Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11016
Peer-review started: April 13, 2022
First decision: July 11, 2022
Revised: July 27, 2022
Accepted: September 14, 2022
Article in press: September 14, 2022
Published online: October 26, 2022
Processing time: 190 Days and 12.5 Hours
The VPS33B (OMIM: 608552) gene is located on chromosome 15q26.1. We found a female infant with autosomal recessive arthrogryposis, renal dysfunction and cholestasis syndrome 1 (ARCS1) caused by mutation in VPS33B. The child was diagnosed with ARCS1 (OMIM: 208085) after the whole exome sequencing revealed two heterozygous mutations (c.96+1G>C, c.242delT) in the VPS33B gene.
We report a Chinese female infant with neonatal cholestasis disorder, who was eventually diagnosed with ARCS1 by genetic analysis. Genetic testing revealed two new mutations (c.96+1G>C and c.242delT) in VPS33B, which is the causal gene. The patient was compound heterozygous, and her parents were both heterozygous.
This study extends the mutational spectrum of the VPS33B gene to provide a molecular basis for the etiological diagnosis of ARCS1 and for genetic counseling of the family.
Core Tip: We report a Chinese female infant with neonatal cholestasis disorder, who was eventually diagnosed with arthrogryposis, renal dysfunction and cholestasis syndrome 1 by genetic analysis. Genetic testing revealed two new mutations (c.96+1G>C, c.242delT) in VPS33B, which are the causal genes. The patient was compound heterozygous, and her parents were both heterozygous. Our paper will expand the mutational spectrum of VPS33B.
- Citation: Yang H, Lin SZ, Guan SH, Wang WQ, Li JY, Yang GD, Zhang SL. Two novel mutations in the VPS33B gene in a Chinese patient with arthrogryposis, renal dysfunction and cholestasis syndrome 1: A case report. World J Clin Cases 2022; 10(30): 11016-11022
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11016.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11016
Arthrogryposis, renal dysfunction and cholestasis syndrome 1 (ARCS1, OMIM: 208085 and 613404) is an autosomal recessive disorder caused by mutations of the VPS33B (OMIM: 608552) and VIPAR (OMIM: 613401) genes[1,2]. We report a patient from a non-consanguineous family with ARCS1, who presented with two main symptoms (arthrogryposis and cholestasis) and ichthyosis at birth.
A 14-d-old female infant presented with jaundice for 5 d, with transcutaneous bilirubin (level 12.1 mg/dL).
The child was brought to the hospital because she had cholestatic jaundice since she was 9-d-old. No special treatment was given, and there was no progressive exacerbation or significant regression. The patient’s body weight was decreasing, and she had a loss of appetite, light yellow stools, and normal urine volume. Probiotics were given, but no improvement was seen. On September 26, 2021, she came to our hospital. There were no obvious abnormalities observed after routine blood examination. Liver function showed: total bilirubin (TBiL), 229.7 µmol/L; direct bilirubin (DBil), 152.4 µmol/L; and total bile acid (TBA), 50.6 µmol/L. Neonatal jaundice was diagnosed.
The child was delivered at 39 + 6W, G2P1. Cesarean section was performed because of unstable fetal heart rate. Her birth weight was 3.1 kg. The child did not have asphyxia or hypoxia at birth. Her mother had premature rupture of membranes. There was no meconium-stained amniotic fluid, intrauterine distress or abnormalities in the umbilical cord or placenta. The Apgar score was unknown.
The parents denied consanguineous marriage. Her father was Han Chinese, 26-years-old and healthy. Her mother was also Han Chinese and 27-years-old with a history of Mediterranean anemia and hypothyroidism and was receiving oral levothyroxine.
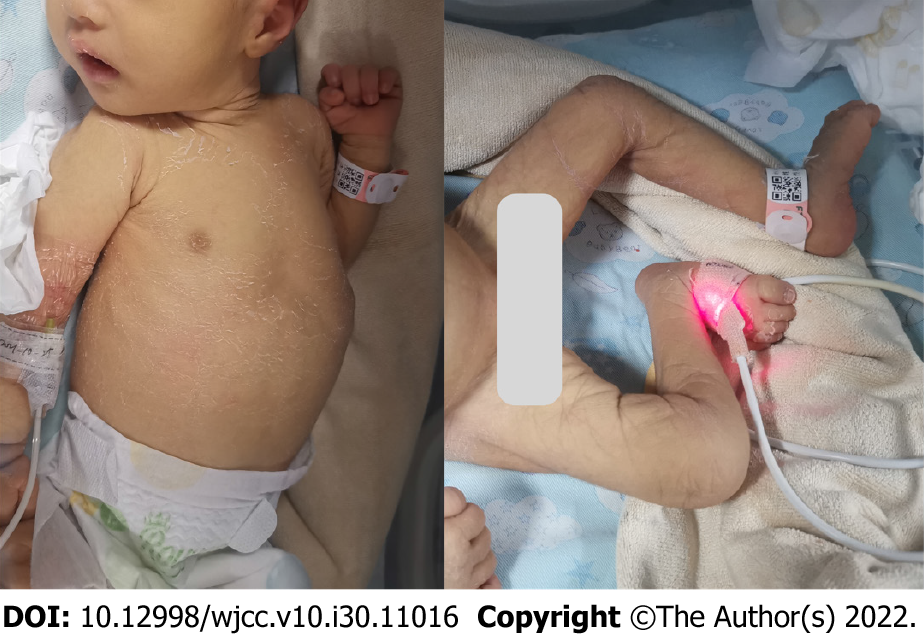
On physical examination at 13 d after birth, her weight was 2.68 kg, length was 50 cm and cranial circumference was 32 cm. The systemic skin mucosa was yellow and dry with ichthyosis. No hemorrhagic spot. The anterior fontanelle was flat and had normal tension. Both pupils were equal in size and were sensitive to light. There was no edema in either eyelid and no congestion in the conjunctiva of either eye. Neonatal hearing screening revealed bilateral deafness. The oral mucosa was smooth. Heart sounds were strong and regular; no pathological murmur was found in the valve areas. The abdomen was soft, no rebound pain was experienced, and the liver or spleen were normal. She had joint contracture and could not stretch straight, with low muscle tone in the limbs. The ends of the limbs were warm, and capillary filling time was approximately 2 s. Primitive reflex could be elicited (Figure 1).
After admission, we examined the patient thoroughly. The patient was blood type O, Rh-positive, and coagulation function was normal. Thalassemia genetic test showed: deletion type: genotype: -α4.2/αα. Her white blood cell count was markedly elevated (356.10/L), urine bilirubin, protein and glucose were positive, and leukocyte esterase was positive (3+). She had a suspected Gram-positive bacterial urinary tract infection. Hepatitis A IgG antibody was positive. Total bilirubin was 157.6 µmol/L, direct bilirubin was 98.7 µmol/L, and total bile acid was 50.6 µmol/L. Urine acid and blood urea nitrogen were normal. Creatinine clearance rate was normal. Thyroid function was evaluated: triiodothyronine, 1.08 nmol/L; total thyroxine, 124.0 nmol/L; free triiodothyronine, 3.39 pmol/L; free thyroxine, 10.60 pmol/L; and thyroid-stimulating hormone, 7.73 mU/L. There were no obvious abnormalities in blood metabolism. The levels of urine organic acids were not raised. Cerebrospinal fluid analysis showed no abnormalities.
Small-organ color Doppler ultrasound showed a bilateral choroid plexus cyst. No significant abnormalities were observed in the abdominal color Doppler ultrasound. Cardiac color Doppler ultrasound showed an atrial septal defect. Brain magnetic resonance imaging showed signs of small cysts in the bilateral ventricles. Active electroencephalography showed that background activity was normal, with no abnormal electrical episodes.
Informed consent was obtained from the parents on behalf of the proband for whole-exome sequencing (WES), mitochondrial sequencing and for publication of photographs. DNA samples were extracted from peripheral blood taken from the child and her parents to detect WESs and whole-genome copy number variations (CNVs). For analysis of genomic DNA, 2 mL of peripheral blood were extracted. WES and whole genome sequencing analysis of CNVs was performed by MGExome.
The second generation sequencer Illumina NextSeqTM 500 (Illumina, San Diego, CA, United States) was used to sequence the captured region at two ends, with a reading length of 150 bp. After sequencing the target region, splicing and low-quality data were removed from the sequencing data. Parental genetic investigation by Sanger sequencing revealed two heterozygous mutations (c.96+1G>C and c.242delT) in the VPS33B gene of the patient.
DNA was obtained from peripheral blood samples from the patient and her parents on October 1, 2021. The American College of Medical Genetics and Genomics sequence variation interpretation standards and guidelines were used for a comprehensive evaluation of the pathogenicity of mutation sites. Other gene mutations associated with the patient’s phenotype were not detected.
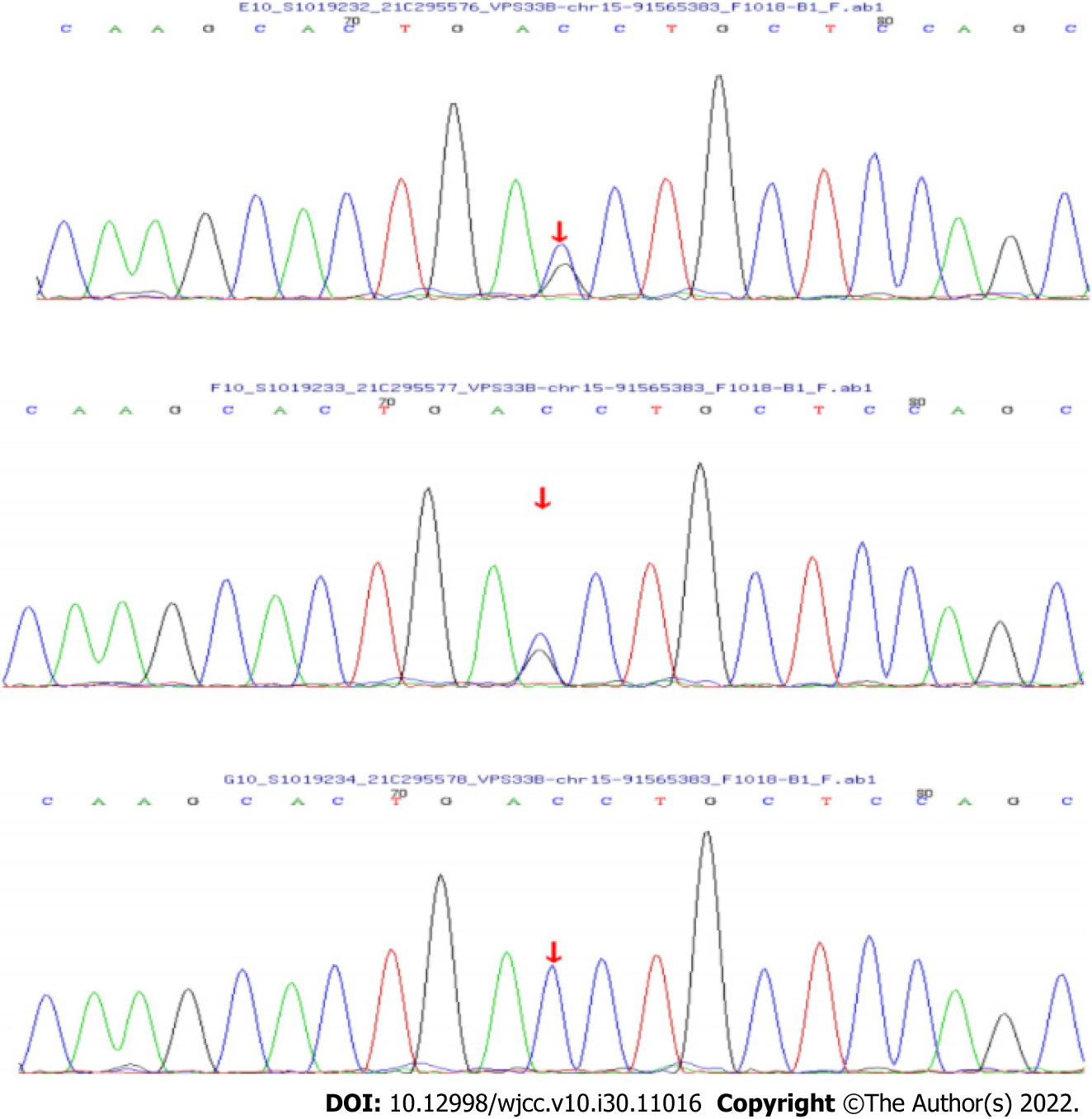
WES showed that there were two compound heterozygous mutations of the VPS33B gene in this patient, c.96+1G>C and c.242delT, which were unreported before. The mutation, c.96+1G>C in exon 1, inherited from the father, is a novel variant. It causes a splicing mutation in aminophenol and might lead to a loss of gene function. The frequency of the variation in the normal population database is unknown, and it is a low-frequency variation. The results of protein function prediction are unknown and are not reported in the Human Gene Mutation Database (HGMD) database. According to Sanger sequencing, the variation originated from the child’s father, and her mother was wildtype (Figure 2). According to the American College of Medical Genetics and Genomics (ACMG) guidelines, the mutation was pathogenic.
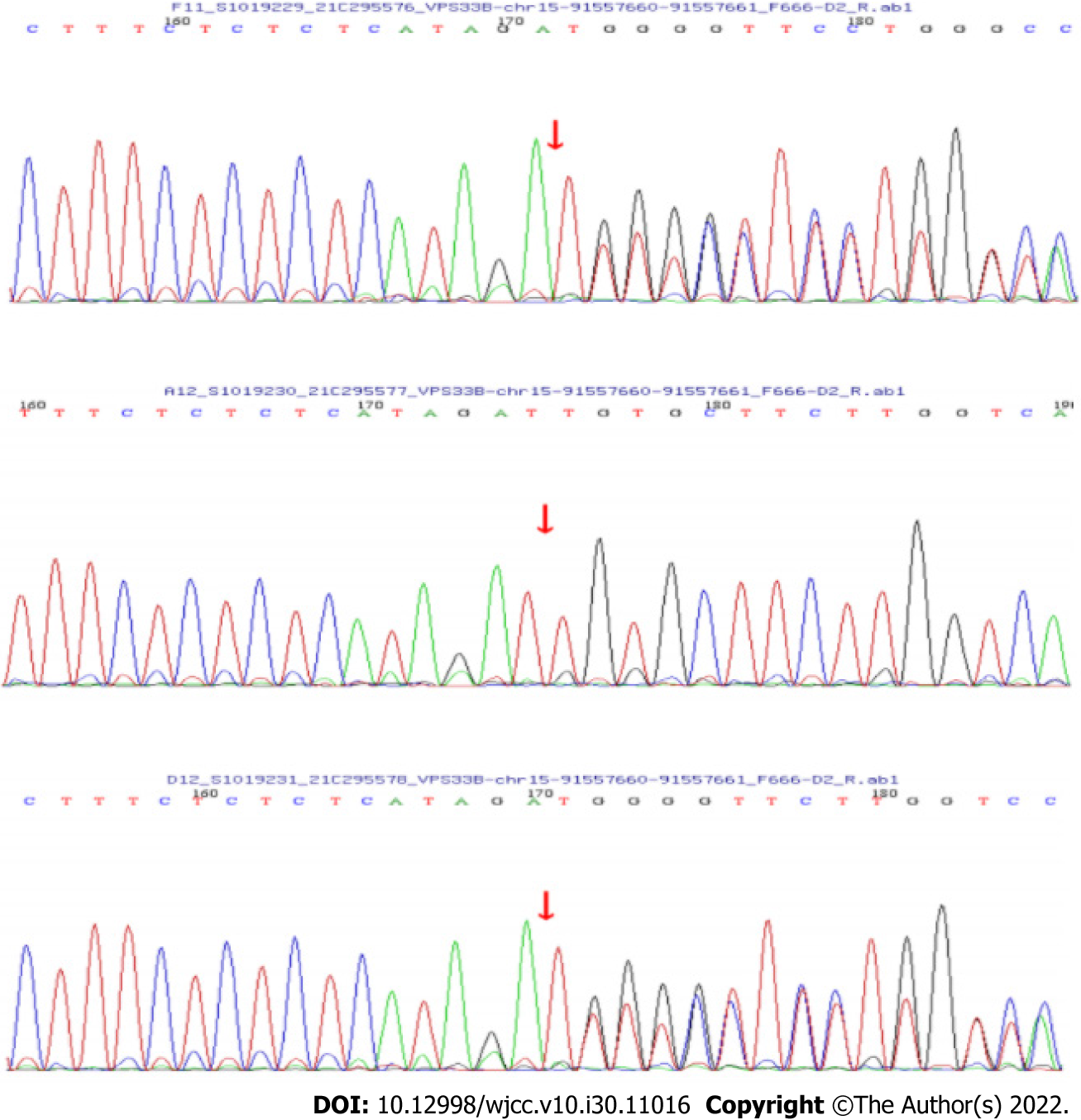
The other was a frameshift mutation c.242delT (p.L81Cfs*33), a deletion of one thymine (T) in exon 4 (Figure 3), and is a rare variant found in < 0.1% of the general population. The mutation was a low-frequency mutation. The results of protein function prediction are unknown and are not reported in the HGMD database. This mutation was heterozygous in the mother, and the paternal gene was wildtype. According to the ACMG guidelines, the clinical significance of the variation was pathogenic.
Laboratory results showed there were two compound heterozygous mutations in the VPS33B gene. Based on clinical presentation, laboratory tests and gene sequencing results, the clinical phenotype of the patient was ARCS1.
Routine blood examination follow-up during hospitalization indicated anemia, and thyroid-stimulating hormone was significantly elevated. Suspensions of red blood cells, levothyroxine and vitamins A, D and E were given to provide symptomatic treatment.
After 25 d of treatment, the child’s weight decreased to 2.71 kg. The whole body skin was still yellow. She had low muscular strength in the limbs; but the symptoms of anemia and hypothyroidism were better than before. The child was alive at age 4 mo. Her skin was still yellow, her weight had not increased, her joints could not straighten, she still had contractures, and she could only slightly raise her head.
The VPS33B gene is located on chromosome 15q26.1, is 23.9 kb long, contains 23 exons and encodes a homolog of the class C yeast Vps33 gene, which contains a Sec1-like domain important in the regulation of vesicle-to-target SNARE complex formation and subsequent membrane fusion[3,4]. VPS33B contributes signaling between cell compartments. VPS33B is ubiquitously expressed in human tissues including both liver and kidneys. A proposed mechanism for the pathogenesis of VPS33B mutations includes failed intracellular trafficking, resulting in abnormal hepatocyte polarity leading to cellular damage and dysfunction[5]. The VPS33B is involved in intracellular protein sorting and ubiquitously expressed in human tissues. Mutations cause widespread errors in protein trafficking and membrane fusion, leading to dysfunction in multiple organ systems[6]. VPS33B dysfunction may lead to disruption of cell polarization in many organs, resulting in multisystem diseases. It can cause life-threatening conditions including serious dehydration, recurrent infection, metabolic acidosis and internal bleeding[7].
Mutation of the VPS33B gene can lead to ARCS1 (OMIM: 208085 and 613404). It is a congenital malfunction with autosomal recessive inheritance with poor prognosis. ARCS1 is an autosomal recessive disorder caused by mutations of the VPS33B and VIPAR (OMIM: 613401) genes[8]. The pathogenesis of ARCS1 was first described by Gissen et al[1] in 2004. VIPAR is another causative gene of ARCS1. It is believed that VPS33B is altered in 75% of cases[9,10]. Additional features of ARCS1 include nephrogenic diabetes insipidus, failure to thrive, anomalies of the corpus callosum with neurodevelopmental delay, ichthyosis, platelet dysfunction, recurrent infections, dysmorphic features, congenital heart disease, hypothyroidism and keratitis[11].
We reported a patient with ARCS1 in China, and the patient carried two novel mutations in the VPS33B gene, which were the causative variations. The child was admitted for neonatal jaundice with clinical symptoms including joint contracture, weight loss, anemia and hypothyroidism. After vitamins, levothyroxine and other treatments, the child’s condition improved, but it was still below normal. Although she had a family history of hypothyroidism, it does not explain all symptoms. To clarify the etiology and provide better treatment, we performed WES in the child.
In our patient, ARCS1 was not clinically suspected initially. The delay in clinical diagnosis was due to the absence of renal dysfunction. The mutation c.96+1G>C in exon 1 inherited from the father, causing a splicing mutation in aminophenol, might lead to a loss of gene function. The other was a frameshift mutation c.242delT (p.L81Cfs*33), a deletion of one thymine (T) in exon 4, which is a rare variant found in < 0.1% of the general population[12].
Compared with the previously reported ARCS1 patients, our child developed manifestations of neonatal jaundice and joint contracture. She had no significant abnormalities in renal function, which was distinguished from the typical symptoms of ARCS1[13-15].
We found that although it is not the typical symptom of ARCS1, ichthyosis has been reported in many previous cases, and it was present in our patient. The child had anemia, which may be associated with thalassemia gene mutations, but ARCS1 can also be accompanied by abnormal blood cell morphology. Therefore, the cause could not be defined. The child had hypothyroidism, and considering the medical history of her mother, there is also no clear evidence that this was an accompanying symptom of the disease.
Treatment of ARCS1 is mainly supportive, consisting of ursodeoxycholic acid and fat-soluble vitamin administration, maintenance of water, acid–base and electrolyte equilibrium and treatment of concurrent infections. For joint contracture in some patients, surgical correction can restore some joint function; however, due to the poor immune function of children with ARCS1, active orthopedic surgery is not recommended[16,17].
We herein reported a patient with ARCS1 caused by two new VPS33B mutations in China. We suggest that VPS33B should be considered in individuals with cholestatic jaundice, hypothyroidism and arthrogryposis features. The identification of compound heterozygotes encourages clinicians to consider ARCS1 in patients with similar clinical features and an unrelated family history. As there is no treatment for this syndrome, early identification and genetic diagnosis are essential to counsel and select for the affected families.
We would like to thank the child and her family members for agreeing to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Batool SN, Pakistan; Eng CSY, Malaysia; Gupta MK, Germany; Malekzadegan A, Iran S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE, McClean P, Lynch SA, Thompson RJ, Lo B, Quarrell OW, Di Rocco M, Trembath RC, Mandel H, Wali S, Karet FE, Knisely AS, Houwen RH, Kelly DA, Maher ER. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;36:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Gissen P, Tee L, Johnson CA, Genin E, Caliebe A, Chitayat D, Clericuzio C, Denecke J, Di Rocco M, Fischler B, FitzPatrick D, García-Cazorla A, Guyot D, Jacquemont S, Koletzko S, Leheup B, Mandel H, Sanseverino MT, Houwen RH, McKiernan PJ, Kelly DA, Maher ER. Clinical and molecular genetic features of ARC syndrome. Hum Genet. 2006;120:396-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Hanley J, Dhar DK, Mazzacuva F, Fiadeiro R, Burden JJ, Lyne AM, Smith H, Straatman-Iwanowska A, Banushi B, Virasami A, Mills K, Lemaigre FP, Knisely AS, Howe S, Sebire N, Waddington SN, Paulusma CC, Clayton P, Gissen P. Vps33b is crucial for structural and functional hepatocyte polarity. J Hepatol. 2017;66:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Sprecher E, Ishida-Yamamoto A, Mizrahi-Koren M, Rapaport D, Goldsher D, Indelman M, Topaz O, Chefetz I, Keren H, O'brien TJ, Bercovich D, Shalev S, Geiger D, Bergman R, Horowitz M, Mandel H. A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am J Hum Genet. 2005;77:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Zhou Y, Zhang J. Arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome: from molecular genetics to clinical features. Ital J Pediatr. 2014;40:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Smith H, Galmes R, Gogolina E, Straatman-Iwanowska A, Reay K, Banushi B, Bruce CK, Cullinane AR, Romero R, Chang R, Ackermann O, Baumann C, Cangul H, Cakmak Celik F, Aygun C, Coward R, Dionisi-Vici C, Sibbles B, Inward C, Kim CA, Klumperman J, Knisely AS, Watson SP, Gissen P. Associations among genotype, clinical phenotype, and intracellular localization of trafficking proteins in ARC syndrome. Hum Mutat. 2012;33:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Hershkovitz D, Mandel H, Ishida-Yamamoto A, Chefetz I, Hino B, Luder A, Indelman M, Bergman R, Sprecher E. Defective lamellar granule secretion in arthrogryposis, renal dysfunction, and cholestasis syndrome caused by a mutation in VPS33B. Arch Dermatol. 2008;144:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Rogerson C, Gissen P. VPS33B and VIPAR are essential for epidermal lamellar body biogenesis and function. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1609-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Adamczyk-Gruszka O, Horecka-Lewitowicz A, Zmelonek-Znamirowska A, Gruszka J, Koziel D, Lewitowicz P. A New Aberration in the VPS33B Gene Leads to Full-Symptom ARCS1. Am J Case Rep. 2021;22:e932769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ilhan O, Ozer EA, Ozdemir SA, Akbay S, Memur S, Kanar B, Tatli MM. Arthrogryposis-renal tubular dysfunction-cholestasis syndrome: a cause of neonatal cholestasis. Case report. Arch Argent Pediatr. 2016;114:e9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Akbar MA, Mandraju R, Tracy C, Hu W, Pasare C, Krämer H. ARC Syndrome-Linked Vps33B Protein Is Required for Inflammatory Endosomal Maturation and Signal Termination. Immunity. 2016;45:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22319] [Article Influence: 2231.9] [Reference Citation Analysis (0)] |
| 13. | Agakidou E, Agakidis C, Kambouris M, Printza N, Farini M, Vourda E, Gerou S, Sarafidis K. A Novel Mutation of VPS33B Gene Associated with Incomplete Arthrogryposis-Renal Dysfunction-Cholestasis Phenotype. Case Rep Genet. 2020;2020:8872294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Duong MD, Rose CM, Reidy KJ, Del Rio M. An uncommon case of arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome and review of the renal involvement: Answers. Pediatr Nephrol. 2020;35:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Del Brío Castillo R, Squires JE, McKiernan PJ. A novel mutation in VPS33B gene causing a milder ARC syndrome phenotype with prolonged survival. JIMD Rep. 2019;47:4-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Götze T, Blessing H, Grillhösl C, Gerner P, Hoerning A. Neonatal Cholestasis - Differential Diagnoses, Current Diagnostic Procedures, and Treatment. Front Pediatr. 2015;3:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Mutlu M, Aslan Y, Aktürk-Acar F, Çakır M, Erduran E, Kalyoncu M. ARC syndrome. Turk J Pediatr. 2017;59:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |