Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11023
Peer-review started: April 16, 2022
First decision: August 4, 2022
Revised: August 15, 2022
Accepted: September 16, 2022
Article in press: September 16, 2022
Published online: October 26, 2022
Processing time: 187 Days and 17.6 Hours
Pisa syndrome (PS) refers to marked lateral flexion of the trunk with a Cobb angle greater than 10°, which is typically mobile and can be resolved by lying down. PS is one of the most common postural deformities secondary to Parkinson’s disease (PD) and can aggravate scoliosis in the advanced stages of PD.
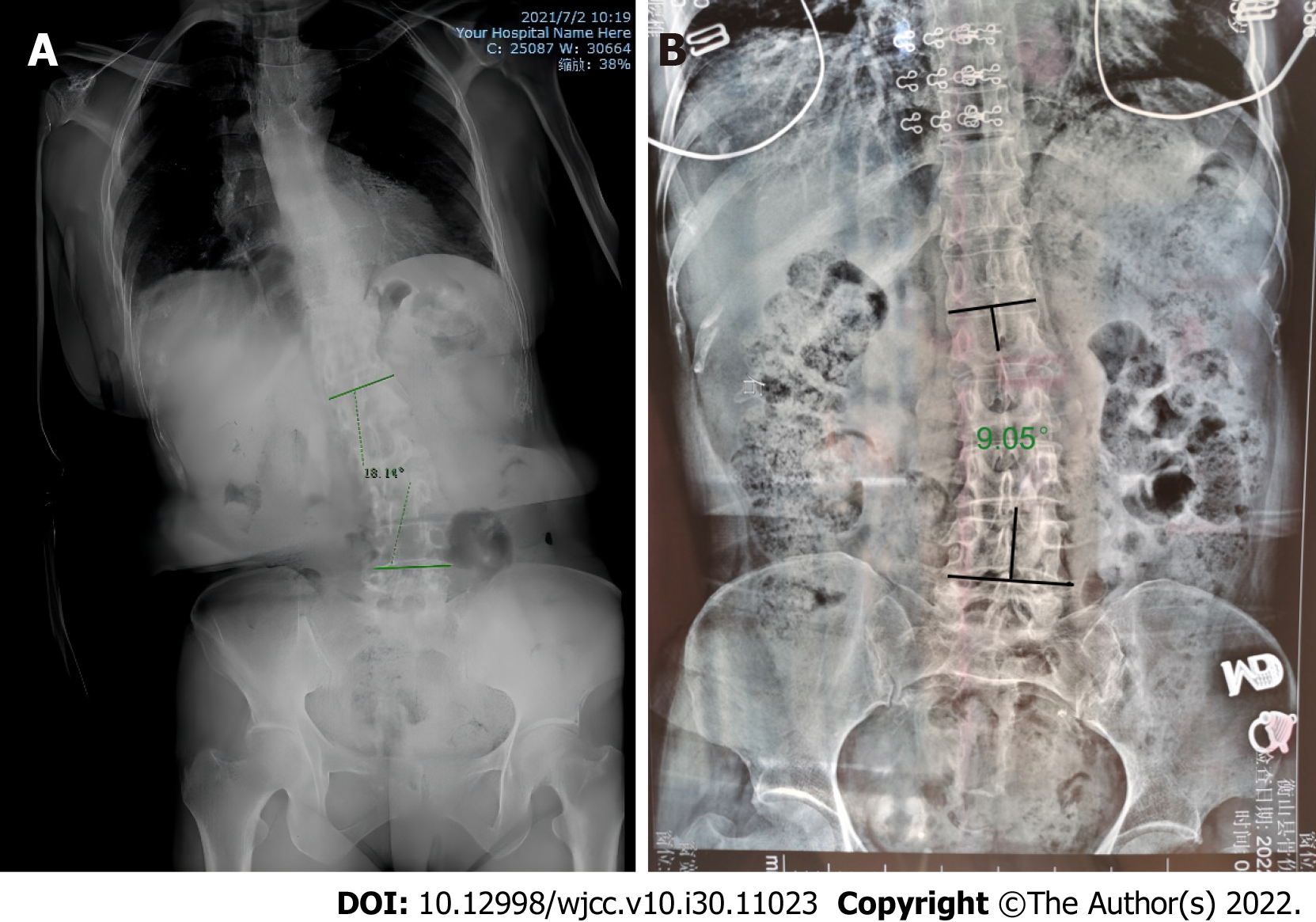
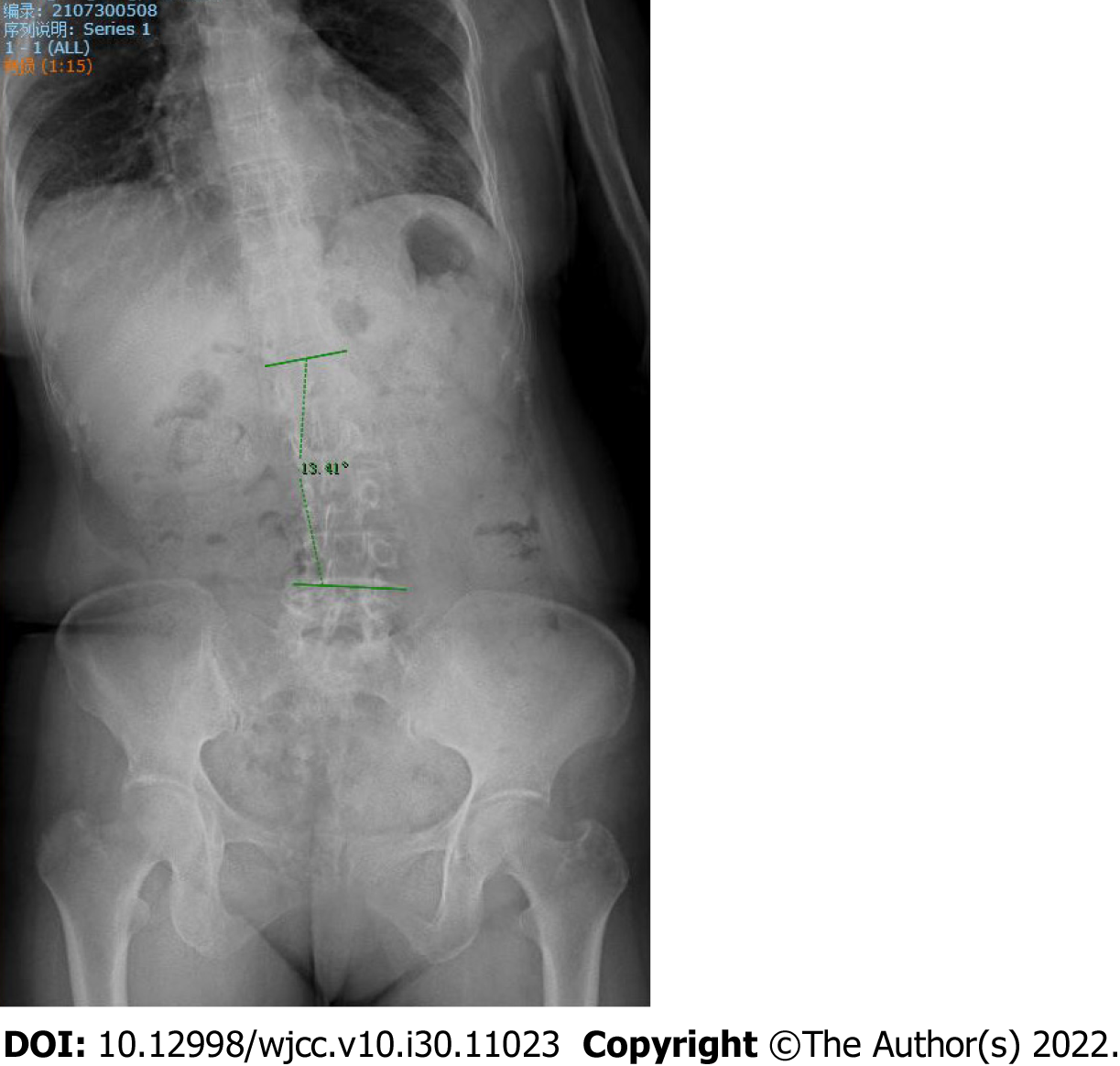
Here, we present the case of a 53-year-old woman who presented with lateral curvature for 6 mo. Full spine X-ray films in the correct position showed that the thoracolumbar spine was bent to the right without any rotation of the vertebrae. The patient was diagnosed with Pisa syndrome. After receiving a month’s treatment with electroacupuncture, the Cobb angle decreased from 18.14° to 13.41°.
This case demonstrates that electroacupuncture can effectively improve Pisa syndrome secondary to PD with few side effects and a low risk of recurrence. Additionally, early accurate diagnosis and timely intervention are meaningful for the prognosis of PS.
Core Tip: Pisa syndrome is common in Parkinson’s disease (PD) patients, can progress to scoliosis and may even be disabling. Currently, there is still a lack of effective treatments without side effects. We propose a new method, electroacupuncture, which may improve lateral curvature by relaxing the paraspinous muscles and modulating brain metabolism. This can ameliorate Pisa syndrome and delay the progression of PD by simultaneously treating the symptoms and disease. Thus, electroacupuncture may be an effective and beneficial treatment option for patients with Pisa syndrome.
- Citation: Lu WJ, Fan JQ, Yan MY, Mukaeda K, Zhuang LX, Wang LL. Effect of electroacupuncture for Pisa syndrome in Parkinson’s disease: A case report. World J Clin Cases 2022; 10(30): 11023-11030
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11023.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11023
Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized by resting tremor, bradykinesia, muscle rigidity and postural instability[1]. When PD is in the moderate or late stages, postural instability can progress to postural deformities which are a disabling complication in patients with PD. Approximately 33.33% of patients with PD experience postural deformities, including bell deformity, anterior cervical deformity, Pisa syndrome (PS) and scoliosis[2]. PS is one of the most common postural deformities and in the advanced stages of PD, it aggravates scoliosis[3]. The pathogenesis of PS is indeterminate, probably multifactorial and may be associated with central and peripheral pathogeneses[4]. As PS is reversible and its aggravation might decrease the quality of life of patients with PD, clinicians should recognize this condition and correct the curvature as early as possible.
Therapeutic measures for PS are limited because patients with PD generally have high muscle tension and low bone quality making conventional treatments for spinal deformity unsuitable for patients with PD[5]. However, electroacupuncture is also effective in the treatment of myotonia and spinal diseases.
Here, we report the case of a patient diagnosed with PS secondary to PD and treated with electroacupuncture who experienced a favorable result with no side effects. Her Cobb angle decreased from 18.14° to 13.41° after 1 mo, with other motor symptoms further improving.
A 53-year-old woman with a 4-year history of PD and lateral curvature for 6 mo presented to the acupuncture department in July 2021.
The patient was diagnosed with PD in January 2017. In January 2021, she experienced low back pain, stiff waist muscles and tremors. Stiffness tended to occur more frequently and seriously, and the spine could not remain upright and bent to the right. Her medication comprised of 125 mg of levodopa and benserazide tablets four times a day, pramipexole 0.375 mg once daily, piribedil 50 mg three times a day, amantadine 100 mg once a day and entacapone 100 mg four times per day.
The patient had a medical history of PD for 4 years and did not have diabetes, hypertension or other underlying diseases.
The patient denied any family history of PD.
The patient's hands trembled and she walked slowly. The patient’s head and lower limbs shook involuntarily. The spine was bent to the right (Figure 1), but this bending was alleviated when the patient adopted the supine position. Her Unified Parkinson's Disease Rating Scale (UPDRS) score was 43, UPDRS III (motor function) score was 19 and Hoehn Yahr stage was 2.5. The muscle on the right side of her lower back was taut and rigid. The muscle tone of the right limb was slightly strengthened, and the tendon reflex of the limbs and Ashworth scale scores were 3.
The patient’s laboratory examination results were unremarkable.
The erect position full-spine X-ray films showed that the thoracolumbar spine was bent to the right, the density of the vertebral body was decreased, the T10 vertebral body was suspicious of wedge-shaped changes and the full spine was degenerative. The Cobb angle at presentation was 18.14° (Figure 2A). Supine position full spine X-ray films showed that the T10 vertebral body was wedge shaped. In this position, the Cobb angle was 9.05° (Figure 2B).
Pisa syndrome secondary to PD.
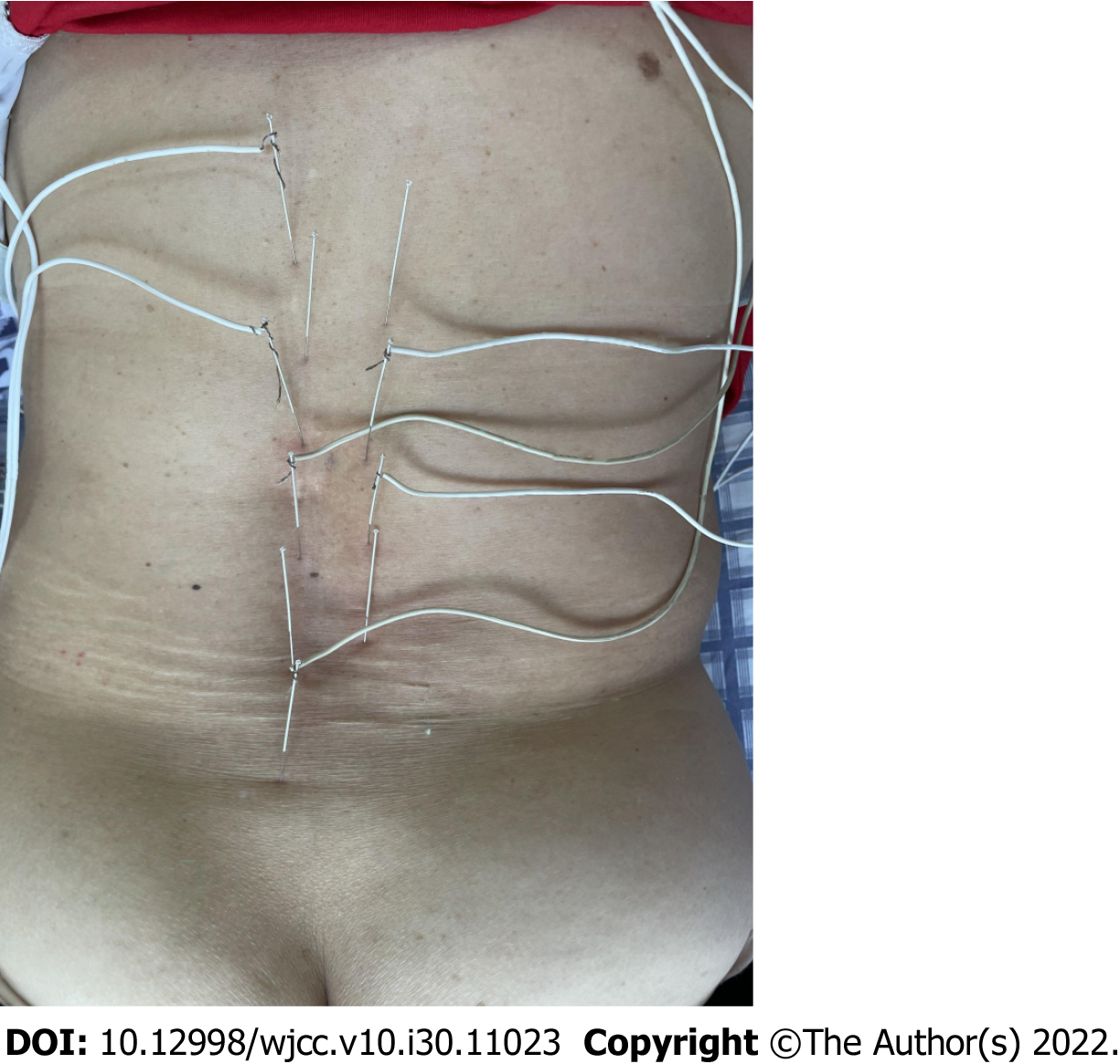
The patient underwent electroacupuncture on July 15, 2021. Along the center of the spine, 1.5-inch (0.30 mm × 40 mm) acupuncture needles were used, punctured from the twelfth thoracic vertebra to the fifth lumbar vertebra (Dumai). The needle feeding angle was 15° and the needle feeding depth was 30 mm. Six needles were evenly spaced and the feeding position of each needle was connected to the tip of the needle. Sanjiao Shu (BL 22), Shen Shu (BL 23), Qihai Shu (BL 24), and Dachang Shu (BL 25) were targeted at the right-side straight-needle (Figure 3). After acupuncture, a g-6850 electroacupuncture therapeutic instrument was used to connect the lumbar acupoints. A density wave was used and the current intensity was based on the patient's tolerance. The needle was maintained for 30 minutes and the treatment was performed three times per week for 4 wk.
After 4 wk of treatment, the problems with leaning forward and bending to the right -side during walking significantly improved (Figure 4). Walking was more flexible than before and the patient experienced no special discomfort. Radiography of the whole spine revealed that the amplitude of lumbar scoliosis was lower than that in the anterior position. Her Cobb angle had decreased to 13.41° (Figure 5) and the UPDRS-Ⅲ (motor function) score was 9 points. After 1 mo of follow-up, no aggravation of scoliosis was observed.
PS refers to a marked lateral flexion of the trunk with a Cobb angle greater than 10°[5]. Patients are typically mobile and their condition can be resolved by lying down[5]. At presentation, the patient’s Cobb angle was 18.14° which decreased in the supine position. Radiography of the entire spine did not show any rotation of the vertebrae which confirmed the diagnosis of PS. PS may be a precursor to the development of scoliosis in PD[6]; thus, it is necessary to distinguish between PS and scoliosis. Scoliosis is defined as a lateral curve of the spine with a Cobb angle of ≥ 10° in the coronal plane, usually combined with rotation of the vertebrae[7]. A previous study showed that scoliosis and Pisa syndrome were not the same, as PS is not always structural and may be caused by intrinsic muscle and soft tissue changes while scoliosis usually involves rotation and collapse of the vertebrae[6].
The mechanisms of PS are likely multifactorial and may be associated with dopamine, basal ganglia, sensorimotor dysfunction, body schema, perception and cognition, and trunk muscles[3]. Studies have shown that PS is correlated with the initiation or change in the dose of dopamine agonists in patients with PD[8,9]. According to movement disorders, central hypotheses play a key role in the pathogenesis of PS, particularly asymmetry in basal ganglia output and altered sensorimotor integration[4]. Tinazzi et al[10] found two different EMG patterns in the trunk muscles of parkinsonian patients under both static and dynamic conditions. The first pattern was characterized by hyperactivity of the lumbar paraspinal muscles contralateral to the leaning side, and the second pattern was thoracic paraspinal hyperactivity, which was contralateral in all the patients. Both patterns indicated that there were bilateral spinal muscle imbalances[10].
There are no specific guidelines for the treatment of patients with PS; therefore, some challenges remain. One study recommended that Parkinson’s drug revision and adjustment should be considered as the first-line intervention. Second-line interventions may include pharmacological, non-pharmacological and surgical strategies[3]. PS has been associated with the initiation and dose changes of anti-Parkinsonian medication[8]; thus, adjusting the administration of medication may be effective. However, there is uncertainty regarding the adjustment of anti-parkinsonian medication, as levodopa may not only reduce Pisa symptoms[11] but can also possibly induce dyskinesias[12]. Current studies have suggested that botulinum toxin injections are effective in small cohorts as a pharmacological treatment for PS[13,14]; however, the effectiveness of botulinum toxin injections for PS is inconsistent between patients and lacks large and multicenter randomized clinical evidence[3,15]. Bartolo et al[16] observed improvements in axial posture and trunk mobility through a 4-wk rehabilitation program; however, trials of this treatment have only been performed in small cohorts and data on the long-term effects are inconsistent[3]. Surgical treatment is the last choice for PS. Currently, effective surgeries include deep brain stimulation (DBS) and spinal realignment surgery[3]. Only a few studies have addressed the effects of DBS on posture because dystonia is the underlying mechanism, but PS is not always related to dystonia; therefore, no definitive studies have yet proven that DBS is efficient for PS[17]. When a PS patient suffers from severe impairment in daily life, surgical intervention can be considered[18]. However, spine surgery should accept the possibility of high rates of complications and reoperations due to muscle dystonia and poor bone quality[19]. In terms of cost, surgical treatment requires a large monetary investment of thousands of dollars which may cause enormous economic burden on patients. In this case, the patient was advised to undergo DBS surgery but refused because of the cost and concerns regarding surgical risks. Therefore, the patient was advised to undergo acupuncture.
No report of acupuncture treatment for PS has yet been published; however, some studies have suggested that acupuncture may be helpful in the management of scoliosis. Liu reported a case treated with acupuncture that achieved a 10° reduction in the Cobb angle[20]. Weiss et al[21] found that acupuncture could influence the deformity of patients with scoliosis by no more than 35 degrees. Both chose the Backshu acupoints to condition the zang-fu and relax the spinal muscles, similar to our study. Additionally, researchers found that acupuncture may reduce neurodegeneration in dopaminergic neurons and regulate the balance of the dopaminergic circuit to delay the progression of PD[22]. As the mechanisms of PS are strongly associated with dopaminergic neural circuits[8], acupuncture may promote the lateral curvature of PS. In addition, acupuncture may ameliorate back pain and tightness by relaxing the paraspinous muscles[23], thus reducing bending. Previous studies have shown that α-synuclein plays critical roles in PD pathogenesis[24]. Researchers have investigated the effect of electroacupuncture on abnormal neurochemical changes and motor symptoms in a mouse neurodegenerative disease model and concluded that electroacupuncture can enhance both anti-inflammatory and antioxidant activities, regulate neuronal autophagy, suppress aberrant glial activation in the diseased sites of the brain and possesses the ability to ameliorate motor abnormalities[1,25]. Based on the results of previous studies[20,21,25], we chose electroacupuncture aimed at the Backshu and Dumai acupoints on the back. The patient received a 4-wk treatment, reducing the Cobb angle by nearly 5°, which cost less than 200 dollars and induced no discomfort. After treatment, her back pain and tightness decreased, and her body movements became more flexible and gradually returned to a normal rhythm. During treatment, there were no adverse events, such as needle fainting and stagnation.
In this case, electroacupuncture improved the lateral curvature by relaxing the paraspinous muscles and balancing the dopaminergic circuit, thus preventing the curvature from developing into scoliosis which may cause disability or being bed-bound. The most important advantage of electroacupuncture is that it not only improves the lateral curvature but also delays the progression of PD[22]. Simultaneously, the cost of electroacupuncture is low and the manipulation is simple, reducing the economic burden on patients. The curvature was well maintained when she visited our clinic for postoperative review 1 mo after the end of treatment.
In conclusion, Pisa syndrome is common in patients with PD, can progress to scoliosis and can even be disabling. Currently, there is a lack of effective treatments without side effects. We propose a new method, electroacupuncture, which may improve lateral curvature by relaxing the paraspinous muscles and modulating brain metabolism. This can ameliorate PS and delay the progression of PD by simultaneously treating the symptoms and disease. Therefore, electroacupuncture may be an effective and beneficial treatment option for patients with PS.
We express our gratitude to the patients for their contribution to this case report. We also thank the Parkinson's Clinic of the First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine for their support in this treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Integrative and complementary medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Adam CA, Romania; Shibata Y, Japan S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Hsu WT, Chen YH, Yang HB, Lin JG, Hung SY. Electroacupuncture Improves Motor Symptoms of Parkinson's Disease and Promotes Neuronal Autophagy Activity in Mouse Brain. Am J Chin Med. 2020;48:1651-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord. 2006;21:1856-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Barone P, Santangelo G, Amboni M, Pellecchia MT, Vitale C. Pisa syndrome in Parkinson's disease and parkinsonism: clinical features, pathophysiology, and treatment. Lancet Neurol. 2016;15:1063-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Castrioto A, Piscicelli C, Pérennou D, Krack P, Debû B. The pathogenesis of Pisa syndrome in Parkinson's disease. Mov Disord. 2014;29:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Doherty KM, van de Warrenburg BP, Peralta MC, Silveira-Moriyama L, Azulay JP, Gershanik OS, Bloem BR. Postural deformities in Parkinson's disease. Lancet Neurol. 2011;10:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 6. | Doherty KM, Davagnanam I, Molloy S, Silveira-Moriyama L, Lees AJ. Pisa syndrome in Parkinson's disease: a mobile or fixed deformity? J Neurol Neurosurg Psychiatry. 2013;84:1400-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Schwab FJ, Smith VA, Biserni M, Gamez L, Farcy JP, Pagala M. Adult scoliosis: a quantitative radiographic and clinical analysis. Spine (Phila Pa 1976). 2002;27:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 332] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Cannas A, Solla P, Floris G, Tacconi P, Serra A, Piga M, Marrosu F, Marrosu MG. Reversible Pisa syndrome in patients with Parkinson's disease on dopaminergic therapy. J Neurol. 2009;256:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Galati S, Möller JC, Städler C. Ropinirole-induced Pisa syndrome in Parkinson disease. Clin Neuropharmacol. 2014;37:58-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Tinazzi M, Juergenson I, Squintani G, Vattemi G, Montemezzi S, Censi D, Barone P, Bovi T, Fasano A. Pisa syndrome in Parkinson's disease: an electrophysiological and imaging study. J Neurol. 2013;260:2138-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Kim JS, Park JW, Chung SW, Kim YI, Kim HT, Lee KS. Pisa syndrome as a motor complication of Parkinson's disease. Parkinsonism Relat Disord. 2007;13:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Turcano P, Mielke MM, Bower JH, Parisi JE, Cutsforth-Gregory JK, Ahlskog JE, Savica R. Levodopa-induced dyskinesia in Parkinson disease: A population-based cohort study. Neurology. 2018;91:e2238-e2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Artusi CA, Bortolani S, Merola A, Zibetti M, Busso M, De Mercanti S, Arnoffi P, Martinetto S, Gaidolfi E, Veltri A, Barbero P, Lopiano L. Botulinum toxin for Pisa syndrome: An MRI-, ultrasound- and electromyography-guided pilot study. Parkinsonism Relat Disord. 2019;62:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Tassorelli C, De Icco R, Alfonsi E, Bartolo M, Serrao M, Avenali M, De Paoli I, Conte C, Pozzi NG, Bramanti P, Nappi G, Sandrini G. Botulinum toxin type A potentiates the effect of neuromotor rehabilitation of Pisa syndrome in Parkinson disease: a placebo controlled study. Parkinsonism Relat Disord. 2014;20:1140-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Jocson A, Lew M. Use of botulinum toxin in Parkinson's disease. Parkinsonism Relat Disord. 2019;59:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Bartolo M, Serrao M, Tassorelli C, Don R, Ranavolo A, Draicchio F, Pacchetti C, Buscone S, Perrotta A, Furnari A, Bramanti P, Padua L, Pierelli F, Sandrini G. Four-week trunk-specific rehabilitation treatment improves lateral trunk flexion in Parkinson's disease. Mov Disord. 2010;25:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11:98-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 18. | Bouyer B, Scemama C, Roussouly P, Laouissat F, Obeid I, Boissière L, Parent FH, Schuller S, Steib JP, Pascal-Moussellard H, Guigui P, Wolff S, Riouallon G. Evolution and complications after surgery for spine deformation in patients with Parkinson's disease. Orthop Traumatol Surg Res. 2017;103:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Galbusera F, Bassani T, Stucovitz E, Martini C, Ismael Aguirre MF, Berjano PL, Lamartina C. Surgical treatment of spinal disorders in Parkinson's disease. Eur Spine J. 2018;27:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Liu CT, Chen KC, Chiu EH. Adult degenerative scoliosis treated by acupuncture. J Altern Complement Med. 2009;15:935-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Weiss HR, Bohr S, Jahnke A, Pleines S. Acupucture in the treatment of scoliosis - a single blind controlled pilot study. Scoliosis. 2008;3:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Zhao Y, Zhang Z, Qin S, Fan W, Li W, Liu J, Wang S, Xu Z, Zhao M. Acupuncture for Parkinson's Disease: Efficacy Evaluation and Mechanisms in the Dopaminergic Neural Circuit. Neural Plast. 2021;2021:9926445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Vickers AJ, Vertosick EA, Lewith G, MacPherson H, Foster NE, Sherman KJ, Irnich D, Witt CM, Linde K; Acupuncture Trialists' Collaboration. Acupuncture for Chronic Pain: Update of an Individual Patient Data Meta-Analysis. J Pain. 2018;19:455-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 471] [Article Influence: 58.9] [Reference Citation Analysis (1)] |
| 24. | Atik A, Stewart T, Zhang J. Alpha-Synuclein as a Biomarker for Parkinson's Disease. Brain Pathol. 2016;26:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Deng J, Lv E, Yang J, Gong X, Zhang W, Liang X, Wang J, Jia J, Wang X. Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic α-synuclein mutant mouse model. J Neuroinflammation. 2015;12:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |