Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11082
Peer-review started: May 29, 2022
First decision: June 27, 2022
Revised: July 8, 2022
Accepted: September 14, 2022
Article in press: September 14, 2022
Published online: October 26, 2022
Processing time: 144 Days and 9.6 Hours
Adenylosuccinate lyase (ADSL) deficiency is a rare autosomal-recessive defect of purine metabolism caused by mutation of the ADSL gene. It can cause severe neurological impairment and diverse clinical manifestations, including epilepsy.
Here, we describe a 3-year-old Chinese boy who had both psychomotor retar
We identified a novel complex heterozygous mutation in the ADSL gene associated with ADSL deficiency, thus expanding the known spectrum of pa
Core Tip: A child presented with comprehensive developmental delay and epilepsy. Whole-exon sequencing revealed the presence of a novel complex heterozygous mutation in the adenylosuccinate lyase (ADSL) gene. Bioinformatics analysis suggested that the mutation caused ADSL deficiency.
- Citation: Wang XC, Wang T, Liu RH, Jiang Y, Chen DD, Wang XY, Kong QX. Child with adenylosuccinate lyase deficiency caused by a novel complex heterozygous mutation in the ADSL gene: A case report. World J Clin Cases 2022; 10(30): 11082-11089
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11082.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11082
Adenylosuccinate lyase (ADSL) deficiency is a rare deficiency of purine metabolism. More than 120 cases have been reported[1], and the global prevalence is 1 in 1.25 million[2]. The most common and prominent clinical manifestations are neurological symptoms, including acute encephalopathy, chronic encephalopathy, and behavioral abnormalities. Approximately half of affected patients have epilepsy[2]. Currently, there is no effective treatment for ADSL deficiency; thus, prenatal genetic testing is important for affected families.
ADSL participates in two purine nucleotide metabolic pathways. It catalyzes the conversion of succinylaminoimidazole carboxamide ribotide to aminoimidazole carboxamide ribotide as well as the conversion of adenylate succinate to adenosine monophosphate. ADSL deficiency results in the accumulation of succinylaminoimidazole carboxamide riboside (SAICAr) and succinyladenosine in various body fluids, particularly cerebrospinal fluid and urine[3]. ADSL deficiency is caused by ADSL gene mutations; two-thirds of confirmed cases involve complex heterozygous mutations[2]. Thus far, more than 150 ADSL gene mutations have been identified, most of which constitute missense mutations[1]; all mutations in the ADSL gene can cause ADSL deficiency.
A 3-year-old boy was admitted to our clinic after he had experienced paroxysmal loss of consciousness for > 1 year.
The patient exhibited loss of consciousness, global lack of muscle tone and presence of cyanotic lips during seizures, and spontaneous remission after 2-3 min. Since the onset of the seizures, he had exhibited poor mentation, additional crying, ingestion of a semiliquid diet, reduced appetite, and worsened sleep. Convulsion episodes had continued during treatment with sodium valproate 9 mL twice daily (ineffective) and topiramate 75 mg twice daily (effective but poor control). Oxazepine was added on January 5, 2021. No seizures were observed, but a rash appeared 4 week later and sleep increased. Oxazepine was discontinued on February 25, 2021, and the rash abated. Perampanel, two tablets once daily, was added on February 26, 2021. However, the patient continued to experience sei
The patient had no abnormal birth history. He had a history of poor growth milestones (e.g., he began to roll over slowly and could not sit unassisted at the age of 9 months) and had been diagnosed with developmental delay. At the time of admission, he could not sit unassisted, could not recognize people, could not speak, and exhibited unconscious pronunciation.
The patient’s parents were healthy, non-blood relatives. The patient was the only child in his family, and neither parent had other offspring. There was no obvious family history of developmental delay or seizures.
Physical examination findings were temperature 37.3 °C, pulse 102 beats/min, respiration 24 breaths /min, and weight 17.7 kg. The patient exhibited a clear mind, poor spirit, frequent crying, limited development, moderate nutrition, pharyngeal congestion, coarse breath sounds in both lungs, and slightly elevated muscle tension.
Laboratory findings were within normal limits.
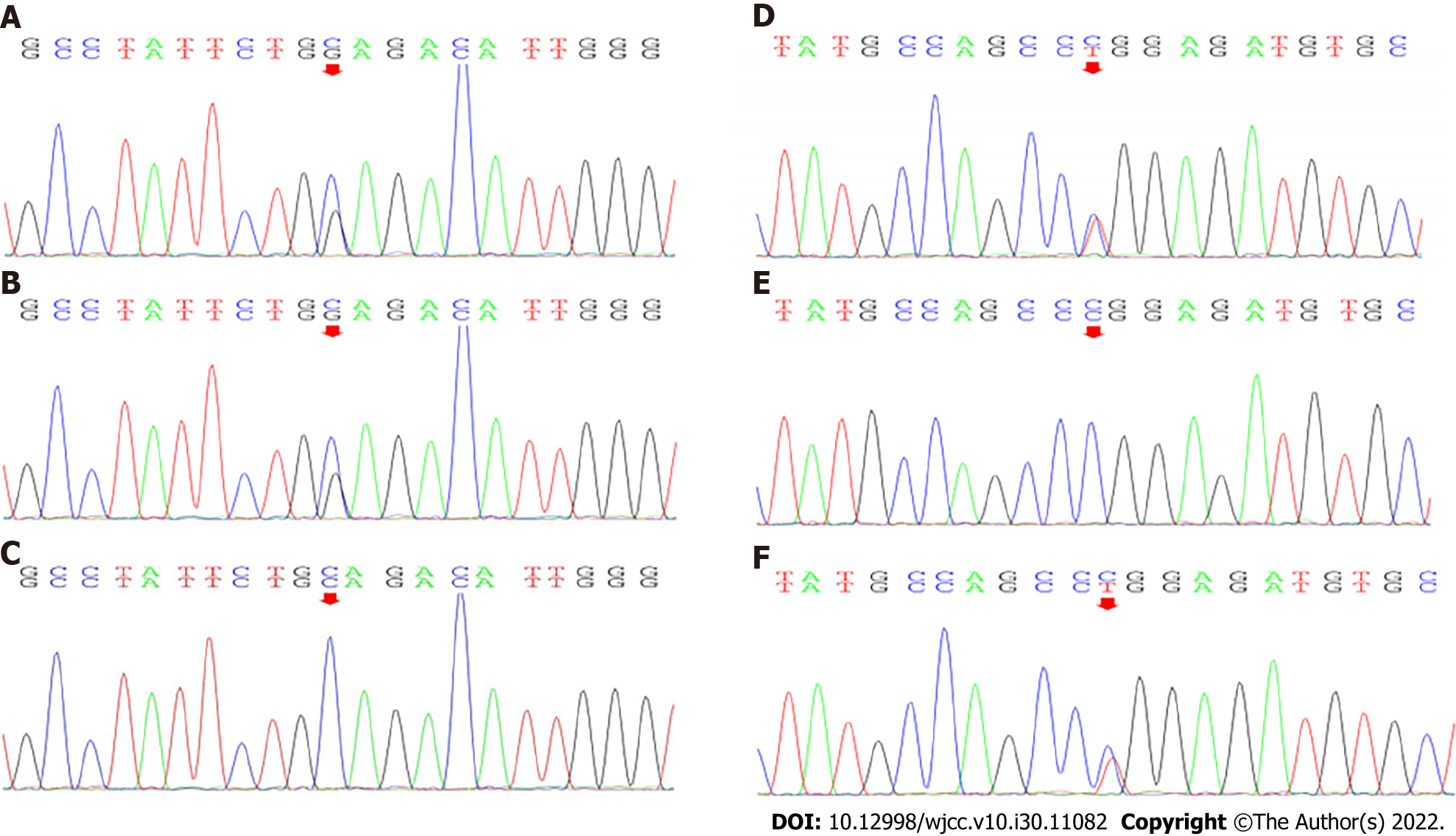
Genetic analysis: Genomic DNA was isolated from peripheral blood that had been collected from the patient and his parents. Candidate mutation sites were examined by Sanger sequencing. Complex heterozygous mutations were found in the patient’s ADSL gene: The splicing mutation c.154-3C>G (present in his father; Figure 1A-C) and the missense mutation c.71C>T (p. Pro24Leu) (present in his mother; Figure 1D-F).
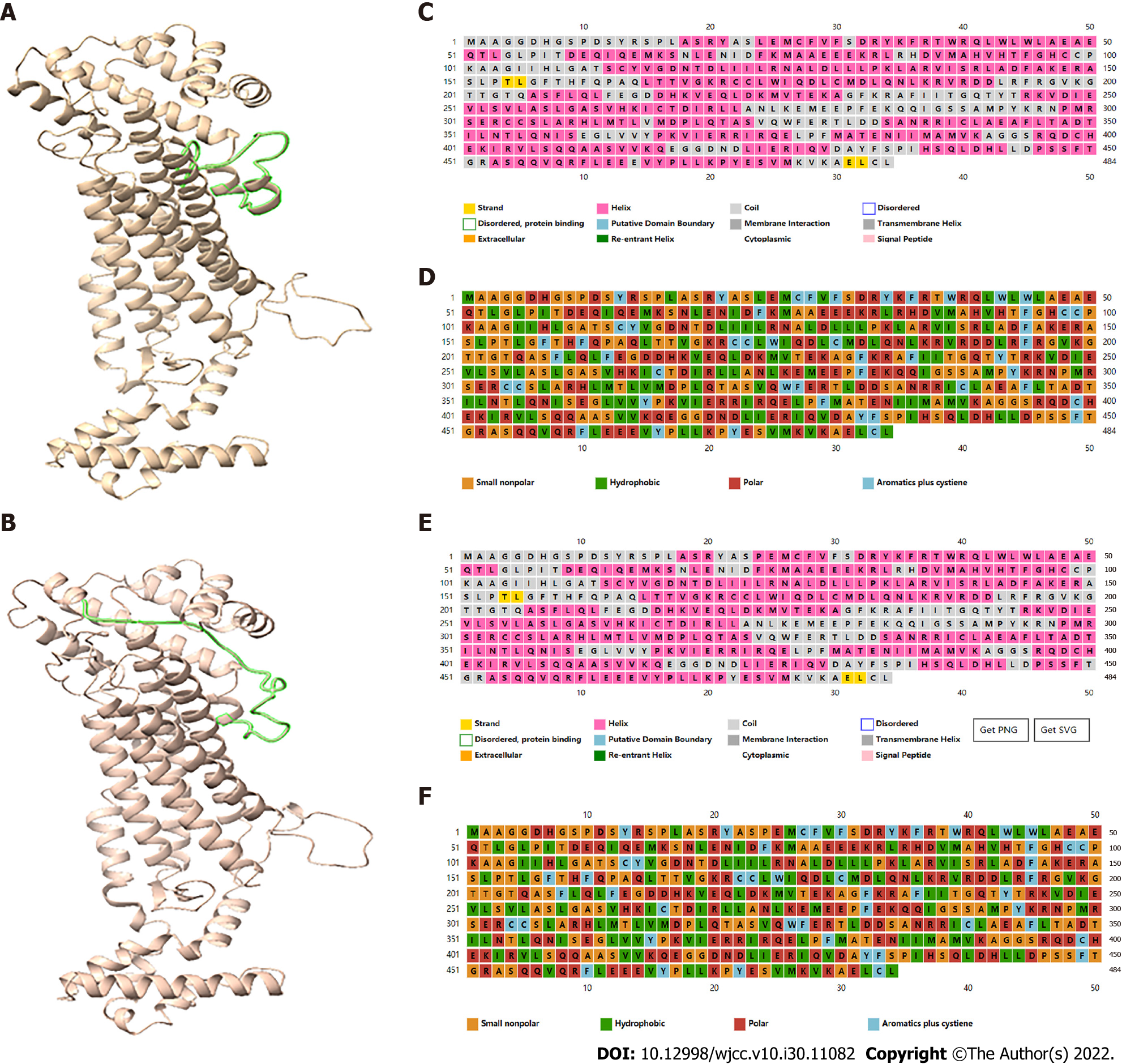
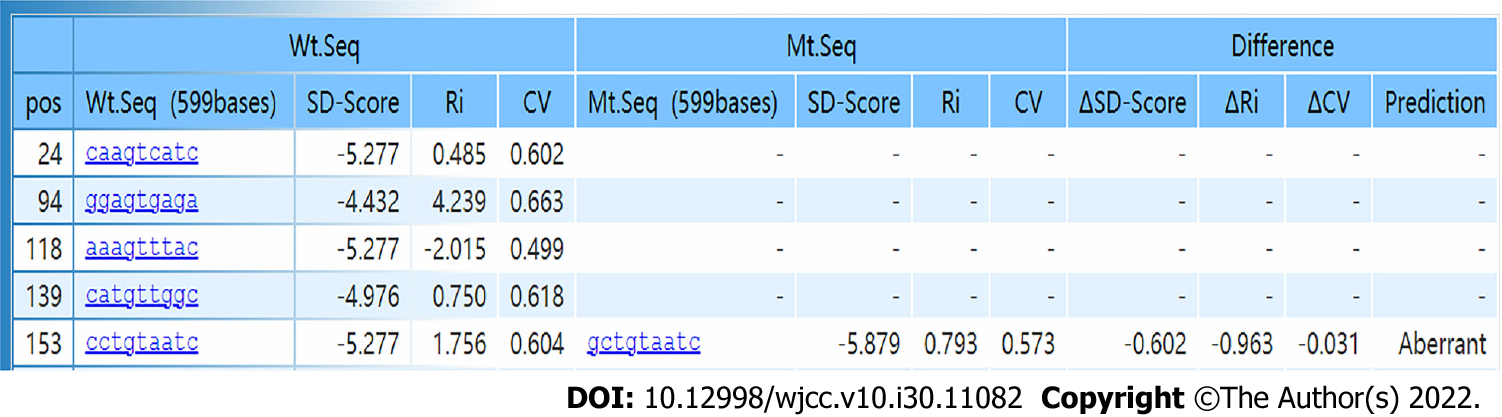
The bioinformatics software programs PSIPRED V4.0 (http://bioinf.cs.ucl.ac.uk/psipred/) and RaptorX (http://raptorx.uchicago.edu) were used to predict the secondary and tertiary structures, respectively, of mutant and wild-type ADSL proteins. The missense mutation c.71C>T causes an amino acid change from proline to leucine (p. Pro24Leu), possibly leading to a change in polarity (Figure 2). The splicing mutation c.154-3C>G was predicted to be pathogenic, according to the SD-Score Algorithm (Figure 3).
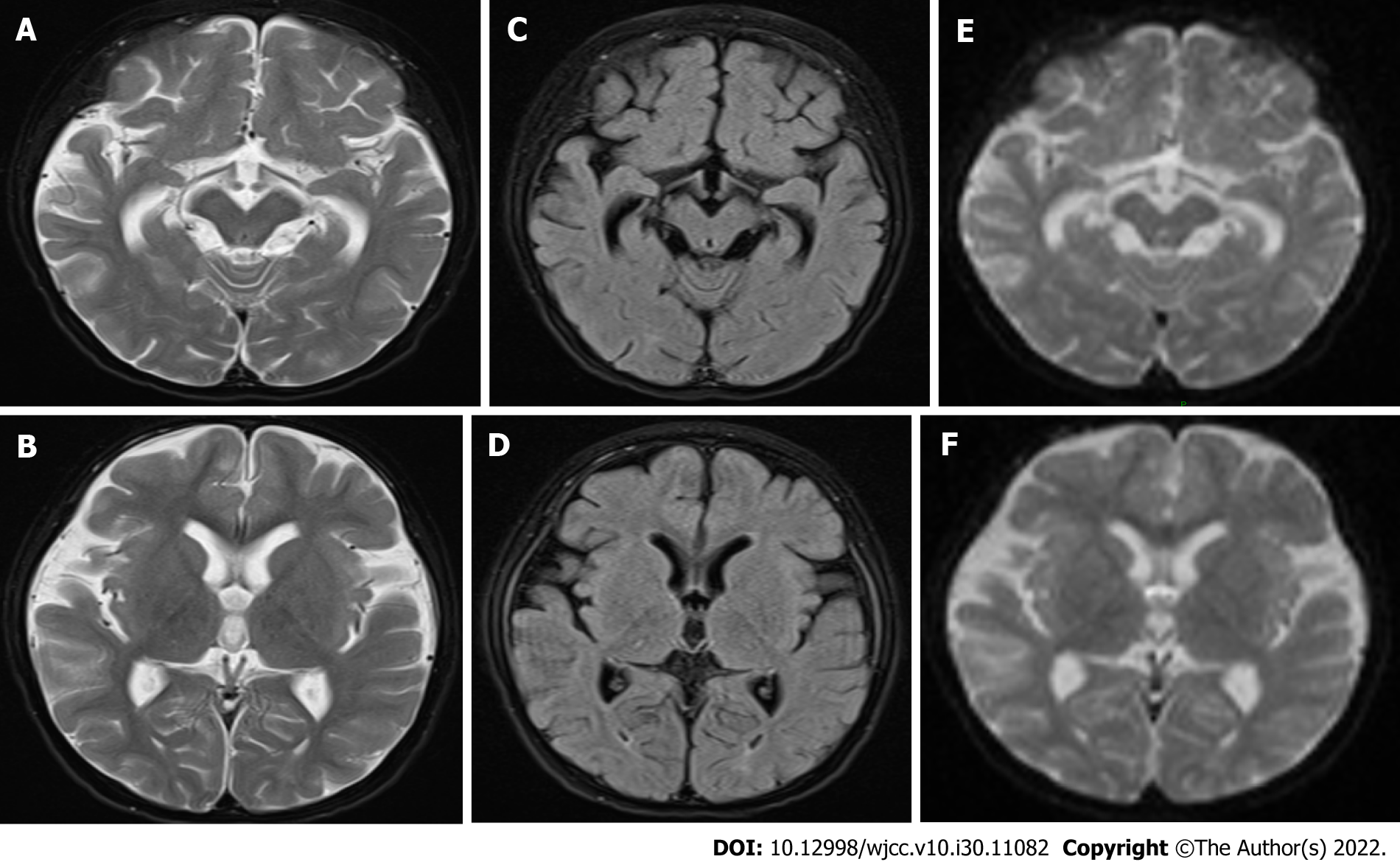
Magnetic resonance imaging in July 2020 showed bilateral external frontal temporal space widening and abnormal signals around the posterior horns of both lateral ventricles, suggestive of myelin hypoplasia; follow-up examination showed similar findings (Figures 4 and 5).
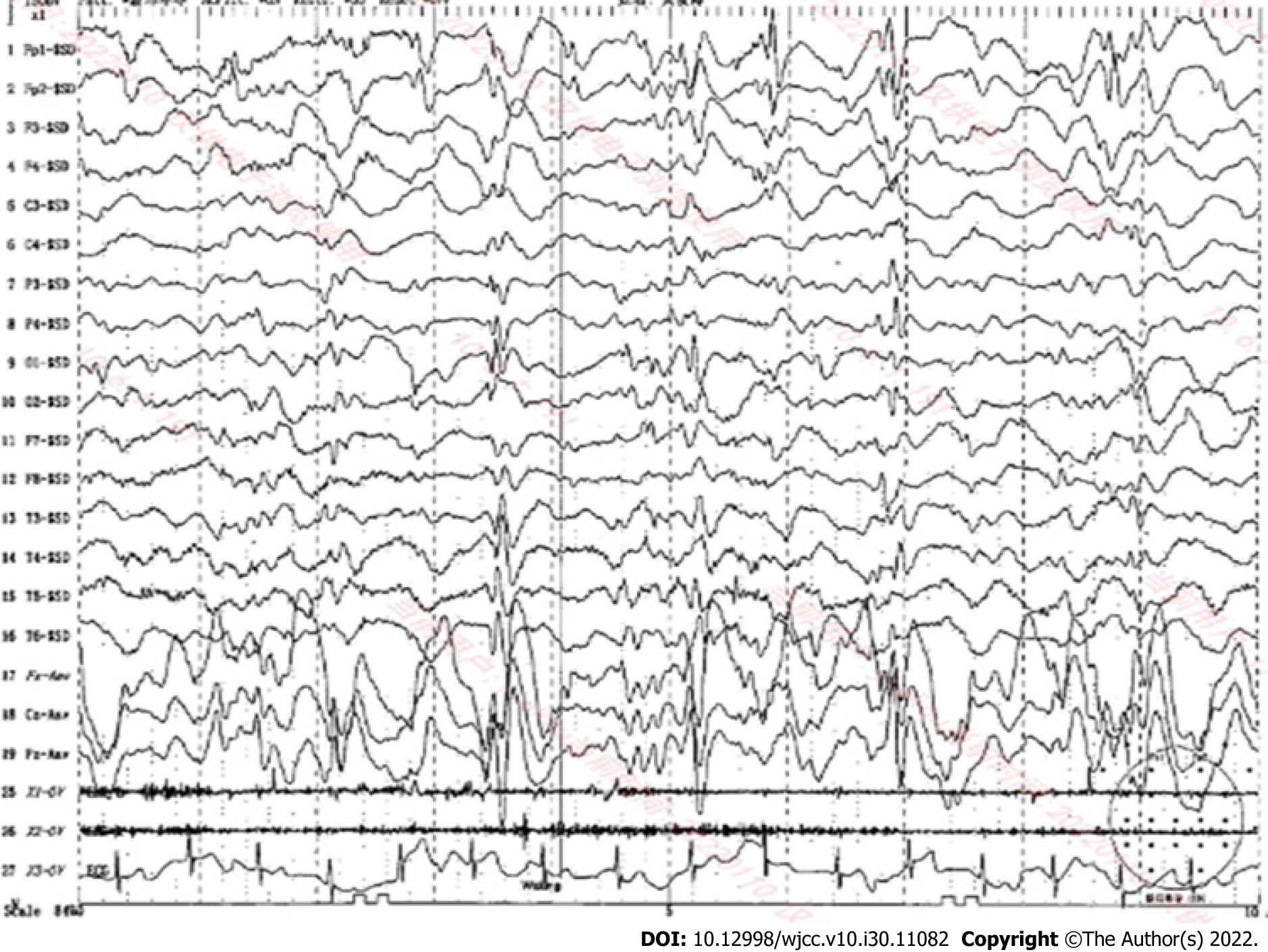
Electroencephalography: Electroencephalography performed in July 2020 revealed an abnormal electroencephalogram with slightly more medium-high amplitude in the left and right frontal regions, a few full-conduction irregular medium-high amplitude spikes, sharp slow waves, and a slow background rhythm. In July 2021, video electroencephalography revealed highly rhythmical patterns during most of the waking period and the entire sleep period. On the basis of irregular slow waves with full diffusion of 2-7 Hz, the patient exhibited multifocal slow waves, spiky slow waves, and polyspinous slow waves, with left and right asymmetry, asynchronous anterior and posterior findings, pronounced anterior activity, and an obvious sleep period (Figure 5).
Based on the patient’s clinical characteristics and the results of genetic tests and bioinformatics analyses, the child was diagnosed with ADSL.
Oral valproate solution, topiramate, and perampanel tablet.
The patient was seizure-free for > 1 year, but he did not show clinically significant improvements in intelligence or motor ability.
There are four types of ADSL deficiency, according to the clinical manifestations. The neonatal type is characterized by fatal neonatal encephalopathy, lack of autonomous movement, respiratory failure, and intractable epilepsy, leading to death within a few weeks after birth. Type I (the most common type) is characterized by severe psychomotor retardation, early epileptic seizures, microcephaly, and autistic features. Type II is a milder form in which symptoms usually develop within a few years after birth; affected patients usually exhibit mild to moderate psychomotor retardation and transient contact disturbances, sometimes accompanied by epilepsy. There is an additional phenotype that solely involves solitary psychomotor retardation or ataxia[1,4].
The phenotypic severity of ADSL deficiency may reflect the structural stability and residual enzymatic activity of the mutant ADSL enzyme complex. The pathogenic effects of biochemically benign and structurally stable mutations may be related to abnormalities that arise only under in vivo conditions in eukaryotic cells, rather than their intrinsic structural and/or catalytic properties[5]. Diffuse cortical atrophy and delayed myelination are the main neuroimaging findings in patients with ADSL deficiency[6].
Type II is a milder clinical phenotype of ADSL deficiency that involves slow disease progression and no specific symptoms. This phenotype was previously suspected to occur in approximately 15%-20% of patients with ADSL deficiency, but there is some evidence that it may be more common[7]. Patients with type II ADSL deficiency have only minor neurological involvement and a low incidence of epilepsy; they also have milder brain anomalies and generally do not exhibit microcephaly[7]. Our patient presented with comprehensive developmental delay and epilepsy, but he lacked autism or microcephaly. His magnetic resonance results were suggestive of myelin dysplasia, genetic analysis demonstrated a complex heterozygous mutation in the ADSL gene, and his clinical manifestations had not substantially progressed in recent years. Thus, he was diagnosed with type II ADSL deficiency.
The pathogenesis of ADSL deficiency is currently unclear. The underlying mutations are presumed to cause enzyme instability, which leads to the accumulation of SAICAr and succinyladenosine; the accumulation of SAICAr then produces neurotoxic effects. Other hypotheses regarding the pathogenesis of ADSL deficiency include a lack of the de novo purine biosynthetic pathway or the absence of a fully functional purine cycle in the muscles and brain[2]. We searched for common ADSL deficiency-related gene mutations in ADSLD (http://www1.lf1.cuni.cz/udmp/adsl) and PubMed (Table 1). c.1277G>A was the most common missense mutation, followed by c.340T>C; c.-49t>C was the most common splicing mutation. We speculate that the complex heterozygous mutation c.71C>T and c.154-3C>G in the ADSL gene, which is present in Chinese families, may be responsible for the phenotype in our patient. The c.71C>T mutation may cause a change in amino acid polarity, such that a hydrophobic leucine replaces a small non-polar proline. This adversely affects ADSL function (Figure 2). c.154-3C>G is a splicing mutation in the intron before nucleotide 154 in the coding region, which may influence the heterogenous nuclear RNA splicing process and produce an altered form of ADSL (Figure 3). We hypothesized that the ADSL alleles in our patient could not produce normal ADSL, leading to the clinical manifestation of ADSL deficiency. Our report of a complex heterozygous mutation in the ADSL gene in a Chinese patient could help expand the known spectrum of mutations and provide guidance for genetic counseling.
| c.1277G>A | c.340T>C | c.-49T>C | c.907C>T | c.736A>G | c.1187G>A | c.569G>A | |
| Mutant protein | p. R426H | p. Y114H | - | p. R303C | p. K246E | p. R396H | p. R190Q |
| Number | 36 | 12 | 5 | 4 | 3 | 3 | 3 |
Epilepsy is a common clinical manifestation of ADSL deficiency, such that it occurs in approximately half of patients with ADSL deficiency. Epilepsy phenotypes in patients with ADSL deficiency include myoclonus, partial seizures, infantile spasm, and epileptic persistence[8]. In patients with type I ADSL deficiency, epilepsy tends to occur early and is mostly refractory. In patients with type II ADSL deficiency, epilepsy usually appears by 2-4 years of age and can be controlled with medication; the mildest forms of epilepsy generally are not accompanied by seizures[4]. Currently, ADSL-related epilepsy is mainly controlled by antiepileptic drugs, but the therapeutic effect depends on the type of seizure[2]. A ketogenic diet has been shown to reduce the frequency of seizures in patients with ADSL deficiency who exhibit refractory epilepsy[9].
There is currently no effective treatment for ADSL deficiency. The interval from onset to diagnosis of this disease is generally long. It is easy to miss the diagnosis or misdiagnose patients with other diseases. Therefore, ADSL deficiency should be considered, and genetic screening should be performed for children with neurological symptoms such as psychomotor retardation and refractory epilepsy along with magnetic resonance imaging findings of diffuse cortical atrophy and delayed myelination.
ADSL deficiency is a rare deficiency of purine metabolism. ADSL deficiency should be suspected in children with psychomotor retardation and refractory epilepsy as well as in patients with magnetic resonance imaging findings of diffuse cortical atrophy and delayed myelination. Genetic testing is necessary to confirm the diagnosis. Metabolic epilepsy caused by ADSL deficiency can be controlled by the administration of antiepileptic drugs.
We thank the patient and his parents for providing relevant information and allowing us to publish this information. We also thank the Affiliated Hospital of Jining Medical University for providing support and assistance with this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gumustas OG, Turkey; Shrestha AB, Bangladesh S-Editor: Chen YL L-Editor: Filipodia P-Editor: Wu RR
| 1. | Dewulf JP, Marie S, Nassogne MC. Disorders of purine biosynthesis metabolism. Mol Genet Metab. 2022;136:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Jurecka A, Zikanova M, Kmoch S, Tylki-Szymańska A. Adenylosuccinate lyase deficiency. J Inherit Metab Dis. 2015;38:231-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Jinnah HA, Sabina RL, Van Den Berghe G. Metabolic disorders of purine metabolism affecting the nervous system. Handb Clin Neurol. 2013;113:1827-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Macchiaiolo M, Buonuomo PS, Mastrogiorgio G, Bordi M, Testa B, Weber G, Bellacchio E, Tartaglia M, Cecconi F, Bartuli A. Very mild isolated intellectual disability caused by adenylosuccinate lyase deficiency: a new phenotype. Mol Genet Metab Rep. 2020;23:100592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Baresova V, Skopova V, Sikora J, Patterson D, Sovova J, Zikanova M, Kmoch S. Mutations of ATIC and ADSL affect purinosome assembly in cultured skin fibroblasts from patients with AICA-ribosiduria and ADSL deficiency. Hum Mol Genet. 2012;21:1534-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Banerjee A, Bhatia V, Didwal G, Singh AK, Saini AG. ADSL Deficiency - The Lesser-Known Metabolic Epilepsy in Infancy. Indian J Pediatr. 2021;88:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Mastrogiorgio G, Macchiaiolo M, Buonuomo PS, Bellacchio E, Bordi M, Vecchio D, Brown KP, Watson NK, Contardi B, Cecconi F, Tartaglia M, Bartuli A. Clinical and molecular characterization of patients with adenylosuccinate lyase deficiency. Orphanet J Rare Dis. 2021;16:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Lundy CT, Jungbluth H, Pohl KR, Siddiqui A, Marinaki AM, Mundy H, Champion MP. Adenylosuccinate lyase deficiency in the United Kingdom pediatric population: first three cases. Pediatr Neurol. 2010;43:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Jurecka A, Zikanova M, Jurkiewicz E, Tylki-Szymańska A. Attenuated adenylosuccinate lyase deficiency: a report of one case and a review of the literature. Neuropediatrics. 2014;45:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |