Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11198
Peer-review started: August 1, 2022
First decision: August 22, 2022
Revised: August 30, 2022
Accepted: September 19, 2022
Article in press: September 19, 2022
Published online: October 26, 2022
Processing time: 80 Days and 20.1 Hours
Tracheoesophageal fistula (TEF) is a congenital anomaly characterized by interruptions in esophageal continuity with or without fistulous communication to the trachea. Anesthetic management during TEF repair is challenging because of the difficulty of perioperative airway management. It is important to determine the appropriate position of the endotracheal tube (ETT) for proper ventilation and to prevent excessive gastric dilatation. Therefore, the tip of the ETT should be placed immediately below the fistula and above the carina.
A full-term, one-day-old, 2.4 kg, 50 cm male neonate was diagnosed with TEF type C. During induction, an ETT was inserted using video laryngoscope and advanced deeply to ensure that the tip passed over the fistula, according to known strategies. The passage of the ETT through the vocal cords was confirmed via video laryngoscope. However, after inflating the ETT cuff, breath sounds were not heard on bilateral lung auscultation. Instead, gastric sounds were heard. Considering that a large fistula (approximately 6.60 mm × 4.54 mm) located 10.2 mm above the carina was confirmed on preoperative tracheal computed tomography, the possibility of unintentional esophageal intubation was highly suspected. Therefore, we decided to uncuff and withdraw the ETT carefully for repositioning, while monitoring auscultation and end-tidal CO2 simultaneously. At a certain point (9.5 cm from the lip), clear breath sounds and proper end-tidal CO2 readings were suddenly achieved, and adequate ventilation was possible.
Preanesthetic anatomical evaluation with imaging studies in TEF is necessary to minimize complications related to airway management.
Core Tip: Anesthetic management in tracheoesophageal fistula (TEF) repair is challenging for anesthesiologists because of the difficulty in airway management. Unexpected events during airway management can occur, resulting in catastrophic outcomes, such as desaturation, hypoxic damage, and even death. In our case, esophageal intubation was unintentionally performed because of the large fistula. We predicted the possibility of this event based on the preceding tracheal computed tomography, which helped us to obtain a better clinical outcome. Evaluating the anatomy of each patient with TEF using imaging studies before induction is essential to minimize complications and facilitate prompt management as necessary.
- Citation: Hwang SM, Kim MJ, Kim S, Kim S. Accidental esophageal intubation via a large type C congenital tracheoesophageal fistula: A case report. World J Clin Cases 2022; 10(30): 11198-11203
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11198.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11198
A tracheoesophageal fistula (TEF) is a group of congenital anomalies characterized by interruptions in esophageal continuity with or without fistulous communication with the trachea. It has an incidence of one in 2500–3000 live births. There are five types of congenital TEF based on the Gross and Vogt classification. The most common type of congenital TEF is esophageal atresia with a distal TEF, namely, Gross type C/Vogt type IIIb (86%)[1]. Congenital TEF has been associated with other anomalies. Up to 10% of neonates with congenital TEF have VATER or VACTERL (vertebral defects, anorectal malformations, cardiac defects, TEF, renal anomalies, radial dysplasia, and limb defects)[2,3].
After birth, neonates with TEF experience recurrent coughing, gagging, choking, reflux, cyanosis during feeding, and excessive salivation. Failure to pass a nasogastric tube is usually the first sign checked at the clinic. On plain chest and abdominal radiographs, the tip of the catheter can appear curled up at the chest or upper neck level. Gas in the stomach and intestines can be found in some cases, suggesting distal TEF[4].
Surgical repair of the defect is the definitive treatment for congenital TEF. It is usually performed within 24–72 h in neonates. Delayed surgical repair can render the neonates more susceptible to pneumonitis due to aspiration of saliva, accumulated in the upper pouch, or gastric acid reflux through the TEF[5].
Successful airway management is essential for anesthetic management. However, it can be challenging in patients with TEF because of anatomical abnormalities of the airway. During airway management of TEF, unexpected events can cause catastrophic outcomes, such as desaturation, hypoxic damage, and death[6-8]. Adequate positioning of the endotracheal tube (ETT) below the fistula and above the carina is important for proper ventilation and prevention of excessive gastric dilatation. This is achieved by advancing the ETT at the level of the carina. Alternatively, it can be inserted into the main bronchus and then slowly withdrawn until equal air entry is confirmed on lung auscultation[9]. However, various types and sizes of TEF make the intubation process more complicated. This study presents a case of unintentional esophageal intubation in a patient with a large TEF.
A full-term, one-day-old, 2.4 kg, 50 cm male neonate was scheduled for surgical correction of a type C TEF.
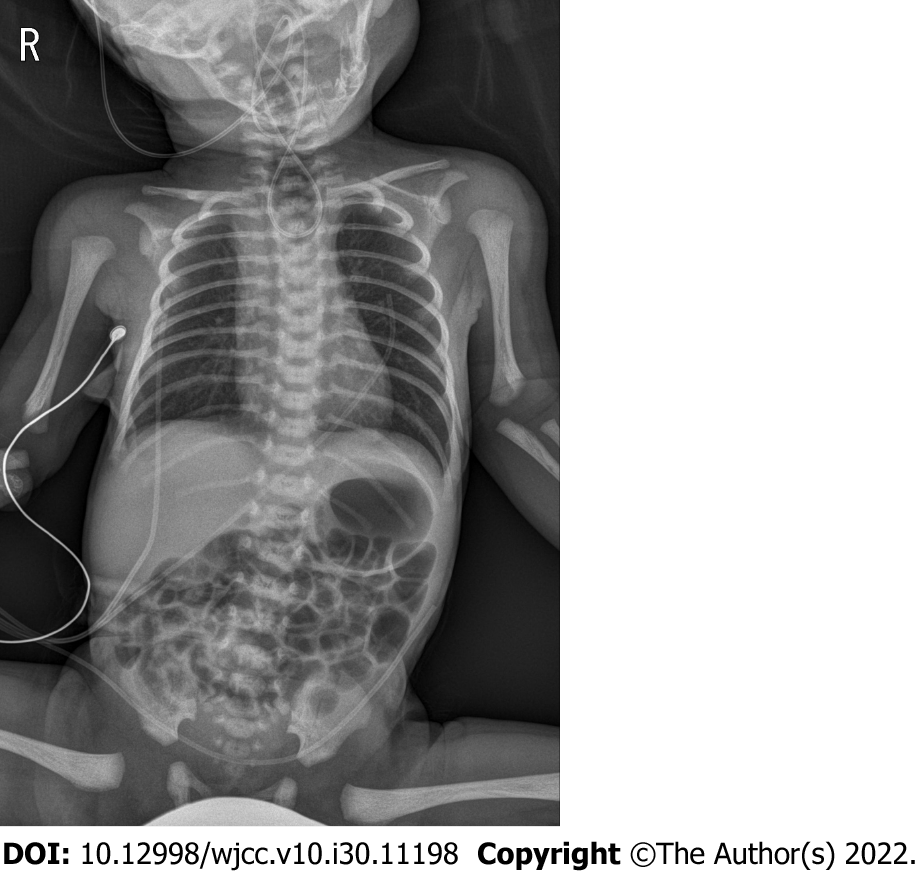
The clinical diagnosis was confirmed via an imaging study, which revealed a connection between the lower esophageal segment and the trachea (Figures 1 and 2).
The patient had a patent ductus arteriosus (PDA) measuring 2–3 mm and an atrial septal defect (ASD) measuring 3–4 mm.
The patient had no relevant family history.
Before anesthetic management in the operating room, the patient was breathing spontaneously on room air, and lung sounds were clear bilaterally on auscultation. There were no symptoms indicative of respiratory abnormalities, and cardiac physical examination revealed unremarkable findings.
Results from preoperative blood tests, including full blood count, liver function test, kidney function test, and electrolyte test results, were within the normal ranges.
Echocardiography performed on day one after birth revealed a PDA measuring 2–3 mm and an ostium secundum ASD measuring 3–4 mm with a left-to-right shunt and without dilatation or hypertrophy of the atria and ventricles. No regurgitation and stenosis of the four heart valves was noted, and the coronary artery system and ventricular function were normal. Before starting anesthetic management, the tracheal computed tomography (CT) images were evaluated. A large fistula (approximately 6.60 × 4.54 mm) was observed 10.2 mm above the carina (Figure 2).
The patient was diagnosed with a type C TEF. For its surgical correction, general anesthesia was induced under standard monitoring (electrocardiography, pulse oximeter oxygen saturation, and blood pressure) using 8% sevoflurane in oxygen without a muscle relaxant. A cuffed ETT with an inner diameter of 3.5 mm (Hi-Contour Oral/Nasal Tracheal Tube Cuffed, ShileyTM) was inserted between the vocal cords using video laryngoscope with a #0 Miller blade. The ETT was gradually advanced up to 12 cm as measured from the lip to ensure that the ETT passes over the fistula. However, after inflating the ETT cuff, end-tidal CO2 readings could not be obtained. On bilateral lung auscultation, breath sounds were not heard bilaterally. Instead, gastric sounds were heard. The patient’s oxygen saturation, measured by pulse oximetry, gradually decreased to 60%. In our case, a large fistula was suspected to be the cause of unintentional esophageal intubation.
Although we confirmed the vocal cords using a video laryngoscope, we decided to remove the ETT immediately after the first intubation attempt. After the ETT was removed, mask ventilation with 100% oxygen was initiated to support spontaneous breathing. Oxygen saturation rapidly recovered to 100%. External pressure was gently applied to the abdomen during mask ventilation to minimize gastric dilatation. In the second attempt, a new cuffed ETT of the same size was inserted using video laryngoscopy with a #0 Miller blade. The ETT was advanced 11 cm from the lip. However, even though we confirmed the vocal cords with a video laryngoscope for the second time, no breath sounds were heard, but gastric sounds were checked again on auscultation. Instead of removing the ETT, the ETT was deflated and slowly withdrawn until 8 cm from the lip. Auscultation of both the lungs and stomach was performed during this process. Subsequently, the ETT was adjusted to 9.5 cm from the lip. Clear breath sounds were heard in both lungs with an adequate end-tidal CO2 readings, and no gastric sounds or dilation were noted. After confirming proper ventilation, a neuromuscular block agent was administered.
Surgical repair was performed with the patient in the left decubitus position. The position of the ETT was confirmed by chest and stomach auscultation after the positional change. Intraoperatively, a large Gross type C (Vogt IIIb) fistula was observed, and surgery was successfully performed. Oxygen saturation was maintained at 99%–100% during anesthesia. Endotracheal intubation was maintained postoperatively for additional care and the patient was transferred to the neonatal intensive care unit. The gastrografin test performed one week postoperatively did not reveal any leakage. Subsequently, the patient was successfully extubated and oral feeding was initiated. The patient had no other complications.
Placing the ETT in an appropriate position is crucial for successful airway management of TEF. Over the decades, several strategies for ETT placement in TEF have been studied[8,10-14]. The traditional technique for positioning ETT in TEF patients required that the tip of the ETT be initially located in the right main bronchus through the carina, and gradually withdrawn to the point at which bilateral lung sounds can be appreciated[10]. The ETT placement was then confirmed by either auscultation or bronchoscopic evaluation.
The video laryngoscope we used could increase the success rate of intubation by confirming the vocal cords. However, it could not inspect the subglottic space. Therefore, we believe that both flexible and rigid bronchoscopes can play an important role in confirming the proper position of an ETT in TEF. Additionally, the bronchoscope provides the benefit when a Fogarty balloon catheter is used. This alternative strategy is designed to block the fistula and ventilate isolately[8,11]. However, our center did not have a Fogarty balloon catheter and a bronchoscope with an external diameter small enough to pass through the pediatric ETT. Therefore, we used the traditional technique for intubating our patient.
Moreover, the cuff of the ETT may play an important role during the intubation process for a TEF. Compared to an uncuffed ETT, a cuffed ETT may provide better ventilation by blocking the fistula when the tip of the ETT is placed distal to it. If ETT without Murphy’s eye is used, positioning the cuffed ETT bevel facing forward is also helpful in blocking the fistula[12,13].
When desaturation episode occurred during the first intubation attempt, we decided to remove the ETT and initiate mask ventilation immediately. It was challenging to determine the main cause of inadequate ventilation and desaturation immediately. Considering that the functional residual capacity tends to be lower in newborns, desaturation can arise faster and result in complications, such as hypoxic damage, or even death. Therefore, immediate mask ventilation was crucial for ensuring adequate ventilation. During this process of securing the airway, maintaining spontaneous breathing is also important. Without spontaneous breathing, continual mask ventilation is required to restore oxygen saturation, and in cases of TEF, gastric distention through the fistula can worsen. The avoidance of neuromuscular blocking agents can help reduce gastric distention and regurgitation[13,14].
In airway management, the sooner the cause of intubation failure and inadequate ventilation is identified, the faster it can be managed. As we confirmed the passage of the ETT through the vocal cords using a video laryngoscope, bronchospasm was first considered. However, there was no wheezing, and only gastric sounds were heard, thus, indicating esophageal intubation. Then, the large size of the fistula was suspected to be the main cause. It was approximately 6.60 mm × 4.54 mm as we preoperatively checked, and seemed big enough that the 3.5 mm cuffed ETT (Outer diameter 4.9 mm) to pass through it. In the second attempt, when the same situation arose, we immediately adjusted the ETT to ensure its proper position. This immediate management was possible because of pre-evaluation of the pre-anesthetic images.
Familiarity with the airway anatomy is essential in every anesthetic case. This is even more important in cases at risk of difficult intubation, such as TEF. Holzki et al[15] reported bronchoscopic findings in 113 neonates with TEF. The fistula was located > 1 cm above the carina in 67%, < 1 cm above the carina in 22%, and below the carina in 11% of patients. Based on type C TEF illustrations in general, the trachea and esophagus are connected perpendicularly[1,13,16]. Therefore, the possibility of esophageal intubation through the fistula appears low. However, Lehavi et al[16] reported a case in which the trachea and esophagus were not connected perpendicularly, but rather, at an obtuse angle. When performing the preanesthetic anatomical evaluation with imaging studies, we focused only on the size and position of the fistula and did not pay much attention to the angle between the trachea and the esophagus. However, fistula size and position were not the only factor that affected the possibility of esophageal intubation. When the tracheal CT was reviewed again postoperatively, the angle between the trachea and fistula was found to be an obtuse angle, at approximately 146° (Figure 2A). We assumed that this obtuse angle played an important role in correlating with the large fistula size. Therefore, as in our case, the possibility of esophageal intubation through the fistula increases when the patient has both a large size and an obtuse angle.
Additionally, preoperative tracheal CT can help predict the appropriate depth of the ETT, which can vary depending on the position of the fistula. In our case, the tracheal CT assessment revealed an ETT depth of 9.8 cm (Figure 2A). Although a measurement bias may exist, it is only within a few millimeters, and it turned out to be a similar value of 9.5 cm in result. Initially, in the traditional strategy, the ETT should be inserted deeply, beyond the fistula. Therefore, the ETT goes deeper than the predicted depth. However, the predicted depth obtained on CT can be helpful when trying to adjust the ETT to an appropriate position.
When evaluating imaging studies including tracheal CT, it is important to obtain information on not only the type of TEF but also the size, position, and angle. In our case, the fistula was large (6.60 mm × 4.54 mm) and located 10.2 mm above the carina and at an obtuse angle of 146° (Figure 2). We believe that preoperative awareness of anatomical conditions makes it possible to assess adverse situations earlier.
Therefore, evaluation of the anatomy of TEF patient using imaging studies is recommended prior to anesthetic management to minimize the possibility of intubation failure and other damaging consequences. In addition to the type and position of the fistula, its size and angle formed by the esophagus and trachea should be considered. In particular, the sagittal view of tracheal CT is essential for determining the angle between the trachea and the fistula. Based on this, anesthesiologists can detect the cause of intubation failure more promptly and minimize complications.
Understanding the anatomic condition using imaging studies is essential for appropriate airway management of TEF. In our case, the possibility of esophageal intubation via a large fistula in a patient with type C congenital TEF was detected on preoperative tracheal CT. The tracheal CT played an important role to obtain information about the type, size, position, and angle of TEF. Preanesthetic anatomical evaluation using imaging studies, such as tracheal CT, is essential for the airway management and the prevention of catastrophic events.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiu H, Taiwan; Gupta N, India; Liu S, China; Yang F, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007;2:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (2)] |
| 2. | Clark DC. Esophageal atresia and tracheoesophageal fistula. Am Fam Physician. 1999;59:910-916, 919. [PubMed] |
| 3. | Al-Rwwi O, Booker PD. Oesophageal atresia and tracheo-oesophageal fistula. Continuing Education in Anaesthesia, Crit Care Pain 2007; 7: 15-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Motshabi P. Anaesthesia for oesophageal atresia with or without tracheo-oesophageal atresia. South Afr J Anaesth Anal 2014; 20: 19-25 . [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Gayle JA, Gómez SL, Baluch A, Fox C, Lock S, Kaye AD. Anesthetic considerations for the neonate with tracheoesophageal fistula. Middle East J Anaesthesiol. 2008;19:1241-1254. [PubMed] |
| 6. | Alabbad SI, Shaw K, Puligandla PS, Carranza R, Bernard C, Laberge JM. The pitfalls of endotracheal intubation beyond the fistula in babies with type C esophageal atresia. Semin Pediatr Surg. 2009;18:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Buchino JJ, Keenan WJ, Pietsch JB, Danis R, Schweiss JF. Malpositioning of the endotracheal tube in infants with tracheoesophageal fistula. J Pediatr. 1986;109:524-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Taneja B, Saxena KN. Endotracheal intubation in a neonate with esophageal atresia and trachea-esophageal fistula: pitfalls and techniques. J Neonatal Surg. 2014;3:18. [PubMed] |
| 9. | Salem MR, Wong AY, Lin YH, Firor HV, Bennett EJ. Prevention of gastric distention during anesthesia for newborns with tracheoesophageal fistulas. Anesthesiology. 1973;38:82-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Desy P, Putu K, Tjokorda GAS. Anesthetic management of patients undergoing one-step surgical tracheoesophageal fistula: Case series. Bali J Anesthesiology 2021; 5: 267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Reeves ST, Burt N, Smith CD. Is it time to reevaluate the airway management of tracheoesophageal fistula? Anesth Analg. 1995;81:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ho AM, Dion JM, Wong JC. Airway and Ventilatory Management Options in Congenital Tracheoesophageal Fistula Repair. J Cardiothorac Vasc Anesth. 2016;30:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Broemling N, Campbell F. Anesthetic management of congenital tracheoesophageal fistula. Paediatr Anaesth. 2011;21:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Goswami D, Kachru N, Pant N. Difficult ventilation in a wide congenital tracheoesophageal fistula. Can J Anaesth. 2012;59:118-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |