Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11010
Peer-review started: February 11, 2022
First decision: June 16, 2022
Revised: July 7, 2022
Accepted: September 19, 2022
Article in press: September 19, 2022
Published online: October 26, 2022
Processing time: 252 Days and 1.8 Hours
This is the first documentation of a spontaneous and nonspecific chemical reaction of an iodinated contrast media with ammonium persulfate used in As3+-Ce4+ catalytic spectrophotometry for urine iodine concentration (UIC) detection.
We herein report an incidental case who had a dual source computed tomography examination for papillary thyroid carcinoma diagnosis. Serial spot urine speci
The following laboratory suggestions should be considered: (1) As3+-Ce4+ catalytic spectrophotometry is only suitable for UIC measurement after confirmed ICM renal clearance; (2) A mass spectrometry-based method can be applied as an alternative during the ICM clearance period; and (3) The UIC baseline can be confirmed after ICM injection by consecutive detection for at least 2 mo.
Core Tip: There has been no report on the nonspecific chemical reaction of an iodinated contrast media with ammonium persulfate used in As3+-Ce4+ catalytic spectrophotometry thus far. We herein report a typical case, which might contribute to improving our understanding of the biochemistry mechanism as well as interpretation of the results of UIC detection during papillary thyroid carcinoma (PTC) diagnosis and treatment. This report also serves as a reminder to establish an individual flowchart to evaluate prognosis during PTC follow-up.
- Citation: Zhang SC, Yan CJ, Li YF, Cui T, Shen MP, Zhang JX. Right time to detect urine iodine during papillary thyroid carcinoma diagnosis and treatment: A case report. World J Clin Cases 2022; 10(30): 11010-11015
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11010.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11010
As the most widely used marker to assess the plasmatic iodine pool, urine iodine concentration (UIC) measurement is recommended to assess the iodine status of a population[1,2]. With the increased use of iodinated contrast media (ICM) for chest and neck computed tomography (CT), excess iodine exposure has increased in patients with thyroid disease (TD). Several studies suggest that ICM increases the total body iodine stores for at least 3 mo following contrast exposure and, in some situations, for as long as 2 years. The introduction of approximately 0.1% inorganic iodine free from ICM into serum might compete with radioactive iodine or disturb thyroid function due to the Wolff‒Chaikoff effect[3,4]. Considering that accurate UIC detection is very important to guide radioactive iodine treatment in patients with TD, it is necessary to understand the effect of excess iodine exposure on UIC after ICM injection in patients with TD.
A 39-year-old female patient was admitted to our hospital on January 11, 2020 for a re-examination of a thyroid nodule.
The thyroid nodule was found 1 year ago. Ultrasound examination revealed a 6.1 cm × 4.9 cm × 6.7 cm hypoechoic area in the right lobe of the thyroid gland. The lesion was classified by the thyroid imaging reporting and data system (TI-RADS) as TI-RADS 4C (highly suspected malignancy).
The patient had a medical history that was free of previous illness.
The patient had no personal or family history.
Upon physical examination, a solid mass was found in the right lobe of the thyroid gland.
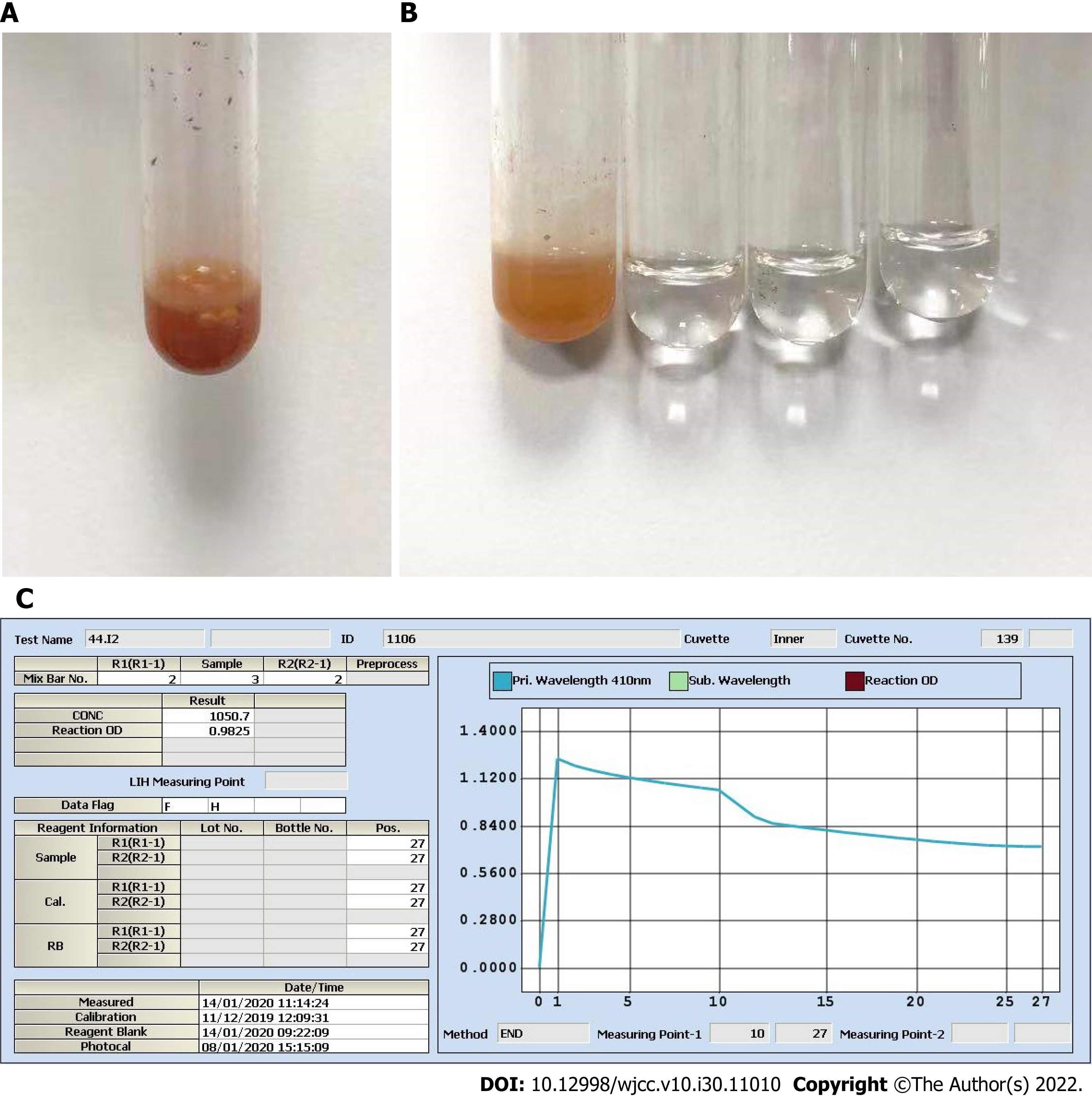
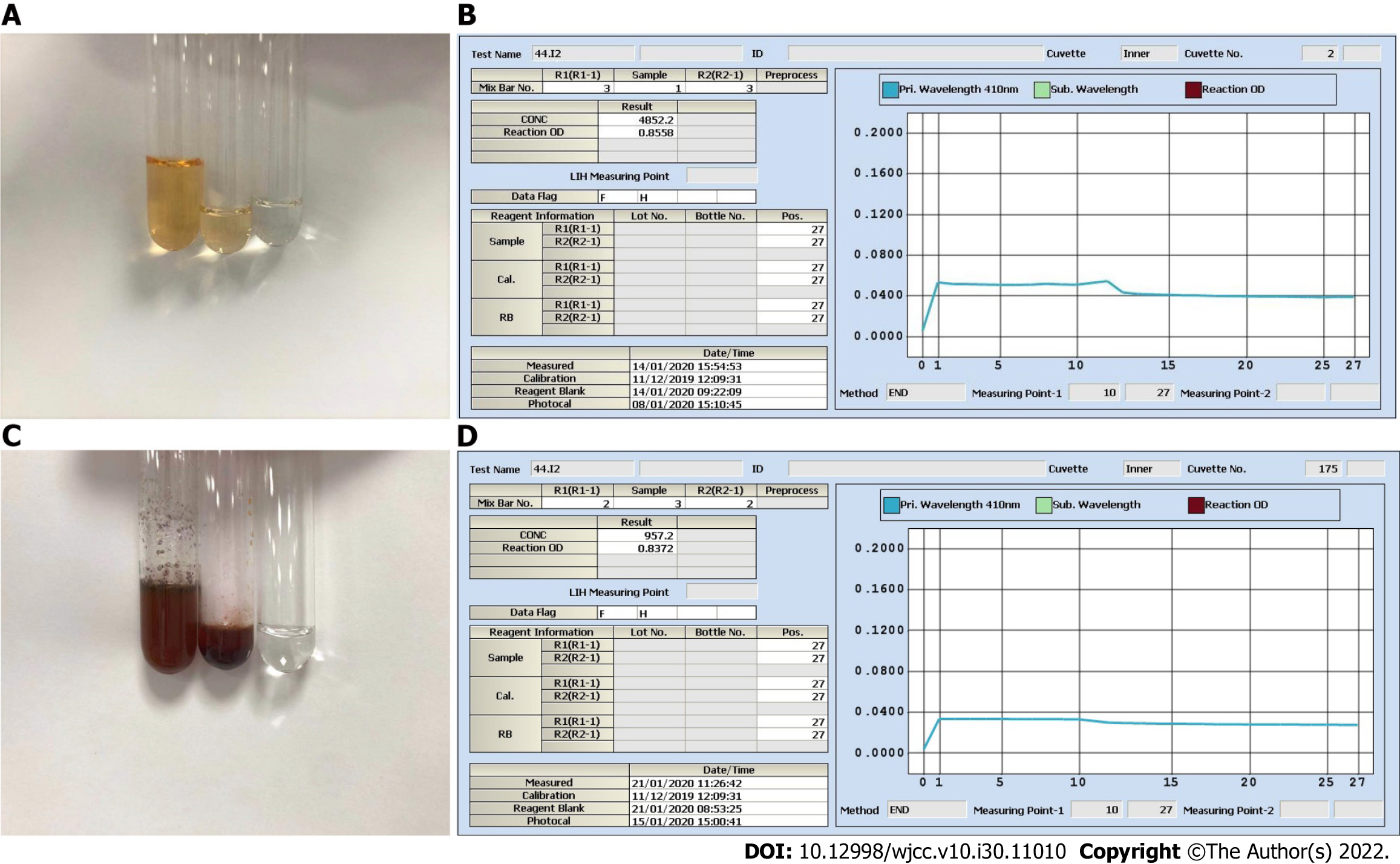
The patient’s laboratory blood test results on January 13, including AFP and thyroid function indicators (free triiodothyronine, free thyroxine, thyroid stimulating hormone, thyroid peroxidase antibody, thyroglobulin antibody, thyroglobulin, and parathyroid hormone), were all within the reference ranges. However, the spot UICs (sUICs) determined on January 14 were reported as errors (Figure 1A-C). We contacted her doctor to obtain new spot urine specimens on January 15 (preoperation) and 17 (1 d before discharge). Each specimen was serially diluted (10-fold, 50-fold, and 100-fold). All sUICs were extremely high (> 3 mg/L, Figure 2). Nevertheless, the reaction curves gradually changed when comparing the same dilution ratio between different specimens over time and finally became valid in 100-fold dilution on January 17. The results indicated that the iodine concentration in the patient was actually dropping.
On January 13, 2020, the patient underwent dual source CT of the thyroid, and the result indicated a low density, ground-glass enhanced lesion near the back side of the right lobe. No obvious abnormality was found in the left lobe or the isthmic portion. There were multiple nodules in the II, III, IV, and VI regions of the bilateral neck with normal shapes that showed no early-stage enhancement.
The final diagnosis of the presented case was PTC.
The patient underwent radical thyroidectomy on January 15, 2020. Histological examination of the frozen sections from the resected lesion confirmed PTC.
The patient had a good recovery. Her thyroid function indicators were normal until this article is written.
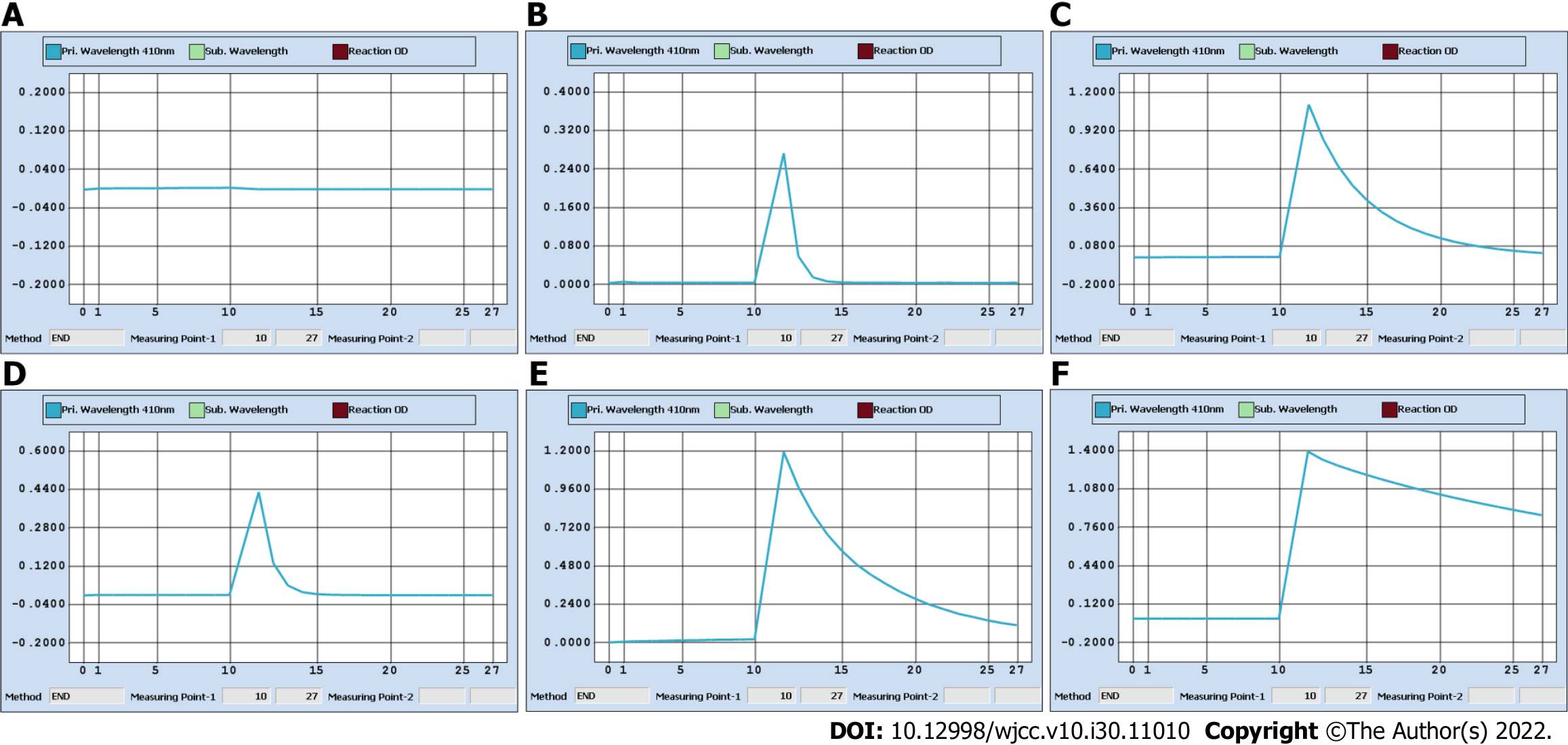
For the determination of iodine in urine, As3+-Ce4+ catalytic spectrophotometry is recommended as the standard assay according to WS/T 107.2-2016 by the National Health and Family Planning Commission in China. The protocol is as follows: (1) Add 600 μL ammonium persulfate to 200 μL urine and digest the mixture for 60 min in a 100 °C incubator; (2) Transfer the digested mixture to a Beckman Coulter AU5800; (3) Add 120 μL reagent 1 containing As3+ to 25 μL digested mixture; (4) Add 36 μL reagent 2 containing Ce4+ to reduce the yellow Ce4+ to colorless Ce3+; and (5) The decreasing absorbance curve of Ce4+ at a wavelength of 410 nm is positively proportional to the concentration of iodine over a designated period of time[5]. On January 14, we incidentally found that the digested solution of a spot urine specimen as well as its 2-fold dilution was “brownish”. We contacted the patient, and she recalled that the specimen was collected within 1 h after intravenous ICM injection on January 13. The ICM was iohexol (35 g I/100 mL). We tested iohexol instead of urine in serial dilutions. The results strongly indicated that a chemical reaction immediately started once iohexol was mixed with ammonium persulfate, even prior to digestion (Figure 3A), and we perfectly reproduced the “brownish” solution as well as the solid purple precipitates (Figure 3B). We believe that this automatic chemical reaction would disrupt the desired reaction of urine iodine with ammonium persulfate and cause inaccurate sUIC values.
There are three important studies that provide convincing and detailed data on urine iodine clearance[6-8]. In general, if a patient undergoes intravenous contrast CT examination, it will take at least 1 mo for the UIC to return to the baseline level. Unfortunately, there is a lack of data for the Chinese population to date. As3+-Ce4+ catalytic spectrophotometry has a sensitivity of 10 μg/L and a reportable range of 0-3000 μg/L in our laboratory. Notably, the color shade of the spot urine specimen on January 14 was between those of 35 mg iohexol (100% renal clearance assuming 200 mL urine output) and 3.5 mg iohexol (90% renal clearance). More importantly, as shown in Figure 1B, the color faded for the 2-fold dilution and disappeared for the 5-fold dilution, which allowed us to speculate that the original urine specimen probably contained at least 17.5 mg iohexol (50% renal clearance). This value was very different from that calculated on January 14, which was 1.05 mg/L (Figure 1C). We also checked the Ce4+ absorbance curve for 3.5 mg iohexol. Consistent with the curve of the 5-fold dilution of urine specimens on January 14, there was a background optical density value indicating a nonspecific reaction (Figure 3C and D). Therefore, the spontaneous and nonspecific chemical reaction of ICM with ammonium persulfate used in As3+-Ce4+ catalytic spectrophotometry can interfere with the accuracy of the UIC results. It is very important to know the period for urine iodine levels to return to baseline after ICM injection and to collect urine specimens on the right time.
We suggest that the following scenarios should be considered for PTC patients who may have UIC measurement: (1) Pre-examination quality control of urine specimen. As3+-Ce4+ catalytic spectrophotometry is only suitable for the determination of the sUIC as well as the 24-h UIC after confirmed ICM renal clearance; (2) Appropriate laboratory methods for UIC detection. A mass spectrometry-based method can be applied as a favorable alternative during the ICM clearance period to evaluate potential ICM impairment; and (3) Optimal UIC detection intervals. The UIC should be detected for at least two consecutive months to confirm the baseline after ICM injection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gluvic Z, Serbia; Tuan Ismail TS, Malaysia S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | Caldwell KL, Miller GA, Wang RY, Jain RB, Jones RL. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003-2004. Thyroid. 2008;18:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Farebrother J, Zimmermann MB, Assey V, Castro MC, Cherkaoui M, Fingerhut R, Jia Q, Jukic T, Makokha A, San Luis TO, Wegmüller R, Andersson M. Thyroglobulin Is Markedly Elevated in 6- to 24-Month-Old Infants at Both Low and High Iodine Intakes and Suggests a Narrow Optimal Iodine Intake Range. Thyroid. 2019;29:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Jarvis C, Simcox K, Tamatea JA, McAnulty K, Meyer-Rochow GY, Conaglen JV, Elston MS. A low incidence of iodine-induced hyperthyroidism following administration of iodinated contrast in an iodine-deficient region. Clin Endocrinol (Oxf). 2016;84:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Luo Y, Kawashima A, Ishido Y, Yoshihara A, Oda K, Hiroi N, Ito T, Ishii N, Suzuki K. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int J Mol Sci. 2014;15:12895-12912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Pino S, Fang SL, Braverman LE. Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin Chem. 1996;42:239-243. [PubMed] |
| 6. | Padovani RP, Kasamatsu TS, Nakabashi CC, Camacho CP, Andreoni DM, Malouf EZ, Marone MM, Maciel RM, Biscolla RP. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012;22:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Nimmons GL, Funk GF, Graham MM, Pagedar NA. Urinary iodine excretion after contrast computed tomography scan: implications for radioactive iodine use. JAMA Otolaryngol Head Neck Surg. 2013;139:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Lee SY, Chang DL, He X, Pearce EN, Braverman LE, Leung AM. Urinary iodine excretion and serum thyroid function in adults after iodinated contrast administration. Thyroid. 2015;25:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |