Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.10997
Peer-review started: June 20, 2022
First decision: August 1, 2022
Revised: August 11, 2022
Accepted: September 6, 2022
Article in press: September 6, 2022
Published online: October 26, 2022
Processing time: 122 Days and 11.1 Hours
Wells’ syndrome (eosinophilic cellulitis) is an uncommon eosinophilic dermatosis of uncertain pathogenesis, characterized by clinical polymorphism and suggestive but nonspecific histopathologic traits. Its course is recurrent, and response to therapy is unpredictable. In a case in which the patient has a number of potential triggers for the manifestation of Wells’ syndrome skin rash, the treating physician must decide or must make an assumption in order to establish the most likely clinical scenario. This is important for the patient’s future treatment plans.
We describe the clinical case of a 46-year-old female with chronic lymphocytic leukemia who had already received treatment for several months with ibrutinib. She was diagnosed with Wells’ syndrome 10 d after an influenza vaccination containing thimerosal. Based on the literature, the patient was treated with a course of oral steroids. Resolution of clinical symptoms and rash were observed in response to the treatment. Ibrutinib was not discontinued.
The etiology of Wells’ syndrome remains unknown. Clinically, it resembles bacterial cellulitis. Lack of response to antibiotic treatment should lead the physician to consider a diagnosis of Wells’ syndrome. Treating the underlying condition is important and may lead to resolution of the syndrome. However, the most common and effective treatment to limit the course of the disease are systemic steroids.
Core Tip: Our patient presented with pruritic rash all over her body. Based on the pathohistological features, a diagnosis of Wells’ syndrome (eosinophilic cellulitis) was established. We considered hematological malignancy, ibrutinib and influenza vaccine as possible triggers. The only new event and therefore most probable trigger for Wells’ syndrome was an influenza vaccination with a vaccine containing thimerosal. Clinically, this is a relatively rare case.
- Citation: Šajn M, Luzar B, Zver S. Wells’ syndrome possibly caused by hematologic malignancy, influenza vaccination or ibrutinib: A case report. World J Clin Cases 2022; 10(30): 10997-11003
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/10997.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.10997
Classic eosinophilic dermatoses include eosinophilic cellulitis (Wells’ syndrome), granuloma faciale, eosinophilic fasciitis (Shulman syndrome) and eosinophilic folliculitis (Ofuji disease). Even though these disorders share the common characteristic of tissue eosinophilia, they have a variety of clinical presentations[1]. Fewer than 200 cases of Wells’ syndrome have been reported in the literature[2]. It is characterized by protean cutaneous manifestations with prominent eosinophilia[3]. The diagnosis is corroborated by histopathological findings from a skin biopsy specimen.
The cause of Wells’ syndrome is not known. In literature specific triggers were implicated, including drugs such as penicillin or infliximab, thimerosal-containing vaccines, and hematological malignancies, such as chronic lymphocytic leukemia (CLL), non-Hodgkin’s lymphoma, chronic myeloid leukemia and polycythemia rubra vera[1,2,4]. The most common and effective treatment is oral steroids. Topical corticosteroids are less effective and should be considered in cases of limited disease or persistent residual lesions[2].
In this case report, a female patient with CLL receiving ibrutinib and recently receiving an influenza vaccination at the time of diagnosis of Wells’ syndrome is presented.
A 46-year-old Caucasian woman presented at the Haematology Outpatient Department with pruritic rash all over her body. Prior to this visit, she had been examined by an infectious diseases specialist at her local hospital, who suspected a scabies-related rash.
The patient complained of pruritic, erythematous, blister-like lesions that had arose over the past 14 d. Because of scrabbing, the blisters were soon replaced with crusts (Figure 1). She reported having noticed no signs of infection, no fevers or chills, and no B-symptoms, and denied having been bitten by an insect or close contact with domestic animals. She was not receiving any medical drugs, with the exception of ibrutinib (started 18 mo earlier) but remembered having received a thimerosal-containing influenza vaccination (VaxigripTetraÒ, Sanofi Pasteur, Lyon, France) 10 d prior to the first skin lesions appearing. She reported never having experienced a similar rash.
The patient had been diagnosed with CLL and treated with allogeneic hematopoietic stem cell transplantation (allo-HSCT) in 2017. To address a persistent CLL clone with bone marrow infiltration of 5%, she had received the above-mentioned ibrutinib. More than 3 years after the allo-HSCT, she had presented for an extra outpatient visit to address the itchy rash.
There is no personal and family history.
On admission, the patient was afebrile. Papulonodular, crusted skin eruptions (2-3 mm in diameter) were prominent on her neck, back, arms and legs. An enlarged painful lymph node (3 cm in diameter) was palpable under the left armpit. The influenza vaccination had been given on that same side.
The patient’s white blood cell and absolute eosinophil counts in the peripheral blood were within normal ranges (7.94 × 109/L and 0.27 × 109/L, respectively). Serological and PCR-based testing ruled out reactivation of herpes simplex virus 1 and 2, varicella zoster virus, cytomegalovirus, and Epstein-Barr virus.
No image inspections are involved.
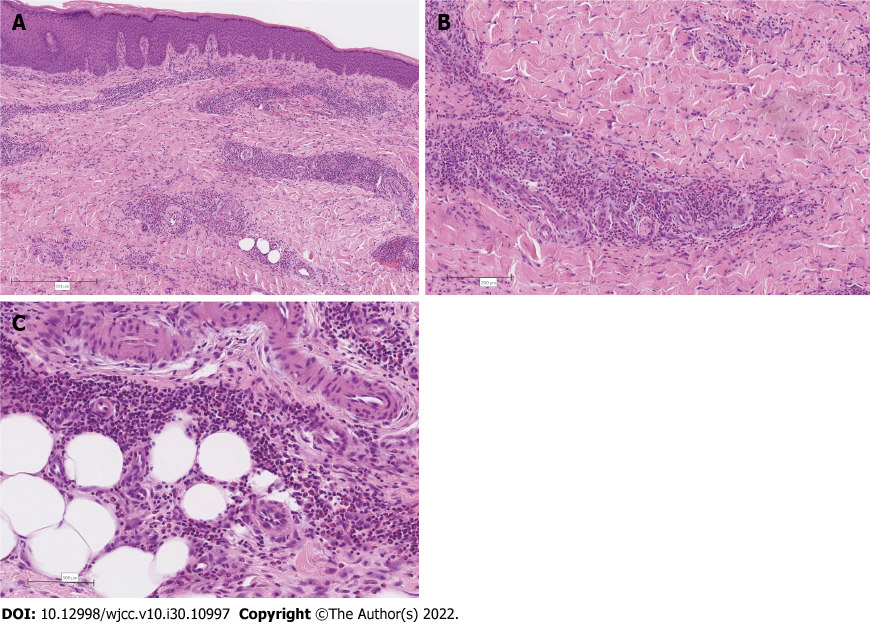
Since the clinical and laboratory examinations did not establish a diagnosis, we opted for skin biopsy. The skin biopsy from the right thigh revealed irregular acanthosis of the epidermis, associated with diffuse superficial and deep perivascular and interstitial inflammatory cell infiltrates composed predominantly of eosinophilic granulocytes admixed with lymphocytes and histiocytes. The inflammatory cell infiltration extended into the septa and lobuli of the subcutaneous fatty tissue (Figure 2).
Wells’ syndrome (eosinophilic cellulitis).
Based on the overall and up-to-date literature, the patient was treated with a course of oral steroid (methylprednisolone 48 mg at 1 mg/kg), administered daily for 1 wk and followed by a rapid tapering until discontinuation within 4 wk.
With the steroid treatment, resolution of the clinical symptoms and rash was observed. The ibrutinib was not discontinued at any point. Repeat bone marrow aspiration flow cytometry and histology findings were consistent with 5% residual infiltration of a CLL clone.
Wells’ syndrome, a recurrent granulomatous dermatitis with eosinophilia was first described by Wells in 1971. It also goes by the name eosinophilic cellulitis and eosinophilic dermatosis. Clinically, the syndrome resembles bacterial cellulitis because patients usually present with a warm erythematous skin lesion. Cellulitis not responding to antibiotic treatment should lead the physician to suspect Well’s syndrome[2,3]. The classic, plaque-type variant has been shown to be the most common clinical pre
The cause of Well’s syndrome is not known. In literature possible triggers were implicated in the syndrome development: insect bites, viral or bacterial infections, drugs, thimerosal-containing vaccines, hematological malignancies and carcinoma. Most of the reported cases suggest a certain trigger, such as an underlying disorder, and rarely does the syndrome appear to be idiopathic in origin. Although the pathogenesis is not well defined, a type IV hypersensitivity reaction to various stimuli may be involved[2,4].
CLL is the most common leukemia in adults in Western countries, with the average age of diagnosis being 72 years. The disease is characterized by an accumulation of monoclonal, mature, CD5+ B cells in the peripheral blood, bone marrow and secondary lymphoid organs[5]. CLL is accompanied by an increased incidence of other malignancies, and patients are prone to cutaneous infections, particularly viral ones, and have exaggerated, vivid reactions to insect bites[6]. Although overall leukemic skin infiltration occurs in 3%-50% of patients within the entire spectrum of leukemias or lymphomas, it is a rare event among patients with CLL[7].
Eosinophilic dermatosis of hematologic malignancy (EDHM) and/or insect bite-like reactions are a rare event, particularly in association with CLL[8]. There has been debate about whether this phe
Our case is unique because a patient who had undergone treatment with allo-HSCT was involved. This had been performed in the context of CLL treatment with adverse prognostic factors (del17, TP53 mutation; IgHV mutation status was not performed) in the young female[13]. However, the treatment was ultimately not curative, and residual disease was still present after immunosuppression withdrawal after the allo-HSCT. The patient was afraid of graft vs host disease[14] and refused a donor lymphocyte infusion, which had been proposed to enhance immune-mediated antitumor activity[15].
Because residual disease is widely associated with a significant risk of CLL progression, ibrutinib, a potent and irreversible small-molecule inhibitor of both Bruton’s tyrosine kinase and IL-2 inducible kinase as well as several other tyrosine kinases, was instituted. Ibrutinib should provide effective CLL treatment of residual disease after allo-HSCT[13,16]. Since a reduced dose of ibrutinib proved effective[17], the initial dosage of 420 mg daily was reduced to 120 mg daily after 1 mo due to severe neu
EGFR inhibitor-induced toxic effects of the skin are well described and are claimed to be a class effect of this substance group. Patients present with macular, papular or pustular lesions in an acneiform distribution, mainly localized in cosmetically-sensitive areas (e.g., regions rich in sebaceous glands, such as the face and upper trunk) but can also extend to the extremities. Severe acute skin reactions show massive neutrophilic infiltration of the epidermis and profound apoptosis[19]. Cutaneous manifestations, including purpuric eruptions, have been reported in 8%-27% of patients receiving ibrutinib, sometimes resulting in treatment delays or even drug discontinuation[21]. The time of rash onset is highly variable, with onset as late as 300-400 d after the ibrutinib initiation in some patients[21,22].
In our case, if ibrutinib was the trigger, then the rash had appeared more than 600 d after the treatment was begun. In a single-center review of patients with ibrutinib-associated rash performed by Iberri et al[21], 4 patients with grade 3 rash underwent biopsy, demonstrating perivascular infiltration of lymphocytes, neutrophils and eosinophils involving the papillary dermis. However, Bullock et al[23] reported a clinical case of eosinophilic skin rash in a patient with CLL who had recently started ibrutinib. The patient presented to the emergency department with a 4-d history of a severely pruritic, eruptive full-body rash. The clinical differential was among EDHM vs drug eruption, given that she had recently started ibrutinib. After treatment with prednisone, topical corticosteroids and antihistamines, the skin lesions resolved. The patient continued to have complete resolution of her cutaneous eruption despite continuing ibrutinib therapy.
In the case of our patient, the only new event was an influenza vaccination 10 d before the clinical presentation of Wells’ syndrome. A case of Wells’ syndrome in an adult 13 d after influenza vaccination was described by Masckauchan et al[24]. There are also some cases of children being diagnosed with Wells’ syndrome post-influenza vaccination and 1 case of an adult being diagnosed after receiving a tetanus vaccination[25-27]. Indeed, all the vaccinations described in the literature include thimerosal, a common preservative[24-27]. In the case of the patient with Wells’ syndrome post-tetanus vaccination, a skin test with thimerosal was found to be positive, demonstrating the possibility that part of the pathophysiology of Wells’ syndrome is related to hypersensitivity reactions[27].
To conclude, in our case of a patient with Wells’ syndrome, we first hypothesized that the cause was EDHM. Prior to the allo-HSCT when the bone marrow infiltration was higher than 5%, she did not experience skin changes associated with EDHM. Next, we considered ibrutinib to be a possible trigger. She had been receiving the drug for more than 2 years and had not develop any skin changes during that period. The only new event, and therefore the most probable trigger of Wells’ syndrome, was an influenza vaccination with a vaccine containing thimerosal. We have no specific or reliable evidence to state that this was the definite triggering event. It is also possible that there was an intertwining of all three reasons: hematological malignancy, ibrutinib and influenza vaccine. However, the skin rash resolution after treatment with methylprednisolone despite ibrutinib continuation and persistent CLL burden in our case, provides evidence that the influenza vaccination was the most likely factor in the appearance of Wells’ syndrome.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: University Medical Centre Ljubljana.
Specialty type: Hematology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hernandez-Caballero A, Mexico; Yikilmaz AS, Turkey S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Peckruhn M, Elsner P, Tittelbach J. Eosinophilic dermatoses. J Dtsch Dermatol Ges. 2019;17:1039-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Sinno H, Lacroix JP, Lee J, Izadpanah A, Borsuk R, Watters K, Gilardino M. Diagnosis and management of eosinophilic cellulitis (Wells' syndrome): A case series and literature review. Can J Plast Surg. 2012;20:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Caputo R, Marzano AV, Vezzoli P, Lunardon L. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Aberer W, Konrad K, Wolff K. Wells' syndrome is a distinctive disease entity and not a histologic diagnosis. J Am Acad Dermatol. 1988;18:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 94] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Burger JA. Treatment of Chronic Lymphocytic Leukemia. N Engl J Med. 2020;383:460-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Agnew KL, Ruchlemer R, Catovsky D, Matutes E, Bunker CB. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Chang HY, Wong KM, Bosenberg M, McKee PH, Haynes HA. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Qiao J, Sun CE, Zhu W, Zhu D, Fang H. Flame figures associated with eosinophilic dermatosis of hematologic malignancy: is it possible to distinguish the condition from eosinophilic cellulitis in patients with hematoproliferative disease? Int J Clin Exp Pathol. 2013;6:1683-1687. [PubMed] |
| 9. | Vassallo C, Passamonti F, Cananzi R, Brazzelli V, Ardigò M, Lazzarino M, Borroni G. Exaggerated insect bite-like reaction in patients affected by oncohaematological diseases. Acta Derm Venereol. 2005;85:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Bairey O, Goldschmidt N, Ruchlemer R, Tadmor T, Rahimi-Levene N, Yuklea M, Shvidel L, Berrebi A, Polliack A, Herishanu Y; Israeli Chronic Lymphocytic Leukemia Study Group (ICLLSG). Insect-bite-like reaction in patients with chronic lymphocytic leukemia: a study from the Israeli Chronic Lymphocytic Leukemia Study Group. Eur J Haematol. 2012;89:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Ozden MG, Yildiz L, Aydin F, Senturk N, Canturk T, Turanli AY. Is it really possible to differentiate insect bite-like reaction and nodular variant of eosinophilic cellulitis in a healthy person? Eur J Dermatol. 2009;19:635-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Barzilai A, Shpiro D, Goldberg I, Yacob-Hirsch Y, Diaz-Cascajo C, Meytes D, Schiby R, Amariglio N, Trau H. Insect bite-like reaction in patients with hematologic malignant neoplasms. Arch Dermatol. 1999;135:1503-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Ryan CE, Sahaf B, Logan AC, O'Brien S, Byrd JC, Hillmen P, Brown JR, Dyer MJ, Mato AR, Keating MJ, Jaglowski S, Clow F, Rezvani AR, Styles L, Coutre SE, Miklos DB. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood. 2016;128:2899-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Filipovich AH. Diagnosis and manifestations of chronic graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11:473-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, Flowers ME, Logan AC, Nakamura R, Blazar BR, Li Y, Chang S, Lal I, Dubovsky J, James DF, Styles L, Jaglowski S. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 17. | Chen LS, Bose P, Cruz ND, Jiang Y, Wu Q, Thompson PA, Feng S, Kroll MH, Qiao W, Huang X, Jain N, Wierda WG, Keating MJ, Gandhi V. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood. 2018;132:2249-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Jensen AB, Stausbøl-Grøn B, Riber-Hansen R, d'Amore F. Ibrutinib-Associated Skin Toxicity: A Case of Maculopapular Rash in a 79-Year Old Caucasian Male Patient with Relapsed Waldenstrom's Macroglobulinemia and Review of the Literature. Dermatol Reports. 2017;9:6976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M. Inhibition of the epidermal growth factor receptor increases expression of genes that stimulate inflammation, apoptosis, and cell attachment. Mol Cancer Ther. 2005;4:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Perez-Soler R, Van Cutsem E. Clinical research of EGFR inhibitors and related dermatologic toxicities. Oncology (Williston Park). 2007;21:10-16. [PubMed] |
| 21. | Iberri DJ, Kwong BY, Stevens LA, Coutre SE, Kim J, Sabile JM, Advani RH. Ibrutinib-associated rash: a single-centre experience of clinicopathological features and management. Br J Haematol. 2018;180:164-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Mannis G, Wu D, Dea T, Mauro T, Hsu G. Ibrutinib rash in a patient with 17p del chronic lymphocytic leukemia. Am J Hematol. 2015;90:179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. |
Bullock T, Steele RB, Weiffenbach A, Fernandez A, Charles K. Eosinophilic Dermatosis of Hematologic Malignancy Presenting as Suspected Drug Eruption in a Patient with Chronic Lymphocytic Leukemia.
Oncol Cancer Case Rep. 2020;6 1-2. Available from: |
| 24. | Safran T, Masckauchan M, Maj J, Green L. Wells syndrome secondary to influenza vaccination: A case report and review of the literature. Hum Vaccin Immunother. 2018;14:958-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Calvert J, Shors AR, Hornung RL, Poorsattar SP, Sidbury R. Relapse of Wells' syndrome in a child after tetanus-diphtheria immunization. J Am Acad Dermatol. 2006;54:S232-S233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Koh KJ, Warren L, Moore L, James C, Thompson GN. Wells' syndrome following thiomersal-containing vaccinations. Australas J Dermatol. 2003;44:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Moreno M, Luelmo J, Monteagudo M, Bella R, Casanovas A. Wells' syndrome related to tetanus vaccine. Int J Dermatol. 1997;36:524-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |