 101 (30.1%) patients were unmarried, and 171 (50.9%) had left-sided cancer. Demographics, clinicopathological characteristics, and therapy are summarized in Table 1.
| Characteristics | n = 336 |
| Age at diagnosis, years, median (range) | 57 (28-100) |
| Marital status, n (%) | |
| Unmarried | 101 (30.1) |
| Married | 235 (69.9) |
| Laterality, n (%) | |
| Left | 171 (50.9) |
| Right | 165 (49.1) |
| Tumor size, cm, n (%) | |
| < 3 | 281 (83.6) |
| ≥ 3 | 55 (16.4) |
| Grade, n (%) | |
| Well-differentiated (I-II) | 103 (30.7) |
| Poorly differentiated (III) | 233 (69.3) |
| Stage, n (%) | |
| I | 146 (43.5) |
| II | 160 (47.6) |
| III | 30 (8.9) |
| T classification, n (%) | |
| T1 | 169 (50.3) |
| T2 | 143 (42.6) |
| T3 | 18 (5.4) |
| T4 | 6 (1.8) |
| N classification, n (%) | |
| N0 | 244 (72.6) |
| N1 | 67 (19.9) |
| N2 | 17 (5.1) |
| N3 | 8 (2.4) |
| Radiotherapy, n (%) | |
| None | 190 (56.6) |
| Yes | 146 (43.4) |
| Chemotherapy, n (%) | |
| None | 109 (32.4) |
| Yes | 227 (67.56) |
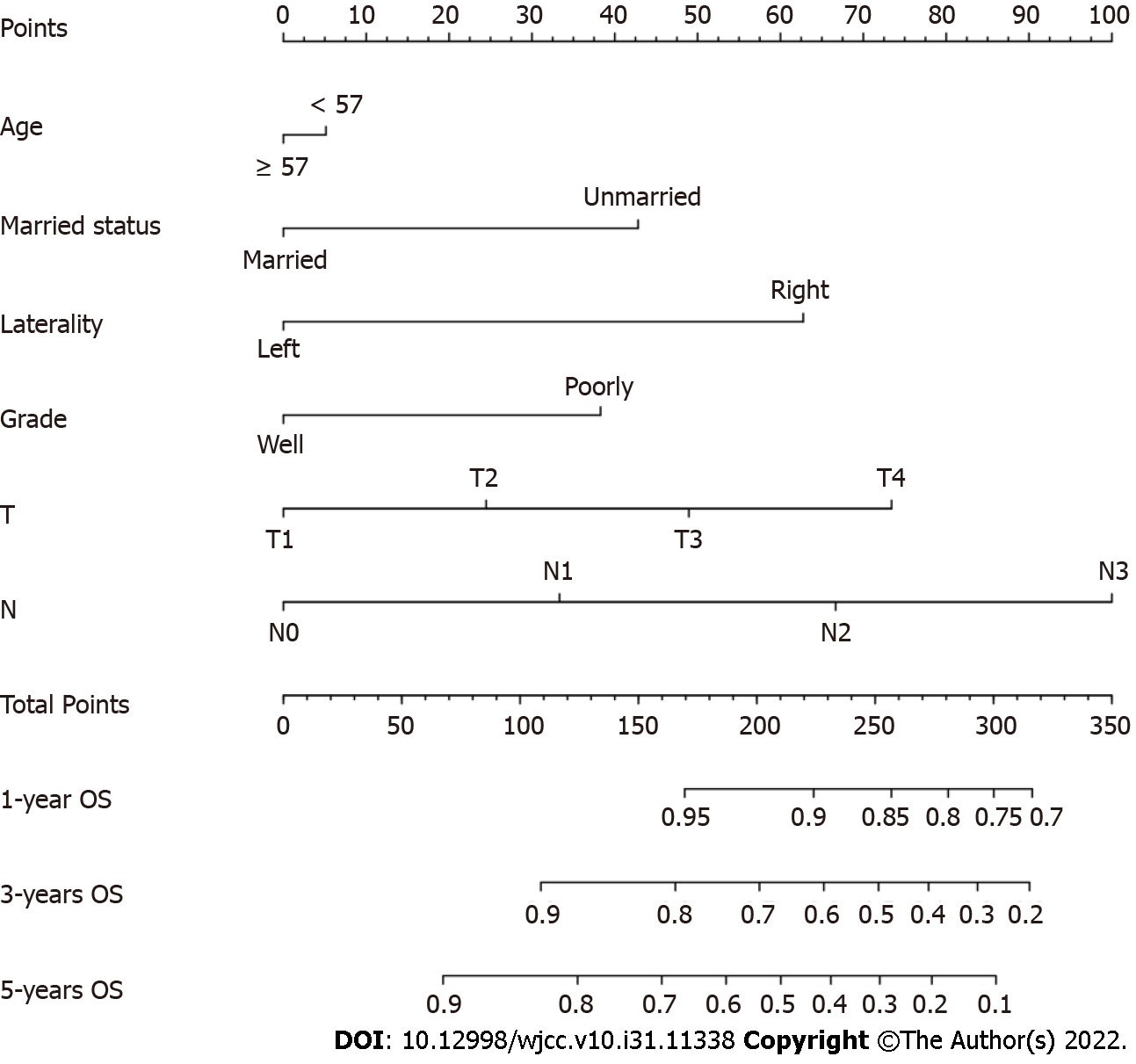
The univariable Cox regression analyses demonstrated that age at diagnosis, marital status, laterality, histological grade, T classification, N classification, and radiotherapy were associated with OS. Unmarried status (P = 0.016) and right-side BC (P = 0.003) were associated with poorer survival (Supplementary Figure 1). These factors were brought into the multivariate Cox regression analysis. Age at diagnosis (P = 0.043), marital status (P = 0.040), tumor localization (P = 0.030), grade (P = 0.035), T classification (P = 0.012), and N classification (P = 0.002) were used to develop the nomogram.
The Nomogram was composed of six independent factors (Figure 2). We gave a score for each category on the point scale axis (Supplementary Table 1). By adding up each score and projecting the total score onto the lowest scale, an overall score was calculated to estimate each patient’s OS at 1, 3 and 5 years.
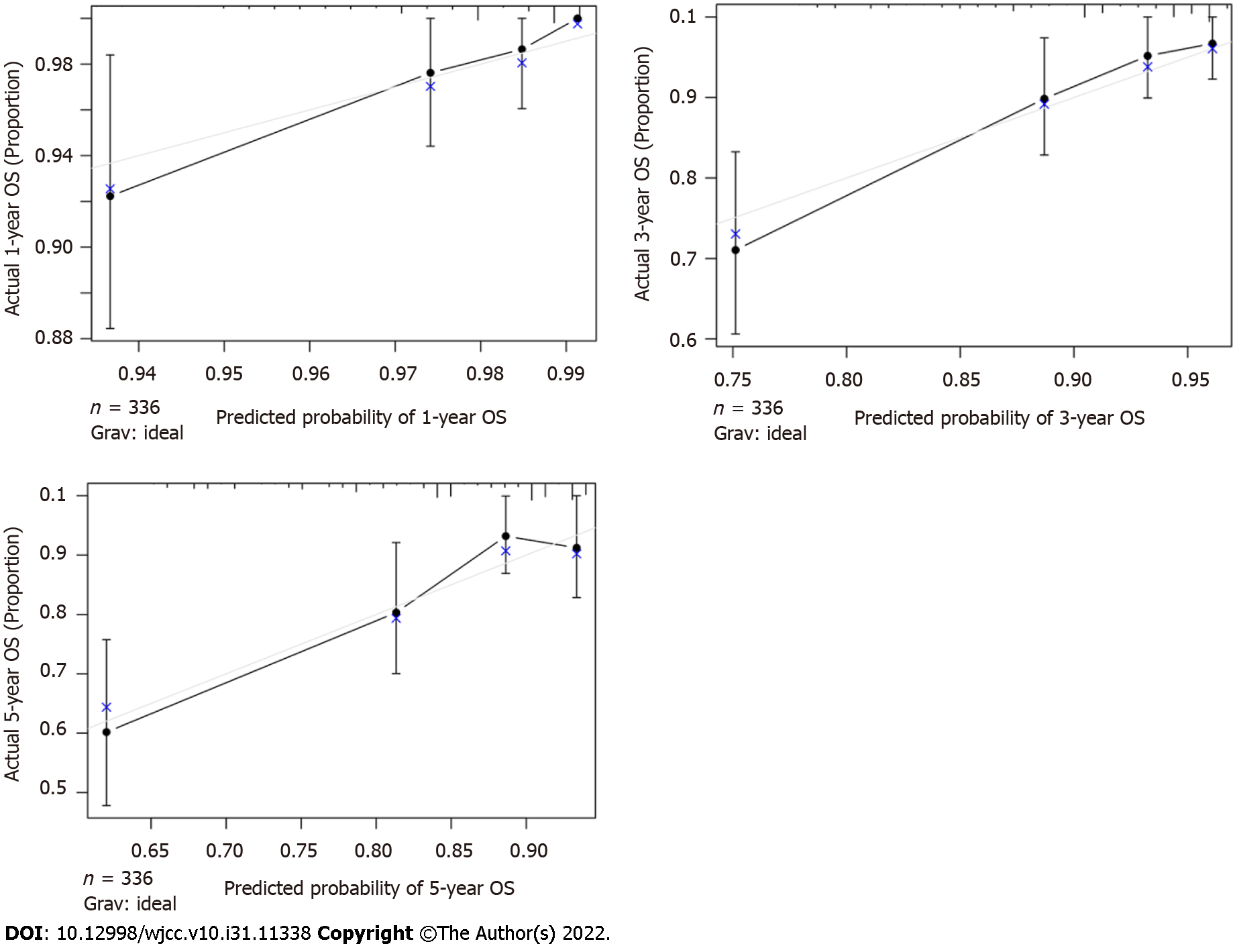
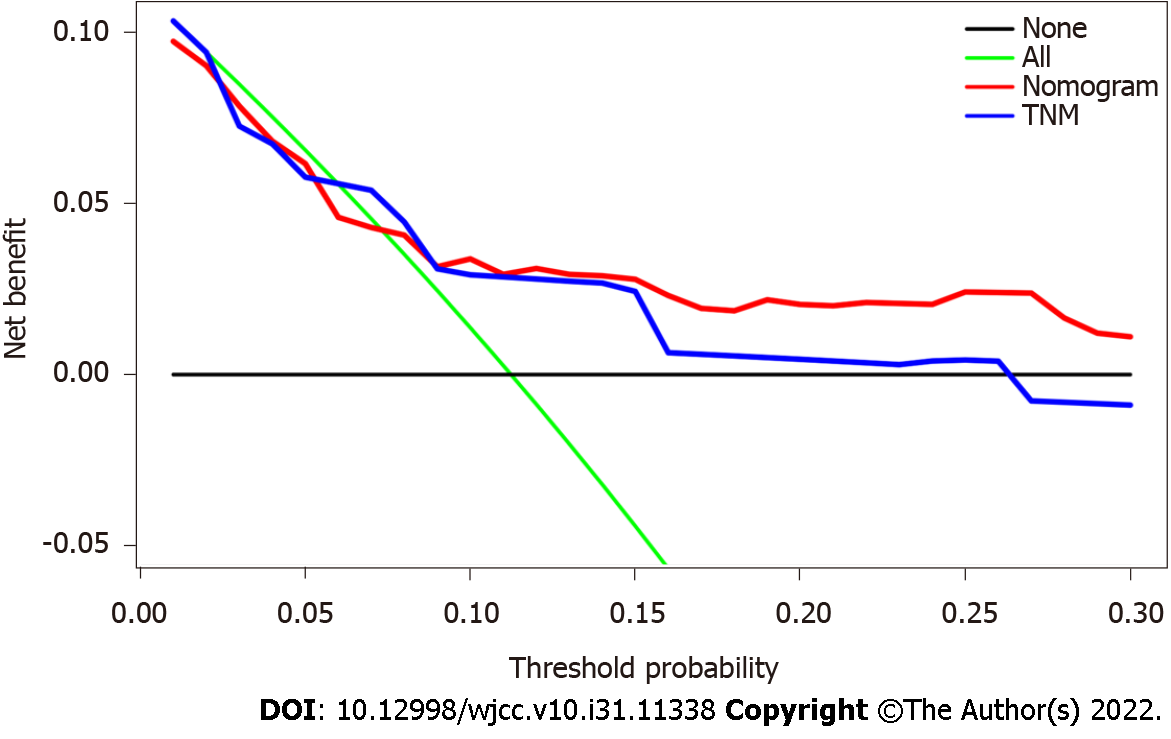
The C-index of the nomogram was higher than the C-index of the 7th version of the American Joint Committee on Cancer tumor, node and metastasis (AJCC TNM) staging system (0.766 vs 0.707, P < 0.01), indicating that predictive accuracy of the nomogram was acceptable. Calibration plots of the nomogram (Figure 3) demonstrated that the predicted OS closely matched the actual observations. Decision curve analysis is an analysis of net benefits that calculates the true-positive rate in relation to the weighted false-positive rate for different thresholds of risk that a clinician or patient may accept. This analysis was used to evaluate the 5-year OS of TNBC patients. All models had higher net returns than the “treat all” strategy (Figure 4). In most of the decision threshold probabilities, the nomogram decision model has a higher net benefit than the TNM phase model.
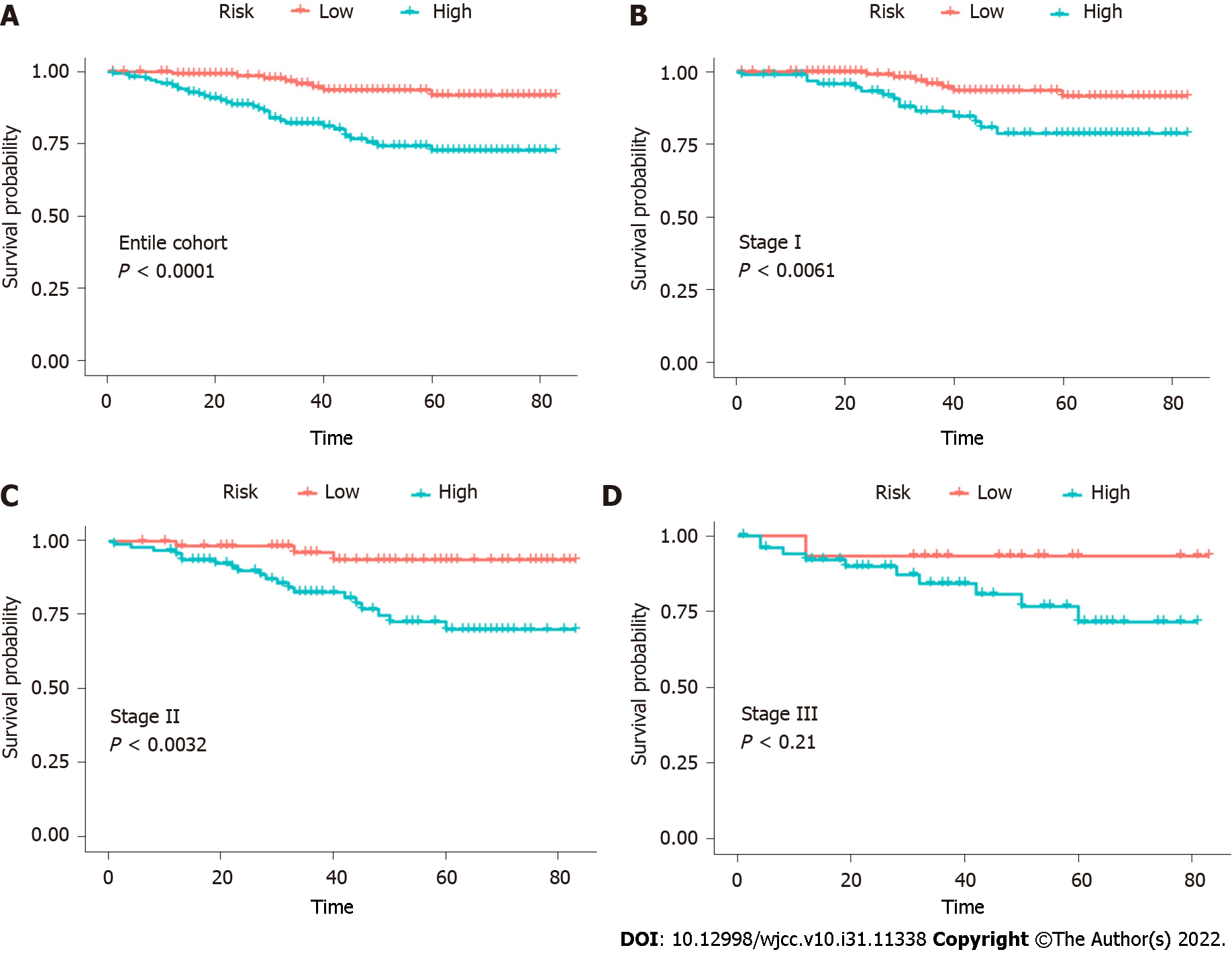
The results supported the predictive efficacy of the nomogram for OS. Therefore, we calculated the total score based on the nomogram prediction score. Patients were divided into two groups based on median: low risk (total score < 101.028) and high risk (total score ≥ 101.028). In the entire cohort, the 5-year OS rates of the patients with low and high risk were 31.9% and 27.8%, respectively (Figure 5). To validate the supplementary role of the risk stratification system to the 7th version of the AJCC-TNM staging system, we stratified the training cohort according to the TNM stage. Therefore, no significant distinction was found for stage III (P = 0.21). Moreover, within each TNM stage, there were significant differences between Kaplan–Meier curves in the survival predicted by the row plots (Figure 5).
The AJCC-TNM staging classification is the most widely used system for risk stratification and the selection of the treatment strategy for cancer patients[27,28]. However, this classical system does not accurately identify survival differences among cancer subtypes[29]. The TNM staging system ignores many factors that were confirmed to be highly associated with OS. At present, there are few nomograms for the prognosis of Chinese patients with TNBC. The aim of this study was to construct and validate a nomogram for OS of Chinese TNBC patients after surgery. The results suggest that the nomogram developed in this study has superior prognostic value than the traditional TNM system has. The nomogram could help clinicians make individual survival or risk prediction for Chinese TNBC patients after surgery and give necessary treatment recommendations.
Few independent studies have been conducted on nomogram survival to predict postoperative survival in Chinese TNBC patients; the sample size was small and the prognostic factors were insufficient[24,25,29]. Thus, the present study used the survival data of the SEER database. The SEER registry, the largest population-based cancer patient database in the USA, covers about 26% of patients diagnosed with cancer[30,31]. Patient data from the latest version of SEER published in 2015 (covering 18 registries, 1973–2015) were collected using SEER*Stat 8.3.5 under strict inclusion and exclusion criteria.
Four independent prognostic factors were identified through univariate and multivariate Cox regression analyses, including age at diagnosis, marital status, laterality, histologic grade, T classification, and N classification. These variables were finally incorporated into the nomogram. The findings were consistent with previous studies on survival risk factors for TNBC patients but not identical. The nomogram by Jiang et al[29] included age, tumor grade, and TNM stage. Yang et al[24] included stromal tumor-infiltrating lymphocytes, tumor size, nodal status, and Ki-67 index. Finally, Shi et al[25] included albumin-to-globulin ratio, neutrophil-to-lymphocyte ratio, positive lymph nodes, and tumor size.
The nomogram developed here showed that N classification made the largest contribution to prognosis. The N3 stage was highly associated with poor prognosis in Chinese TNBC patients. A previous study demonstrated that among lymph node-positive TNBCs, the lymph node ratio appeared to be a stronger predictor of mortality than pathological lymph nodes stage (hazard ratio: 0.80 for pN3 vs pN1, and 3.05 for > 0.65 vs < 0.21 lymph node ratio)[13].
This study also found that the survival of Chinese TNBC patients was associated with tumor laterality. Left-sided cancer had better OS than right-sided cancer. There is a slight excess of BC in the left breast compared with the right[32,33], but previous studies have been hampered by small numbers or limited geographic or demographic populations. Since the laterality difference is small, it has been difficult to appreciate the clinical or biological characteristics associated with left BC predominance[34]. Several studies have linked marital status to cancer outcomes in soft tissue sarcoma and small intestinal adenocarcinoma rectal cancer[35,36]. The present study also had clear survival discrimination in patients with different marital statuses, probably caused by reproductive factors[2].
The necessary identification and calibration evaluations are necessary to ensure the validity of nomograms, to maximize their generalizability and avoid overfitting. Discrimination is usually evaluated with the C-index, and calibration is assessed by comparing the agreement between predicting and actual patient survival. The results suggested that the nomogram developed in this study had better predictive and discriminative ability compared with traditional staging systems. Decision curves analysis was performed to examine the clinical net benefit in Chinese TNBC patients’ prognosis when using the nomogram. The results showed that model improved the clinical net benefit of all threshold probabilities[37,38].
Finally, a system was constructed to classify the patients into two risk subgroups based on the predicted total scores. When we applied the risk stratification system to evaluate patients at the same stage, we could discriminate OS in stages I and II but not in stage III patients. One important reason might be the small sample size. Thus, the nomogram risk stratification system is an accurate and reliable prognostic model supported by all results. It is beneficial for clinicians to identify high-risk patients and conduct necessary adjunctive therapy.
There were some limitations to this study. First, this was a retrospective study based on the SEER database, which has its limitations[39]. There was a possible selection bias regarding the variables included in the nomogram. The SEER database does not contain information on modern gene-array technology or some important molecular factors, such as programmed death ligand 1 expression or microsatellite status, which have been associated with OS in colon cancer[40]. Subsequent analysis might improve the study design in the future. Finally, the sample of Chinese patients with TNBC was small in the SEER database. In addition, they were individuals of Chinese descent but living in the USA. Therefore, they do not completely represent the Chinese population living in China. Future studies should aim at validating the results in China.
A novel nomogram for predicting OS after surgery in Chinese TNBC patients was developed and validated. The included factors are all easy to obtain in the clinical setting, and the prognostic model is convenient to use. This nomogram provides a clear prognostic superiority over the AJCC-TNM 7 system. It is the first model that is suitable for risk stratification in long-term survival for Chinese TNBC patients.
Triple-negative breast cancer (TNBC) represents 15%–20% of all BCs and has more aggressive biology, earlier onset of metastatic disease, visceral metastases, rapidly progressive disease, inefficacy of endocrine therapy, short response duration to available therapies, and inferior survival outcomes. Nomograms are useful and convenient tools for cancer patients to quantify and predict risk and prognosis. At present, there are few nomograms for Chinese TNBC patients. Thus, the present study aimed to establish and validate a nomogram for TNBC in Chinese patients after surgery. This tool could help improve the management of TNBC in Chinese women.
To explore a nomogram for predicting overall survival in Chinese TNBC patients after surgery.
To construct and validate a nomogram for the overall survival (OS) of Chinese TNBC patients after surgery.
This study used the data of SEER*stat 8.3.5 and selected Chinese patients with TNBC operated upon between 2010 and 2015. Univariate and multivariate Cox proportional hazard regression models were used. A predictive nomogram and risk stratification model was constructed by integrating the identified variables; it was assessed with C-indexes and calibration curves.
The median and maximal OS of the 336 patients was 39 and 83 mo, respectively. The multivariate analysis showed that age (P = 0.043), marital status (P = 0.040), tumor localization (P = 0.030), grade (P = 0.035), T classification (P = 0.012), and N classification (P = 0.002) were independent prognostic factors. The six variables were combined to construct a 1-, 3- and 5-year OS nomogram. The C-indexes of the nomogram to predict OS were 0.766, which was higher than that of the seventh edition tumor, node, metastasis (TNM) staging system (0.766 vs 0.707, P < 0.001). Using the average score of each patient in the nomogram, the risk stratification model was developed to group the patients into two prognostic groups. There was a significant difference between the Kaplan–Meier curves of the entire cohort and each disease stage according to the nomogram. Future studies should aim at validating the results in China.
A novel nomogram for predicting OS after surgery in Chinese TNBC patients was developed and validated. The included factors are all easy to obtain in the clinical setting, and the prognostic model is convenient to use. This nomogram provides a clear prognostic superiority over the TNM system. It is the first model suitable for risk stratification in long-term survival for Chinese TNBC patients.
The sample of Chinese patients with TNBC was small in the SEER database. In addition, they were individuals of Chinese descent but living in the USA. Therefore, they do not completely represent the Chinese population living in China. Future studies should aim at validating the results in China.
The authors are very grateful to the data providers of the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atoum M, Senchukova M S-Editor: Liu JH L-Editor: Kerr C P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55823] [Article Influence: 7974.7] [Reference Citation Analysis (132)] |
| 2. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 4.2021. Fort Washington: National Comprehensive Cancer Network, 2021. |
| 3. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer Risk Reduction. Version 1.2021. Fort Washington: National Comprehensive Cancer Network, 2021. |
| 4. | Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1648] [Article Influence: 206.0] [Reference Citation Analysis (0)] |
| 5. | Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F; ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v8-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1105] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 6. | Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso MJ, Carey L, Corneliussen-James D, Curigliano G, Dieras V, El Saghir N, Eniu A, Fallowfield L, Fenech D, Francis P, Gelmon K, Gennari A, Harbeck N, Hudis C, Kaufman B, Krop I, Mayer M, Meijer H, Mertz S, Ohno S, Pagani O, Papadopoulos E, Peccatori F, Penault-Llorca F, Piccart MJ, Pierga JY, Rugo H, Shockney L, Sledge G, Swain S, Thomssen C, Tutt A, Vorobiof D, Xu B, Norton L, Winer E. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017;28:16-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 282] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 7. | Geenen JJJ, Linn SC, Beijnen JH, Schellens JHM. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin Pharmacokinet. 2018;57:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 8. | Gadi VK, Davidson NE. Practical Approach to Triple-Negative Breast Cancer. J Oncol Pract. 2017;13:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293:247-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 485] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 10. | Kim C, Gao R, Sei E, Brandt R, Hartman J, Hatschek T, Crosetto N, Foukakis T, Navin NE. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell. 2018;173:879-893.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 727] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 11. | Lyons TG, Traina TA. Emerging Novel Therapeutics in Triple-Negative Breast Cancer. Adv Exp Med Biol. 2019;1152:377-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Sporikova Z, Koudelakova V, Trojanec R, Hajduch M. Genetic Markers in Triple-Negative Breast Cancer. Clin Breast Cancer. 2018;18:e841-e850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 13. | Urru SAM, Gallus S, Bosetti C, Moi T, Medda R, Sollai E, Murgia A, Sanges F, Pira G, Manca A, Palmas D, Floris M, Asunis AM, Atzori F, Carru C, D'Incalci M, Ghiani M, Marras V, Onnis D, Santona MC, Sarobba G, Valle E, Canu L, Cossu S, Bulfone A, Rocca PC, De Miglio MR, Orrù S. Clinical and pathological factors influencing survival in a large cohort of triple-negative breast cancer patients. BMC Cancer. 2018;18:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Triple negative breast cancer - prognostic factors and survival. Radiol Oncol. 2011;45:46-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Pistelli M, Pagliacci A, Battelli N, Santinelli A, Biscotti T, Ballatore Z, Berardi R, Cascinu S. Prognostic factors in early-stage triple-negative breast cancer: lessons and limits from clinical practice. Anticancer Res. 2013;33:2737-2742. [PubMed] |
| 16. | Jin C, Cao J, Cai Y, Wang L, Liu K, Shen W, Hu J. A nomogram for predicting the risk of invasive pulmonary adenocarcinoma for patients with solitary peripheral subsolid nodules. J Thorac Cardiovasc Surg. 2017;153:462-469.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Veale D, Miles S, Bramley S, Muir G, Hodsoll J. Am I normal? BJU Int. 2015;115:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Wu J, Qiu J, Jiang W, Zhang L, Zhao R, Yu C. Development and validation of a nomogram predicting the probability of type a aortic dissection at a diameter below 55 mm: A retrospective cohort study. Int J Surg. 2018;60:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, Nam SJ, Kim SW, Lee JE, Yu JH, Lee SK, Choi M, Cho SY, Cho EY. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat. 2019;173:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Luo WQ, Huang QX, Huang XW, Hu HT, Zeng FQ, Wang W. Predicting Breast Cancer in Breast Imaging Reporting and Data System (BI-RADS) Ultrasound Category 4 or 5 Lesions: A Nomogram Combining Radiomics and BI-RADS. Sci Rep. 2019;9:11921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Lim DW, Giannakeas V, Narod SA. Survival Differences in Chinese Versus White Women With Breast Cancer in the United States: A SEER-Based Analysis. JCO Glob Oncol. 2020;6:1582-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Maskarinec G, Sen C, Koga K, Conroy SM. Ethnic differences in breast cancer survival: status and determinants. Womens Health (Lond). 2011;7:677-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Ding YC, Steele L, Warden C, Wilczynski S, Mortimer J, Yuan Y, Neuhausen SL. Molecular subtypes of triple-negative breast cancer in women of different race and ethnicity. Oncotarget. 2019;10:198-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Yang Y, Wang Y, Deng H, Tan C, Li Q, He Z, Wei W, Zhou E, Liu Q, Liu J. Development and validation of nomograms predicting survival in Chinese patients with triple negative breast cancer. BMC Cancer. 2019;19:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Shi H, Wang XH, Gu JW, Guo GL. Development and Validation of Nomograms for Predicting the Prognosis of Triple-Negative Breast Cancer Patients Based on 379 Chinese Patients. Cancer Manag Res. 2019;11:10827-10839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Wang H, Sun X, Fang Y, Lu SS, Ding SN, Chen XS, Shen KW. A Risk Stratification Model for Predicting Overall Survival and Surgical Benefit in Triple-Negative Breast Cancer Patients With de novo Distant Metastasis. Front Oncol. 2020;10:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Fouad TM, Barrera AMG, Reuben JM, Lucci A, Woodward WA, Stauder MC, Lim B, DeSnyder SM, Arun B, Gildy B, Valero V, Hortobagyi GN, Ueno NT. Inflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol. 2017;18:e228-e232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Nam SH, Bae MR, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, Kim SY. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol. 2018;87:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Jiang S, Zhao R, Li Y, Han X, Liu Z, Ge W, Dong Y, Han W. Prognosis and nomogram for predicting postoperative survival of duodenal adenocarcinoma: A retrospective study in China and the SEER database. Sci Rep. 2018;8:7940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11933] [Article Influence: 2983.3] [Reference Citation Analysis (4)] |
| 31. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 32. | Roychoudhuri R, Putcha V, Møller H. Cancer and laterality: a study of the five major paired organs (UK). Cancer Causes Control. 2006;17:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Senie RT, Rosen PP, Lesser ML, Snyder RE, Schottenfeld D, Duthie K. Epidemiology of breast carcinoma II: factors related to the predominance of left-sided disease. Cancer. 1980;46:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Onibokun O, Killelea BK, Chagpar AB, Horowitz NR, Lannin DR. The left sided predominance of breast cancer is decreasing. Breast J. 2015;21:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Buja A, Lago L, Lago S, Vinelli A, Zanardo C, Baldo V. Marital status and stage of cancer at diagnosis: A systematic review. Eur J Cancer Care (Engl). 2018;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Wang X, Cao W, Zheng C, Hu W, Liu C. Marital status and survival in patients with rectal cancer: An analysis of the Surveillance, Epidemiology and End Results (SEER) database. Cancer Epidemiol. 2018;54:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Goodacre S, Horspool K, Shephard N, Pollard D, Hunt BJ, Fuller G, Nelson-Piercy C, Knight M, Thomas S, Lecky F, Cohen J. Selecting pregnant or postpartum women with suspected pulmonary embolism for diagnostic imaging: the DiPEP diagnostic study with decision-analysis modelling. Health Technol Assess. 2018;22:1-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ, Steyerberg EW. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur Urol. 2018;74:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 761] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 39. | Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park). 2009;23:288-295. [PubMed] |
| 40. | Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, Qu D, Wang X, Lan B, Yang B, Wang P, Zhang H, Yang Q, Jiao Y. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients with Advanced Esophageal Carcinoma. Clin Cancer Res. 2018;24:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |