Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11155
Peer-review started: July 1, 2022
First decision: August 1, 2022
Revised: August 12, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: October 26, 2022
Processing time: 111 Days and 18.9 Hours
Struma ovarii is a type of monodermal mature teratoma composed entirely or mainly of thyroid tissue, accounting for 1% to 3% of all ovarian teratomas and 0.3% to 1.0% of all ovarian tumors. Of which, struma ovarii with ascites and pleural effusion, called pseudo-Meigs’syndrome and raised cancer antigen-125 levels (CA 125) is even rarer.
This paper reports the diagnosis and treatment of a patient of struma ovarii with pseudo-Meigs’syndrome, presenting with the clinical features of ovarian carci
The diagnosis and treatment process of this case suggests that the clinical symptoms of struma ovarii with pseudo-Meigs’syndrome are lack specificity, which is easily misdiagnosed. Clinicians should improve the understanding of this disease, enhance the awareness of early screening, and improve the level of diagnosis and treatment.
Core Tip: Struma ovarii with pseudo-Meigs’syndrome and elevated serum cancer antigen-125 is easily preoperatively misdiagnosed as ovarian cancer, leading to unnecessary extended surgery. In this case, the patient of giant struma ovarii with pseudo-Meigs’syndrome underwent conservative surgery in the form of a right salpingo-oophorectomy, as there was no evidence of malignancy according to the preoperative biopsy and intraoperative frozen analysis. Besides, this patient was premenopausal and to our knowledge, she is the youngest with this disease.
- Citation: Liu Y, Tang GY, Liu L, Sun HM, Zhu HY. Giant struma ovarii with pseudo-Meigs’syndrome and raised cancer antigen-125 levels: A case report. World J Clin Cases 2022; 10(30): 11155-11161
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11155.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11155
Struma ovarii, a special type of ovarian teratoma, is a highly differentiated monodermal teratoma that arises from ovarian primordial germ cells and is defined as mature teratoma composed of a minimum of 50% of thyroid tissue by World Health Organisation, accounting for 1% to 3% of all ovarian teratomas and 0.3% to 1.0% of all ovarian tumors[1,2]. Meigs’syndrome represents a solid benign ovarian neoplasm, such as fibroma, granulosa cell tumor or thecoma with hydrothorax and ascites which are completely resolved spontaneously after surgical removal of the tumour[3]. When ascites and hydrothorax are associated with other ovarian tumors, it is defined as pseudo-Meigs’syndrome[4]. Struma ovarii is rare, but struma ovarii with pseudo-Meigs’syndrome is even rarer and it is easily misdiagnosed in clinical practice[5]. In order to deepen clinicians' understanding of this disease, here, we present a case of benign struma ovarii associated with pseudo-Meigs’syndrome and elevated cancer antigen-125 (CA 125).
A 37-year-old, Chinese woman, premenopausal, presented to gynecologic clinic with a complaint of abdominal bulge for 4 mo.
Symptoms started 4 mo before presentation with abdominal bulge, without abdominal pain.
She had a history of breast fibroma surgery 6 years ago.
The patient denied any family history of malignant tumours.
Physical examination revealed obvious abdominal distension, positive mobility voiced sounds, positive fluid wave tremor and weak bowel sounds. Besides, the vital signs were as follows: Body temperature, 37.2 °C; blood pressure, 122/83 mmHg; pulse, 102 beats per min; respiratory rate, 18 breaths per min. Furthermore, the right breast had old surgical scars. Gynecological examination: an irregular mass, with a diameter of 12 cm, was found on the right ovary; left ovary and uterus had no obvious abnormalities.
Tumor marker carbohydrate antigen 199 was not elevated (33.87 U/mL, reference, 0-37), but CA 125 was 1492.23 U/mL (reference, 0-35). Besides, thyroid function tests were within normal limits: free triiodothyronine, 6.24 pmol/L (reference, 3.5-6.5); free thyroxine, 19.63 pmol/L (reference, 11.5-22.7); thyroid-stimulating hormone, 1.44 μIU/mL, (reference, 0.55-4.78). No abnormality was found in routine blood analyses.
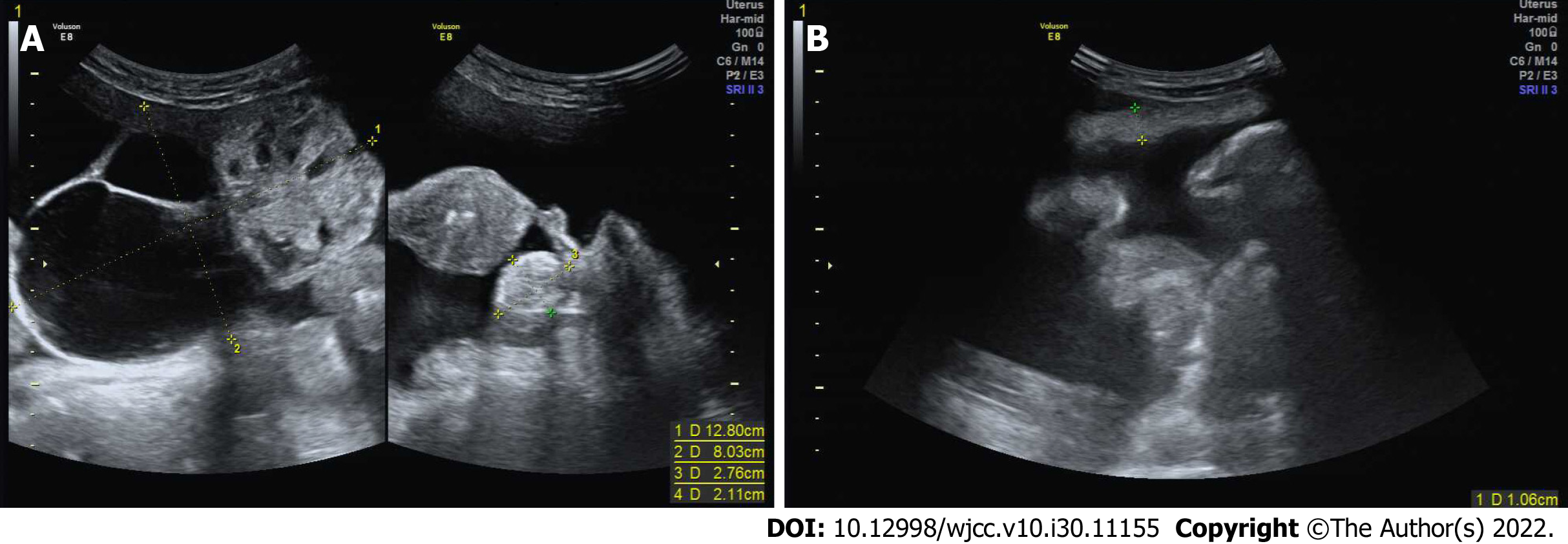
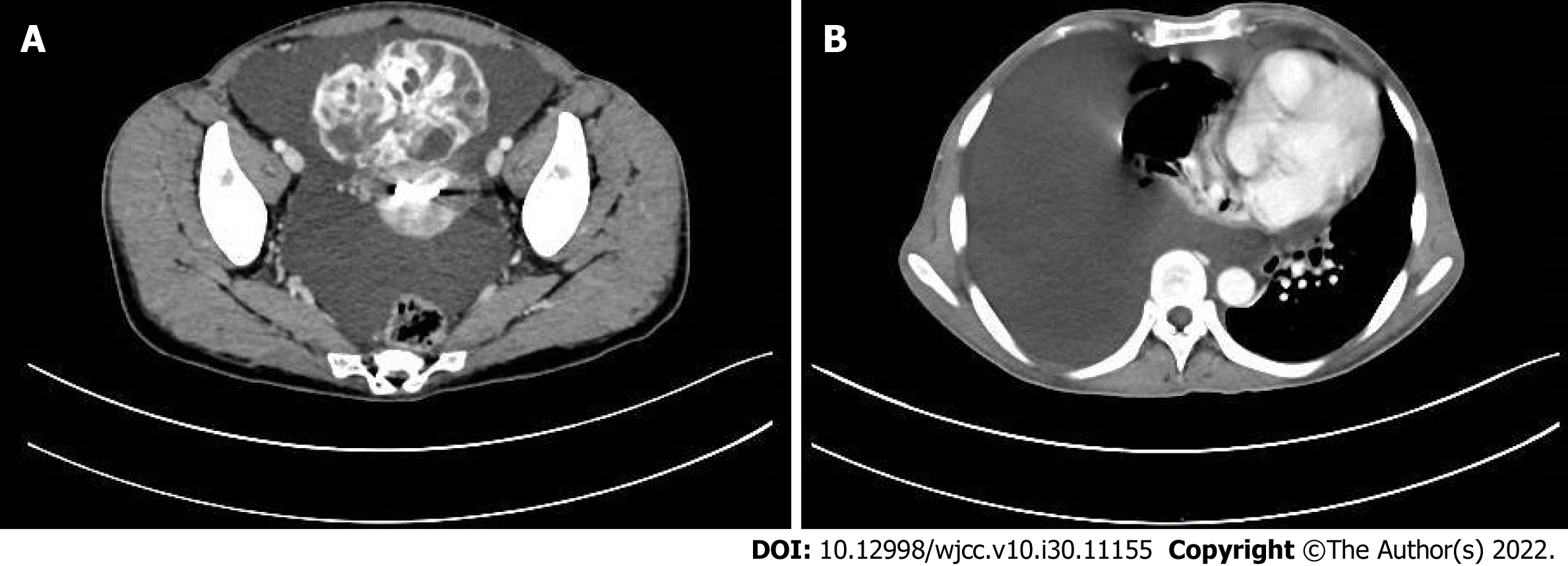
Ultrasonography showed a 12.8 cm × 8.0 cm right adnexal mass containing solid and cystic components with abundant vascularization and 2.8 cm × 2.1 cm solid left adnexal mass. Besides, there was a large amount of free peritoneal fluid and thickened greater omentum (Figure 1). Computed tomography (CT) scan of the chest, abdomen, and pelvis revealed right lung atelectasis with a large right pleural effusion, gross ascites, and a large complex cystic pelvic mass (Figure 2). Overall, the radiological findings were suspicious of ovarian cancer.
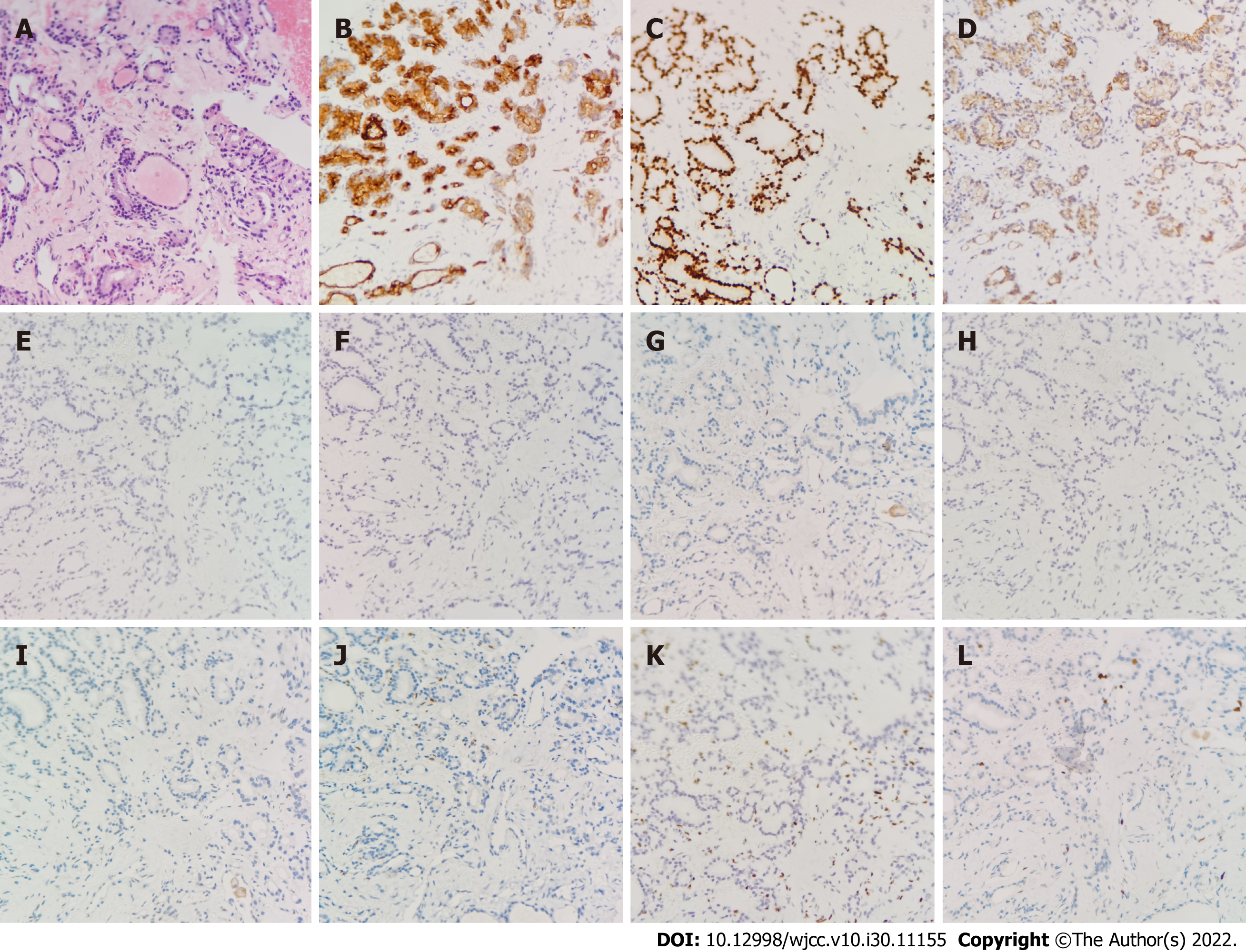
Cytological examination of pleural fluid and ascites indicated only reactive mesothelial cells with a few lymphocytes, histiocytes and neutrophils with no malignant cells identified. Then pathological histology of percutaneous biopsy of the pelvic mass showed hyperplastic fibrous tissue and mature thyroid follicles, without cellular and structural atypia, which was suspecious of struma ovarii combined with immunohistochemistry (Figure 3). Combined with the analysis of pathological histology and immunohistochemistry of the biopsies, the preoperative diagnosis was highly suspecious of struma ovarii.
The final histopathology revealed a mature left-sided ovarian teratoma and struma ovarii of right adnexal mass (Figure 4).
The patient was arranged for an exploratory laparotomy for diagnostic and therapeutic purposes on October 22, 2020. During the operation, 3000 mL of straw-colored ascites was drained. A large solid neoplasm (20 cm × 10 cm × 5 cm) originating from the right ovary was twisted clockwise for half a turn together with the right fallopian tube and part of the intestinal canal was adherent to the mass. Besides, the left ovary was slightly atrophic, containing a cystic mass, with the size of 3 cm × 2 cm × 0.1 cm. Intraoperative examination of all abdominal and pelvic organs did not show any additional lesions. The patient subsequently underwent right salpingo-oophorectomy and resection of the left ovarian mass and intestinal adhesiolysis and the excised specimens were sent for frozen analysis to rule out malignancy.
The patient recovered uneventfully and pleural effusion disappeared 5 d after surgery. Besides, CA 125 returned to normal range level (27.26 U/mL) 1 mo after surgery. The patient was followed up for 1 year after operation and there were no signs of obvious abnormality.
Struma ovarii, as a highly specific mature teratoma, is mostly benign, with malignant transformation only occurring in 0.5%-10 % of cases[1,6]. Struma ovarii can occur in female patients of any age, but perimenopause is the peak period of the disease, and it is usually asymptomatic, whereas patients with large struma ovarii may show abdominal distention, as in our case[7]. Because there are no obvious specificities in ultrasound, CT or magnetic resonance imaging (MRI) for struma ovarii, it is difficult to differentiate from ovarian cancer on imaging, especially for struma ovarii accompanied by ascites and pleural effusion, called pseudo-Meigs’syndrome and elevated CA 125, which can mimic ovarian malignancy. Fujiwara et al[8] reported positron emission tomography/CT combined with thyroid scintigraphy may be useful to define the diagnosis in struma ovarii with pseudo-Meigs’syndrome. Up to now, accurate preoperative diagnosis for struma ovarii by conventional imaging alone remains challenging and postoperative pathology is still required to confirm the diagnosis.
In the literature, 13 cases have been published on struma ovarii combined with pseudo-Meigs’syndrome and elevated CA 125, we describe another case of struma ovarii combined with pseudo-Meigs’syndrome and elevated CA 125[5,8-18]. Of all the cases, most of the patients were in their fifth or sixth decade when diagnosed with struma ovarii and almost 78.6% (11/14) of cases were postmenopausal women. Then, the most common presenting symptom was abdominal distension and the tumour sizes ranged between 5-23 cm in the large dimension, with an average size of 12 cm. Besides, most cases were preoperatively misdiagnosed as ovarian cancer and were treated by hysterectomy and bilateral salpingo-oophorectomy[5,8-17] and only two patients (including this case) underwent conservative surgery[18]. The ascites and hydrothorax disappeared completely and CA 125 levels returned to normal following surgery and all the cases had good prognosis. There are some unique features in our patient. Firstly, she was premenopausal, and to our knowledge, she is the youngest with this disease. Secondly, considering young age of our patient in order to avoid postoperative hormonal substitution, she underwent conservative surgery in the form of a right salpingo-oophorectomy as there was no evidence of malignancy according to analysis of percutaneous biopsy of the pelvic mass and frozen examination.
In summary, we confirm that struma ovarii is difficult to characterize on conventional imaging modalities and such patients should be diagnosed based on imaging features combined with pathology. In addition, more precise preoperative diagnosis should be performed to avoid unnecessary extended surgery.
We would like to thank our patient for participating in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hassan SA, United States; Paparoupa M, Germany S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Oudoux A, Leblanc E, Beaujot J, Gauthier-Kolesnikov H. Treatment and follow-up of malignant struma ovarii: Regarding two cases. Gynecol Oncol Rep. 2016;17:56-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Gobitti C, Sindoni A, Bampo C, Baresic T, Giorda G, Alessandrini L, Canzonieri V, Franchin G, Borsatti E. Malignant struma ovarii harboring a unique NRAS mutation: case report and review of the literature. Hormones (Athens). 2017;16:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | MEIGS JV. Fibroma of the ovary with ascites and hydrothorax; Meigs' syndrome. Am J Obstet Gynecol. 1954;67:962-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 163] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Amant F, Gabriel C, Timmerman D, Vergote I. Pseudo-Meigs' syndrome caused by a hydropic degenerating uterine leiomyoma with elevated CA 125. Gynecol Oncol. 2001;83:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Rana V, Srinivas V, Bandyopadhyay S, Ghosh SK, Singh Y. Bilateral benign non functional struma ovarii with Pseudo-Meigs' syndrome. Indian J Pathol Microbiol. 2009;52:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Brusca N, Del Duca SC, Salvatori R, D'Agostini A, Cannas P, Santaguida MG, Virili C, Bianchi L, Gargano L, Centanni M. A case report of thyroid carcinoma confined to ovary and concurrently occult in the thyroid: is conservative treatment always advised? Int J Endocrinol Metab. 2015;13:e18220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Nurliza Binti Md Nor, Kusumoto T, Inoue S, Nakamura K, Seki N, Hongo A, Kodama J, Hiramatsu Y. Three cases of struma ovarii underwent laparoscopic surgery with definite preoperative diagnosis. Acta Med Okayama. 2013;67:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Fujiwara S, Tsuyoshi H, Nishimura T, Takahashi N, Yoshida Y. Precise preoperative diagnosis of struma ovarii with pseudo-Meigs' syndrome mimicking ovarian cancer with the combination of 131I scintigraphy and 18F-FDG PET: case report and review of the literature. J Ovarian Res. 2018;11:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Bethune M, Quinn M, Rome R. Struma ovarii presenting as acute pseudo-Meigs syndrome with an elevated CA 125 Level. Aust N Z J Obstet Gynaecol. 1996;36:372-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Long CY, Chen YH, Chen SC, Lee JN, Su JH, Hsu SC. Pseudo-Meigs syndrome and elevated levels of tumor markers associated with benign ovarian tumors-two case reports. Kaohsiung J Med Sci. 2001;17:582-585. [PubMed] |
| 11. | Huh JJ, Montz FJ, Bristow RE. Struma ovarii associated with pseudo-Meigs' syndrome and elevated serum CA 125. Gynecol Oncol. 2002;86:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Uehara T, Sawada M. Struma ovarii associated with Meigs syndrome. Jpn J Clin Oncol. 2007;37:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Obeidat BR, Amarin ZO. Struma ovarii with pseudo-Meigs' syndrome and elevated CA125 Levels. J Obstet Gynaecol. 2007;27:97-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Mitrou S, Manek S, Kehoe S. Cystic struma ovarii presenting as pseudo-Meigs' syndrome with elevated CA125 Levels. A case report and review of the literature. Int J Gynecol Cancer. 2008;18:372-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Jiang W, Lu X, Zhu ZL, Liu XS, Xu CJ. Struma ovarii associated with pseudo-Meigs' syndrome and elevated serum CA 125: a case report and review of the literature. J Ovarian Res. 2010;3:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Mostaghel N, Enzevaei A, Zare K, Fallahian M. Struma ovarii associated with Pseudo-Meig's syndrome and high serum level of CA 125; a case report. J Ovarian Res. 2012;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Jin C, Dong R, Bu H, Yuan M, Zhang Y, Kong B. Coexistence of benign struma ovarii, pseudo-Meigs' syndrome and elevated serum CA 125: Case report and review of the literature. Oncol Lett. 2015;9:1739-1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Loizzi V, Cormio G, Resta L, Fattizzi N, Vicino M, Selvaggi L. Pseudo-Meigs syndrome and elevated CA125 associated with struma ovarii. Gynecol Oncol. 2005;97:282-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |