Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11090
Peer-review started: May 30, 2022
First decision: July 29, 2022
Revised: August 5, 2022
Accepted: September 22, 2022
Article in press: September 22, 2022
Published online: October 26, 2022
Processing time: 143 Days and 17.3 Hours
Axillary thoracotomy and muscle flap are muscle- and nerve-sparing methods among the surgical approaches to bronchopleural fistula (BPF). However, in patients who are vulnerable to a nerve compression injury, nerve injury may occur. In this report, we present a unique case in which the brachial plexus (div
A 52-year-old man with a history of ankylosing spondylitis with shoulder joint contractures presented with right arm weakness and sensory impairment immediately after axillary thoracotomy and latissimus dorsi muscle flap surgery for BPF closure. During the surgery, the patient was positioned in a lateral decubitus position with the right arm hyper-abducted for approximately 6 h. Magnetic resonance imaging and ultrasound revealed subclavius muscle injury or myositis with brachial plexus (BP) compression and related neuropathy. An electrodiagnostic study confirmed the presence of BP injury involving the whole-division level, long thoracic, and suprascapular nerve injuries. He was treated with medication, physical therapy, and ultrasound-guided injections. Ultrasound-guided steroid injection at the BP, hydrodissection with 5% dextrose water at the BP and suprascapular nerve, and intra-articular steroid and hyaluronidase in
Clinicians should consider the possibilities of multiple nerve injuries in patients with joint contracture, and treat each specific therapeutic target.
Core Tip: We report a rare case of brachial plexus (division level), suprascapular, and long thoracic nerve injury after axillary thoracotomy and latissimus dorsi muscle flap surgery for bronchopleural fistula. The patient was diagnosed via the clinical course, magnetic resonance imaging, ultrasound, and electrodiagnostic study. This case recommends that clinicians should pay attention to patients’ underlying conditions, which are related to nerve complications such as severe multiple joint contractures, and prevent the complications.
- Citation: Go YI, Kim DS, Kim GW, Won YH, Park SH, Ko MH, Seo JH. Recovery of brachial plexus injury after bronchopleural fistula closure surgery based on electrodiagnostic study: A case report and review of literature. World J Clin Cases 2022; 10(30): 11090-11100
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11090.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11090
The nerves susceptible to damage during thoracic surgery include the vagus nerve, recurrent laryngeal nerve, stellate ganglion or sympathetic trunk, and brachial plexus (BP), and the anatomical position of the nerve and the occurrence of perioperative nerve injury are closely related to each other[1]. The brachial plexus is responsible for the overall motor and sensory functions of the upper extremity, and when an injury occurs, it causes various symptoms, from minor sensory loss to large loss of functional ability; thus, an accurate understanding of anatomy during surgery is important[1]. There are various causes of BP injury during surgery; however, perioperative mechanical forces, such as stretching, compression, and laceration, are the representative contributors. Stretching and compression are typically caused by poor limb padding and positioning during surgery, excessive surgical retractor use, prolonged immobility, and hematoma around the nerve, while laceration is often caused by direct damage from the blade or needle[2]. The risk factors for intraoperative BP injury are inappropriate positioning, especially with arms abducted > 90°, old age (> 60 years), prolonged operation time (316 ± 62 min), hypotension, and hypothermia[3,4].
Axillary thoracotomy is a surgical method that approaches the axillary area, which includes the first rib or second and third rib resection, and smaller muscle transections are made compared to those in the original thoracotomy, which requires transection of the latissimus dorsi or serratus anterior muscle[5]. It is mainly one of the surgical methods for the apical lung, pleural cavity, thoracic cavity, heart, and esophagus and is performed by exposing the axilla by flexing the ipsilateral arm in the lateral decubitus position. In patients with bronchopleural fistula (BPF) due to complications of suppurative pleuropulmonary disease, along with medical treatments, surgical closure, including muscle flap, is performed to provide fistula coverage[6]. Axillary thoracotomy has a lower risk for muscle or nerve injury than the original thoracotomy, although there has been a case of injury to the intercostobrachial nerve or long thoracic nerve at the incision site or a very rare case of BP injury[7,8]. We report a patient who simultaneously suffered from BP injury, whole-division level, suprascapular nerve, and long thoracic nerve injury immediately following BPF closure surgery under general anesthesia in thoracic surgery. Herein, we examine the causes and mechanisms that cause nerve damage during surgery and review the related literature.
A 52-year-old man complained of muscle weakness and paresthesia in the right arm immediately after BPF closure surgery.
During the surgery, the patient was placed in the left lateral decubitus position under general anesthesia, his right shoulder was abducted to approximately 90°, and his elbow was placed on a padded board with 90° of flexion and pronation. First, an incision was made in the right axillary region for entry, and subsequently, partial resection of the second and third ribs to expose the cavitary lesion and debridement were performed. An additional skin incision was performed to expose the latissimus dorsi muscle and dissect the muscle to a length that is sufficient to cover the cavity. After the latissimus dorsi myocutaneous flap was prepared, the wound cavity area was covered with this flap, and the surgical site was completely closed to complete the operation. The operation was performed for approximately 6 h, and there were no complications, such as excessive bleeding, hypotension, decreased oxygen saturation, or hypothermia during the operation.
The patient had a history of old pulmonary tuberculosis, newly detected aspergillosis at the BPF lesion, and asthma. In addition to pulmonary diseases, the patient was diagnosed with ankylosing spondylitis and underwent hip arthroplasty due to bilateral hip arthritis, and multiple joint angles were limited due to bilateral shoulder and elbow arthritis and bamboo spine.
The patient had no personal or family history of neurologic diseases.
On the physical examination performed on postoperative day (POD) 1, the passive range of motion (ROM) of right shoulder flexion, extension, abduction, internal rotation, and external rotation were limited to 80°, 20°, 70 °, 0°, and 30°, respectively. Manual muscle testing (MMT) scores were 1 for shoulder flexion and extension, elbow flexion and extension, wrist flexion and extension, and finger flexion and extension. Sensation was decreased throughout the right arm; in particular, the patient complained of hypoesthesia and paresthesia (visual analog scale of 4) over the medial side of the upper arm, forearm, and hand.
The routine blood tests, including complete blood count, electrolyte profile, infection indexes, and routine urine tests, were within the normal range.
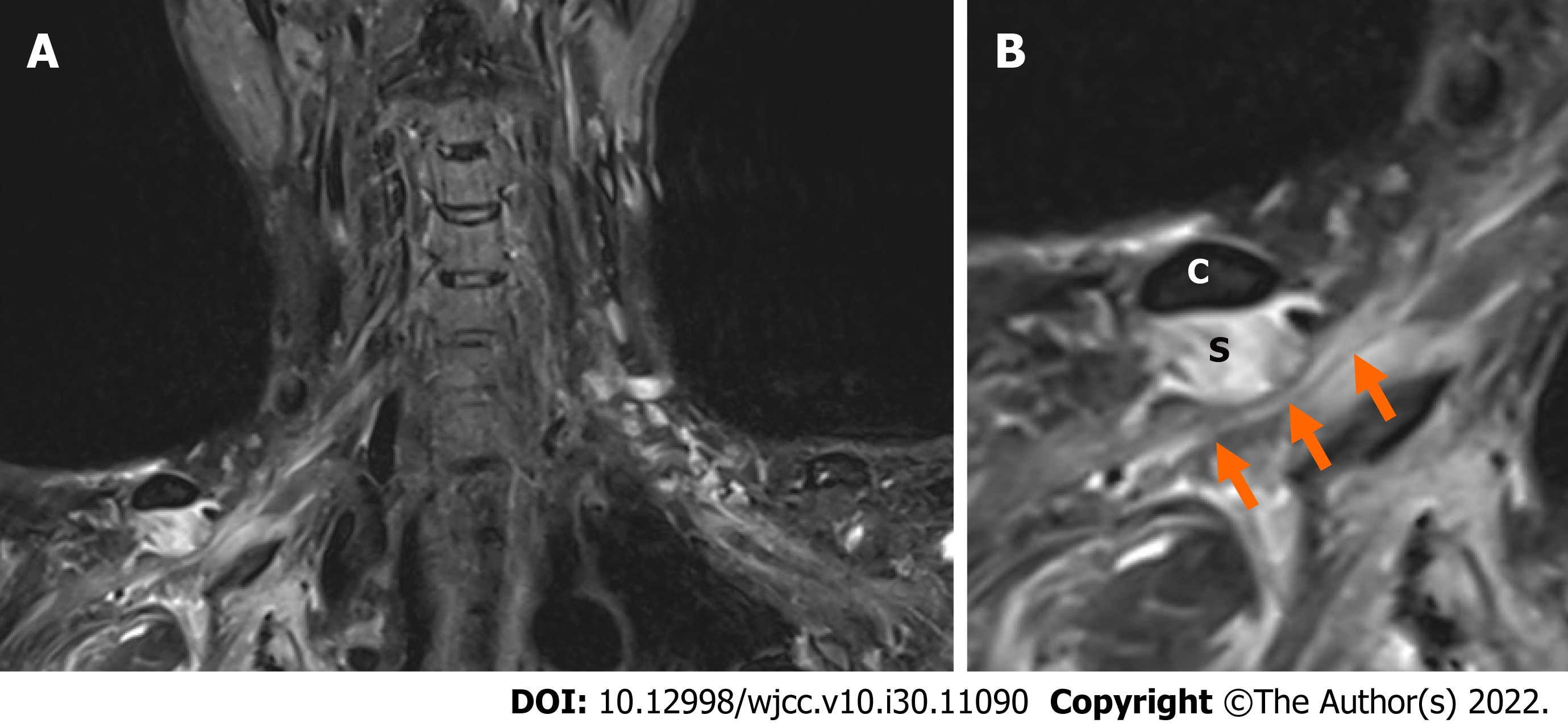
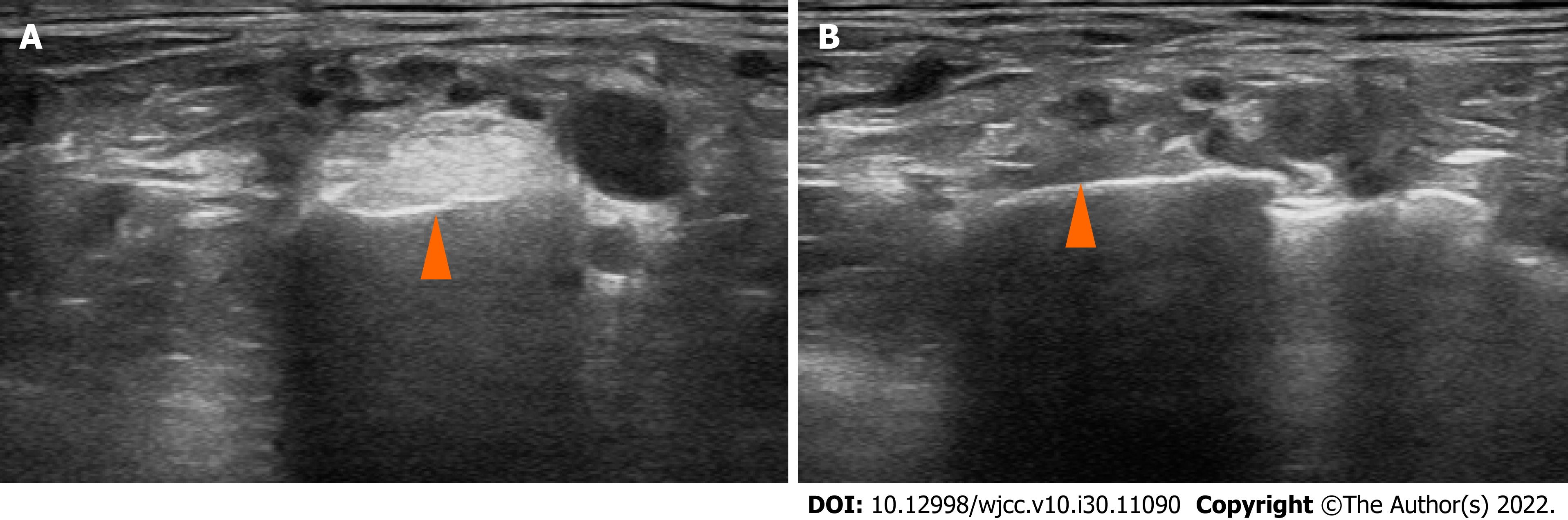
On BP magnetic resonance imaging (MRI) performed on POD 4, the signal intensity of the subclavius muscle was increased on T2WI, and heterogeneous enhancement was observed, suggesting myositis or muscle injury. In addition, the BP was compressed at the division level by the hypertrophied subclavius muscle (Figure 1). Similarly, in the musculoskeletal ultrasound performed on POD 6, clear hyperechogenicity was observed in the right subclavius muscle, and marked swelling was observed when compared to the left (Figure 2).
In the nerve conduction study (NCS) on POD 9 and needle electromyography on POD 14, the results showed right BP injury, whole-division level, right long thoracic nerve injury (severe degree), and right suprascapular nerve injury (moderate degree) (Tables 1 and 2).
| Nerve condu-ction (motor) | Recor-ding site | Stimu-lation site | Latency (ms) | Ampli-tude (mV) | Condu-ction velocity (m/s) | Nerve condu-ction (sensory) | Recor-ding site | Stimu-lation site | Latency (ms) | Ampli-tude (mV) | Condu-ction velocity (m/s) |
| Right median | APB | Wrist | 2.75 | 11.7 | Right median | 3rd finger | Palm | 1.55 | 9.2 | 45.2 | |
| Elbow | 7.00 | 10.1 | 56.5 | Wrist | 3.05 | 7.8 | 46.7 | ||||
| Left median | APB | Wrist | 2.70 | 15.0 | Left median | 3rd finger | Palm | 1.25 | 36.5 | 56.0 | |
| Elbow | 6.90 | 13.4 | 5.71 | Wrist | 2.55 | 25.5 | 53.8 | ||||
| Right ulnar | ADM | Wrist | 2.45 | 16.8 | Right ulnar | 5th finger | Wrist | 2.60 | 23.5 | 53.8 | |
| Below elbow | 6.15 | 16.1 | 59.5 | Below elbow | 6.05 | 14.2 | 58.0 | ||||
| Left ulnar | ADM | Wrist | 2.70 | 14.3 | Left ulnar | 5th finger | Wrist | 2.55 | 22.2 | 54.9 | |
| Below elbow | 6.40 | 13.1 | 59.5 | Below elbow | 6.15 | 18.1 | 55.6 | ||||
| Right radial | EIP | Forearm | 1.65 | 8.9 | Right superficialradial | Thumb | Forearm | 1.75 | 35.5 | 57.1 | |
| Above elbow | 5.60 | 8.9 | 63.3 | Left superficialradial | Thumb | Forearm | 1.80 | 58.8 | 55.6 | ||
| Left radial | EIP | Forearm | 1.65 | 8.9 | Right medialantecubital | Forearm | Forearm | 2.45 | 11.3 | 53.1 | |
| Above elbow | 5.50 | 13.1 | 7.10 | Left medial antecubital | Forearm | Forearm | 2.30 | 14.3 | 56.5 | ||
| Right axillary | Deltoid | Erb’s point | 5.15 | 5.4 | Right lateral antecubital | Forearm | Forearm | Absent | |||
| Left axillary | Deltoid | Erb’s point | 3.50 | 37.5 | Left lateral antecubital | Forearm | Forearm | 1.90 | 18.5 | 63.2 | |
| Right musculo | Biceps | Erb’s point | 7.25 | 2.1 | |||||||
| Left musculo | Biceps | Erb’s point | 3.75 | 24.0 | |||||||
| Right longtho | 5.6th rib | Erb’s point | Absent | ||||||||
| Left long thoracic | 5.6th rib | Erb’s point | 3.15 | 4.1 | |||||||
| Right dorsal scapular | Rhomboideus | Erb’s point | 3.55 | 3.7 | |||||||
| Left dorsal scapular | Rhomboideus | Erb’s point | 4.10 | 2.6 | |||||||
| Right suprascapular | Supraspinatus | Erb’s point | 4.40 | 5.3 | |||||||
| Infraspinatus | Erb’s point | 4.80 | 4.6 | ||||||||
| Left suprascapular | Supraspinatus | Erb’s point | 2.30 | 19.8 | |||||||
| Infraspinatus | Erb’s point | 4.00 | 13.4 |
| Muscle (right) | Abnormal spontaneous activity (positive sharp wave) | MUAP | Recruitment pattern |
| Cervical paraspinal | None | Normal | Normal |
| Rhomboid | None | Normal | Normal |
| Supraspinatus | None | Normal | Reduced |
| Infraspinatus | 2+ | Normal | Reduced |
| Teres major | 1+ | Normal | Reduced |
| Serratus anterior | 2+ | Normal | Reduced |
| Latissimus dorsi | 1+ | Normal | Reduced |
| Pectoralis major | 1+ | Normal | Reduced |
| Deltoid | 1+ | Normal | Reduced |
| Biceps brachii | 3+ | Normal | Reduced |
| Triceps | 1+ | Normal | Reduced |
| Pronator teres | 2+ | Normal | Reduced |
| Flexor carpi ulnaris | 1+ | Normal | Reduced |
| Extensor indicis proprius | 1+ | Normal | Reduced |
| Abductor pollicis brevis | 1+ | Normal | Reduced |
The final diagnosis of the presented case was right brachial plexus injury (division level), suprascapular nerve, and long thoracic nerve injury based on MRI, ultrasound, and electrodiagnostic study.
The patient received physical therapy five times a week from POD 2 to 28. Passive, active-assisted ROM exercises, strengthening exercises, and neuromuscular electrical stimulation were performed to improve right arm weakness and shoulder ROM. For neuropathic pain control, gabapentin was increased from an initial dose of 600 mg orally per day to 1200 mg/d. On POD 6, ultrasound-guided perineural injection at the right BP below the clavicle was administered with 5 mg of dexamethasone and 4 mL of normal saline. On POD 16, ultrasound-guided hydrodissection at the right BP below the clavicle and suprascapular nerve were performed with 10 mL and 5 mL of 277.53 mmol/L dextrose solution, respectively.
After medication, ultrasound-guided injection, and physical therapy, right arm paresthesia was slightly improved, and the patient discontinued gabapentin on POD 62. However, right shoulder pain and the limited ROM of the right shoulder were continuously present, and an ultrasound intra-articular injection at the right shoulder was performed on POD 108. On the follow-up NCS performed on POD 61, the compound muscle action potential amplitude of the right long thoracic nerve was slightly improved, which was absent in the initial test. In addition, there was no significant difference (Table 3). Manual muscle testing scores improved to 2 for shoulder flexion and extension, 3 for elbow flexion and extension, 3 for wrist flexion and finger extension, and 4 for wrist extension and finger flexion. On POD 133, there was a marked improvement in MMT scores, which were 4 for flexion and extension of the whole right arm and limited ROM of the right shoulder joint. The grip power of the right hand, measured using a hand dynamometer, was 24 kg, which was improved compared to 12.7 kg on POD 17. On POD 194, the follow-up NCS showed recovery within the normal range (Table 4). The treatment was terminated because there was no significant hindrance to the patient’s daily activities.
| Nerve condu-ction (motor) | Recor-ding site | Stimu-lation site | Latency (ms) | Ampli-tude (mV) | Condu-ction velocity (m/s) | Nerve condu-ction (sensory) | Recor-ding site | Stimu-lation site | Latency (ms) | Ampli-tude (mV) | Condu-ction velocity (m/s) |
| Right median | APB | Wrist | 2.95 | 9.7 | Right median | 3rd finger | Palm | 1.40 | 11.9 | 50.0 | |
| Elbow | 7.20 | 9.1 | 54.1 | Wrist | 3.10 | 8.2 | 41.2 | ||||
| Right ulnar | ADM | Wrist | 2.20 | 12.6 | Right ulnar | 5th finger | Wrist | 2.35 | 19.1 | 59.6 | |
| Below elbow | 6.05 | 12.3 | 55.8 | Below elbow | 5.80 | 14.0 | 60.9 | ||||
| Right radial | EIP | Forearm | 1.85 | 7.3 | Right superficial radial | Thumb | Forearm | 1.75 | 27.4 | 57.1 | |
| Above elbow | 5.00 | 5.3 | 61.9 | Right medial antecubital | Forearm | Forearm | 1.95 | 9.9 | 66.7 | ||
| Right axillary | Deltoid | Erb’s point | 4.60 | 11.2 | Right lateral antecubital | Forearm | Forearm | Absent | |||
| Right musculo | Biceps | Erb’s point | 6.25 | 12.4 | |||||||
| Right longtho | 5.6th rib | Erb’s point | 2.95 | 0.8 | |||||||
| Right dorsalscapular | Rhomboideus | Erb’s point | 3.45 | 6.6 | |||||||
| Right suprascapular | Supraspinatus | Erb’s point | 1.65 | 5.5 | |||||||
| Infraspinatus | Erb’s point | 1.90 | 3.7 |
| Nerve condu-ction (motor) | Recor-ding site | Stimu-lation site | Latency (ms) | Ampli-tude (mV) | Condu-ction velocity (m/s) | Nerve condu-ction (sensory) | Recor-ding site | Stimu-lation site | Latency (ms) | Ampli-tude (mV) | Condu-ction velocity (m/s) |
| Right median | APB | wrist | 2.85 | 17.0 | Right median | 3rd finger | Palm | 1.30 | 14.8 | 53.8 | |
| antecubital | 7.50 | 16.8 | 50.5 | Wrist | 2.35 | 9.5 | 66.7 | ||||
| Right ulnar | ADM | wrist | 2.35 | 16.7 | Right ulnar | 5th finger | Wrist | 2.40 | 36.3 | 58.3 | |
| Below elbow | 6.15 | 15.9 | 55.3 | Below elbow | 5.65 | 32.3 | 61.5 | ||||
| Right radial | EIP | forearm | 1.75 | 8.7 | Right superficial radial | Thumb | Forearm | 1.70 | 29.2 | 58.8 | |
| Above elbow | 5.65 | 8.1 | 65.4 | Right medialantecubital | Forearm | Forearm | 2.40 | 11.6 | 54.2 | ||
| Right axillary | Deltoid | Erb’s point | 3.75 | 31.6 | Right lateralantecubital | Forearm | Forearm | 1.25 | 11.4 | 64.0 | |
| Right musculo | Biceps | Erb’s point | 5.80 | 17.7 | |||||||
| Right longtho | 5.6th rib | Erb’s point | 3.40 | 1.5 | |||||||
| Right dorsalscapular | Rhomboideus | Erb’s point | 4.25 | 6.3 | |||||||
| Right suprascapular | Supraspinatus | Erb’s point | 1.60 | 12.7 | |||||||
| Infraspinatus | Erb’s point | 2.40 | 9.5 |
In this study, we report a unique case of a patient with BPs injuries at the whole-division level, suprascapular, and long thoracic nerve, which presented various symptoms and severities, which occurred immediately after BPF closure. The well-known mechanisms of injury are compression by the clavicle during retraction in median sternotomy, compression by the thorax and humeral head in the lateral decubitus position, and stretching of the upper BP root by arm abduction and external rotation[2]. However, the pathology of the injury in the current patient is different from the known pathology because it is caused by hypertrophied subclavius muscle. BP passes through the root and trunk at the supraclavicular level based on the clavicle and is divided into cords and branches at the infraclavicular level[9], and this nerve bundle is located below the subclavius muscle in the costoclavicular space[10]. The subclavius muscle originates in the first rib and first costal cartilage and is inserted into the inferior middle third of the clavicle, which depresses the shoulder and pulls the clavicle in the anteroinferior direction[10,11]. During shoulder abduction, downward movement of the scapula and coracoid occurs, and upward movement of the clavicle causes traction of the subclavius muscle[12,13]. Hypertrophy may have occurred as a result of continuous excessive muscle tension while maintaining shoulder abduction posture during the operation period[14]. Ankylosing spondylitis is a chronic inflammatory rheumatic disease that mainly affects the axial skeleton, with shoulder involvement in approximately 7%–33% of patients[15]. The patient previously had severe shoulder joint ROM limitation, and a greater force would have been required to abduct the arm for > 90° during the surgery, and more stress would have been applied to the subclavius muscle. These conditions might cause secondary BP injuries due to the subclavius muscle injury during the surgery.
Suprascapular nerve palsy is caused by nerve entrapment by the anterior coracoscapular ligament and suprascapular ligament, compression by a lipoma-like mass, intraosseous ganglion cyst, or paralabral cyst, and repetitive overhead activity; however, injuries that occur during surgery are rarely reported[16,17]. In this patient’s case, considering the incision site, the possibility of direct nerve transection is relatively low. On the other hand, secondary nerve stretching due to the subclavius muscle injury might be considered according to imaging and electrodiagnostic studies. In particular, mechanical stretching due to nerve kinking from the origin of the upper trunk of the Erb’s point[18] or stretching (or compression) of the nerve by the superior transverse scapular ligament occurs at the suprascapular notch; the so-called sling effect[19] is the most likely mechanism. These nerve injuries are particularly vulnerable to shoulder hyperflexion, hyperabduction, and external rotation postures[18-20]. Furthermore, glenohumeral stiffness reportedly induces a compensatory wider scapulothoracic excursion, making the suprascapular nerve more susceptible to traction injury at the suprascapular notch and more easily irritated[21].
In the case of long thoracic nerve injury, direct nerve injury and traction injury were suspected during axillary thoracotomy or the latissimus dorsi muscle flap. The long thoracic nerve originates from the C5-C7 spinal nerve, passes down the clavicle and the first and second ribs, runs distally along the midaxillary line, and finally innervates the serratus anterior muscle[22]. Unlike other nerve injuries, considering the course of the nerve, the long thoracic nerve transection may have occurred during the second and third rib resection (Figure 3). Alternatively, when flapping the latissimus dorsi muscle, transection and traction damage in the process of exposing and dissecting the muscle to the flap may occur. The pattern was different from the BP injury or suprascapular nerve in that the response was absent on the initial NCS; however, it showed improvement on the follow-up NCS and was considered a partial nerve injury due to direct nerve transection or traction.
As axonal regeneration of the peripheral nerve occurs at a rate of 1–2 mm/day in general, three months of conservative treatment is recommended for stretch/compression injury without laceration; however, exploration is recommended if there is no subsequent improvement[23,24]. In the case of peripheral nerve injury, early rehabilitation is important, and the main goal of treatment is to prevent muscle atrophy and secondary deformity of the upper arm and to improve pain and somatosensory deficit[25]. During the initial post-injury period, applying sling/splint and passive ROM exercises are recommended to maintain upper extremity joint mobility, and active assist or active ROM exercise is optional depending on the patient’s strength gain. Strengthening exercise with electrical stimulation of the denervated muscle using direct-current stimulation is also recommended[25-27]. According to the Neuropathic Pain Special Interest Group guidelines and Canadian Pain Society guidelines, tricyclic antidepressants, gabapentin, and pregabalin are the first-line therapy for pain management, and opioid analgesics or tramadol are recommended as second- or third-line options[25,28]. If non-operative treatment fails, minimally invasive treatment can be attempted, and perineural injection using anesthetic medications with steroids, such as bupivacaine and methylprednisolone, help relieve symptoms, and glenohumeral joint hydrodilatation is helpful for patients with adhesive capsulitis[29,30].
In the electrodiagnostic study, Wallerian degeneration was observed in the area below the BP division level, in the suprascapular nerve, and in the long thoracic nerve, which showed progressive improvement in the follow-up examination. In T2WI on BP MRI, a signal increase due to compression of the BP at the division level was observed; thus, axonotmesis of the BP may have occurred according to the Seddon Sunderland classification[9,31]. Based on these studies, incomplete nerve injury was diagnosed, and axonal regeneration and spontaneous improvement could be expected. Ben-David and Stahl presented prognostic milestones for 1 wk and 6–8 wk for patients with intraoperative BP injury and noted a long-term residual neurological deficit if there is no sign of motor improvement within 6–8 wk[3]. In our patient, the motor showed gradual improvement on POD 61, and almost full motor recovery was observed on POD 133, which is consistent with the previous literature.
This case involved multiple nerve injuries of the ipsilateral arm immediately after axillary thoracotomy and latissimus dorsi muscle flap for pulmonary tuberculosis and BPF caused by aspergillosis in a patient with underlying ankylosing spondylitis and severe multiple joint contractures. Based on the initial symptoms alone, it can be misdiagnosed as a simple BP injury at the root level. However, by observing the characteristics of the incision site, postoperative electrodiagnostic and imaging studies (musculoskeletal ultrasound and MRI), symptom progression, and prognosis, it was confirmed that suprascapular nerve and long thoracic nerve injuries occurred independently of BP injury. In addition, very rarely, compressive BP injury and whole-division level due to subclavius muscle swelling were observed. Along with ultrasound-guided injection, physical therapy, and drug treatment, numbness in the upper extremities and muscle weakness started to improve two months after the onset. Conversely, suprascapular nerve or long thoracic nerve injury did not show as much rapid improvement as BP in symptoms or electrodiagnostic study results and started to show improvement at three months after the onset of physical therapies and ultrasound-guided injections for each nerve.
In case of nerve damage after surgery, efforts should be made to find the cause using electrodiagnostic and imaging studies, and injection therapy, drug therapy, and physical therapy are needed according to the cause and pathophysiology of each nerve damage. In addition, if there are multiple joint angle restrictions due to underlying diseases, such as in this case, it is important to recognize the risk of nerve damage, especially during chest surgery, and efforts, such as intraoperative nerve monitoring, reducing the operating time, and excessive attention to patient posture, are needed to prevent nerve damage.
The authors thank all the members of the Department of Physical Medicine & Rehabilitation, Jeonbuk National University Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nayak S, India; Papazafiropoulou A, Greece S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Auchincloss HG, Donahue DM. Prevention and Management of Nerve Injuries in Thoracic Surgery. Thorac Surg Clin. 2015;25:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Lalkhen AG, Bhatia K. Perioperative peripheral nerve injuries. Contin Educ Anaesth Crit Care Pain. 2012;12:38-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Ben-David B, Stahl S. Prognosis of intraoperative brachial plexus injury: a review of 22 cases. Br J Anaesth. 1997;79:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Canbaz S, Turgut N, Halici U, Sunar H, Balci K, Duran E. Brachial plexus injury during open heart surgery--controlled prospective study. Thorac Cardiovasc Surg. 2005;53:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Irons ML, Kucharczuk JC. Muscle-sparing axillary thoracotomy. Oper Tech Thorac Cardiovasc Surg. 2017;22:122-133. [DOI] [Full Text] |
| 6. | Dal Agnol G, Vieira A, Oliveira R, Ugalde Figueroa PA. Surgical approaches for bronchopleural fistula. Shanghai Chest. 2017;1:14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Yeh C-M, Chou C-M. Brachial plexus injury during axillary thoracotomy. Formos J Surg. 2012;45:55-58. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Isono T, Mori S, Kusumoto H, Shiono H. Winged scapula following axillary thoracotomy with long thoracic nerve preservation. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Dumitru D. Brachial Plexopathies and Proximal Mononeuropathies. In: Dumitru D, Amato A, Zwarts M. Electrodiagnostic medicine, 2nd ed. Philadelphia: Hanley & Belfus, 2001; 785. |
| 10. | Liu Y, Zhang Z, Wang J, Wu G, Yu W, Cui S. Improved functional outcome in NTOS patients following resection of the subclavius muscle with radiological signs of nerve impingement: indication of participation of the subclavius in brachial plexus compression. J Neurosurg. 2018;1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Hyland S, Charlick M, Varacallo M. Anatomy, shoulder and upper limb, clavicle. In: StatPearls (Internet). Treasure Island (FL): Statspearl Publishing 2021. [PubMed] |
| 12. | Atasoy E. Thoracic outlet syndrome: anatomy. Hand Clin. 2004;20: 7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Ludewig PM, Behrens SA, Meyer SM, Spoden SM, Wilson LA. Three-dimensional clavicular motion during arm elevation: reliability and descriptive data. J Orthop Sports Phys Ther. 2004;34:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Souza Jd, Gottfried C. Muscle injury: review of experimental models. J Electromyogr Kinesiol. 2013;23:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Eksioglu E, Bal A, Gulec B, Aydog E, Cakci A. Assessment of shoulder involvement and disability in patients with ankylosing spondylitis. Rheumatol Int. 2006;27:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Economides CP, Christodoulou L, Kyriakides T, Soteriades ES. An unusual case of suprascapular nerve neuropathy: a case report. J Med Case Rep. 2011;5:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Kostretzis L, Theodoroudis I, Boutsiadis A, Papadakis N, Papadopoulos P. Suprascapular Nerve Pathology: A Review of the Literature. Open Orthop J. 2017;11:140-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Safran MR. Nerve injury about the shoulder in athletes, part 1: suprascapular nerve and axillary nerve. Am J Sports Med. 2004;32:803-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Cummins CA, Messer TM, Nuber GW. Suprascapular nerve entrapment. J Bone Joint Surg Am. 2000;82:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Ijiri T. The Function of Scapular Muscle. In: Suzuki T, editor. Clinical Physical Therapy [Internet]. London: IntechOpen; 2017. [DOI] [Full Text] |
| 21. | Weaver HL. Isolated suprascapular nerve lesions. Injury. 1983;15:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Safran MR. Nerve injury about the shoulder in athletes, part 2: long thoracic nerve, spinal accessory nerve, burners/stingers, thoracic outlet syndrome. Am J Sports Med. 2004;32:1063-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Kim DH, Cho YJ, Tiel RL, Kline DG. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 664] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 25. | Smania N, Berto G, La Marchina E, Melotti C, Midiri A, Roncari L, Zenorini A, Ianes P, Picelli A, Waldner A, Faccioli S, Gandolfi M. Rehabilitation of brachial plexus injuries in adults and children. Eur J Phys Rehabil Med. 2012;48:483-506. [PubMed] |
| 26. | de Santana Chagas AC, Wanderley D, de Oliveira Ferro JK, Alves de Moraes A, Morais de Souza FH, da Silva Tenório A, Araújo de Oliveira D. Physical therapeutic treatment for traumatic brachial plexus injury in adults: A scoping review. PM R. 2022;14:120-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kinlaw D. Pre-/postoperative therapy for adult plexus injury. Hand Clin. 2005;21:103-108, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 507] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 29. | Leider JD, Derise OC, Bourdreaux KA, Dierks GJ, Lee C, Varrassi G, Sherman WF, Kaye AD. Treatment of suprascapular nerve entrapment syndrome. Orthop Rev (Pavia). 2021;13:25554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Chang KV, Mezian K, Naňka O, Wu WT, Lin CP, Özçakar L. Ultrasound-guided interventions for painful shoulder: from anatomy to evidence. J Pain Res. 2018;11:2311-2322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Mitchell CH, Brushart TM, Ahlawat S, Belzberg AJ, Carrino JA, Fayad LM. MRI of sports-related peripheral nerve injuries. AJR Am J Roentgenol. 2014;203:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |