Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.10984
Peer-review started: July 18, 2022
First decision: August 6, 2022
Revised: August 19, 2022
Accepted: September 19, 2022
Article in press: September 19, 2022
Published online: October 26, 2022
Processing time: 94 Days and 12.2 Hours
Liver cirrhosis (LC) is currently the 11th most common cause of death and 15th cause of morbidity globally. The treatment of LC is mainly aimed at etiological intervention, lifestyle intervention, prevention and treatment of complications and nutritional treatment. Nutritional treatment of LC mainly includes increasing dietary intake, food intake time and branched-chain amino acids (BCAAs). Despite the recommendation of BCAAs in some guidelines, adverse effects have been reported in studies so the efficacy and safety of BCAAs remain controversial. Currently, BCAAs have been widely used in chronic liver disease, while the summary of the effect of BCAAs on long-term prognosis is rare.
To determine the effects of BCAAs in patients with LC.
The PubMed, Cochrane Library, Embase and Web of Science databases were searched. The retrieval deadline was 1 October 2021 and there were no language restrictions set in the retrieval. The study was performed in strict accordance with the inclusion and exclusion criteria. Nine studies were finally included. The primary outcome was complications of LC. The secondary outcomes were nutri
The analysis included nine studies that consisted of 1080 patients (554 in the BCAA groups and 526 in the control groups). The nine studies were randomized control trials (RCTs). The quality of the studies was assessed using the risk of bias method recommended by the Cochrane Collaboration. BCAAs reduced the rate of complications in LC patients [Risk ratio: 0.70, 95% confidence interval (CI): 0.56-0.88, P = 0.002] and improved patients’ albumin levels [std mean difference SMD: 0.26, 95%CI: 0.12-0.40, P = 0.0002]. Meanwhile, BCAAs significantly ameliorated the levels of alanine transaminase (SMD: -2.03, 95%CI: -2.52 to -1.53, P < 0.00001) and aspartate aminotransferase (SMD: -1.8, 95%CI: -2.14 to -1.46, P < 0.00001). Meanwhile, glucose in the LC was signifi
BCAAs reduce the incidence of complications in patients with LC and ameliorate nutritional status.
Core Tip: Liver cirrhosis (LC) is currently the 11th most common cause of death and the 15th cause of morbidity globally. Nutritional treatment of LC mainly includes increasing dietary intake, food intake time and branched chain amino acids (BCAAs). The efficacy and safety of BCAAs remain controversial. We performed a meta-analysis and nine studies were finally included. The primary outcome was complications of LC. The secondary outcomes were nutritional status and liver function. The conclusion is that branched-chain amino acids reduce the incidence of complications in patients with liver cirrhosis and ameliorate nutritional status.
- Citation: Du JY, Shu L, Zhou YT, Zhang L. Branched-chain amino acids supplementation has beneficial effects on the progression of liver cirrhosis: A meta-analysis. World J Clin Cases 2022; 10(30): 10984-10996
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/10984.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.10984
As the 11th leading cause of death and 15th leading cause of morbidity worldwide, liver cirrhosis (LC) is the end stage of liver diseases[1]. It is the top 20 causes of disability-adjusted life years and years of life lost and accounts for 1.6% and 2.1% of the worldwide burden. Asrani et al[2] summarized that LC causes two million deaths, one million deaths from cirrhosis complications and one million deaths from viral hepatitis and hepatocellular carcinoma annually.
For the high mortality and poor prognosis, much research has reported the following indicators of poor prognosis of LC[3-6]. Although liver biopsy and hepatic venous pressure gradient are currently recommended invasive indicators to predict the prognosis of LC[3,4], noninvasive prediction tools are commonly used in clinical work. Child Pugh and the model for end-stage liver disease (MELD), including creatinine, International Normalized Ratio and bilirubin are two of the most recommended forecasting tools in recent years[7]. Child Pugh scores included encephalopathy, ascites, urine volume, bilirubin, albumin and prothrombin time[5]. MELD scores included creatinine, international normalized ratio and bilirubin[6]. In our study, nutritional status (serum albumin), the occurrence of complications, and liver functions [aspartate aminotransferase (AST), alanine transaminase (ALT), bilirubin] were chosen as indicators to evaluate and predict the prognosis of LC. The disease progresses to deco
At present, the treatment of LC is mainly for the cause of intervention, lifestyle intervention, and the prevention and treatment of complications[15]. Toshikuni et al[16] mentioned that nutritional therapy for LC mainly included increasing dietary intake, the timing of food intake and branched-chain amino acids (BCAAs). In recent years, BCAAs have been found to have a unique effect on LC[17-24]. BCAAs are a set of essential amino acids including leucine, isoleucine and valine. It was considered that the end stage of liver disease is characterized by a low concentration of BCAAs and a high concentration of aromatic amino acids (phenylalanine, tyrosine and tryptophan)[21]. Suzuki et al[25] found that in patients with compensated cirrhosis, amino acid imbalance also occurs. Hyperinsulinemia and hyperammonemia are thought to lead to changes in the amino acid ratio in patients with LC[26,27]. The decrease in BCAA levels is considered to be a crucial pathogenic factor in LC[28]. Consequently, studies have reported that oral BCAAs can ameliorate patients’ nutritional status[17,19-21,23,24], reduce the incidence of complications[17,19] and ameliorate liver function[20,22,23]. Although BCAAs have been recommended in some guidelines[29,30], adverse reactions have been reported in recent studies and the effectiveness and safety of BCAAs are still controversial[31,32]. Kobayashi et al[31] considered that BCAAs have no inhibitory effect on the progression from compensatory cirrhosis to decompensated cirrhosis. In addition, the effect of BCAAs on the overall condition of cirrhosis is less well studied. Therefore, we conducted a meta-analysis of these studies to evaluate the effect of its application in LC.
This analysis’s ultimate goal was to demonstrate the patients’ treatment effect with LC using BCAAs.
Studies that conformed to the following criteria were included in our meta-analysis: (1) Randomized controlled studies; (2) the patient was diagnosed with cirrhosis; and (3) the intervention factor was BCAAs.
Studies were excluded if they met at least one of the following exclusion criteria: (1) The patient used BCAAs or other nutritional agents; (2) the patient had a high suspicion of liver neoplasms or had developed liver neoplasms; and (3) the patient had other major non-hepatic diseases.
In addition, filtering studies, abstracts, letters, reviews without original data, expert opinions, editorials, case reports and studies lacking control groups were excluded.
We selected articles from PubMed, Cochrane Library, Embase and Web of Science. The retrieval deadline was 1 October 2021, and there were no language restrictions set in the retrieval. Search terms were utilized in the title, abstract, mesh fields, and the following keywords and their combinations were applied: (((liver cirrhosis[MeSH Terms])) OR (((hepatic[All Fields])) OR (liver)) AND ((cirrhosis[All Fields])) OR (fibrosis)) AND ((Amino Acids, Branched-Chain [MeSH Terms])) OR (((((((Acids, Branched-Chain Amino[All Fields])) OR (Branched-Chain Amino Acids)) OR (Amino Acids, Branched Chain)) OR (Branched-Chain Amino Acid)) OR (Acid, Branched-Chain Amino)) OR (Amino Acid, Branched-Chain)) OR (Branched Chain Amino Acid).
The outcomes of the meta-analyses were the occurrence of complications, nutritional status and liver function. These data included albumin, alanine transaminase, aspartate aminotransferase, bilirubin, glucose and the occurrence of ascites, hepatic encephalopathy or esophagogastric varices.
Reviewers independently reviewed the quality and qualification of these studies according to the inclusion and exclusion criteria and the second reviewer (corresponding author) was allowed to intervene.
This meta-analysis used the Review Manager, version 5 statistical package (Cochrane Collaboration, Oxford, England) for analysis. A risk ratio (RR) value with a 95% confidence interval (CI) was used for binary variables. Mean difference (MD) or Std MD (SMD) values with a 95%CI are used for continuous variables. The overall effects were measured using a z score with a significance set at P < 0.05. If P ≥ 0.05, there was no significant difference in the results. In contrast, the results are significantly different. Statistical heterogeneity was evaluated using chi-square and I-square (I2) tests with significance set at P ≤ 0.1. Values of P ≤ 0.1 and I2 > 50% were considered to be significantly heterogeneous. For the articles with I2 > 0, we used the random effect model and sensitivity analysis or subgroup analysis, and for the articles with I2 = 0, we used the fixed-effect model.
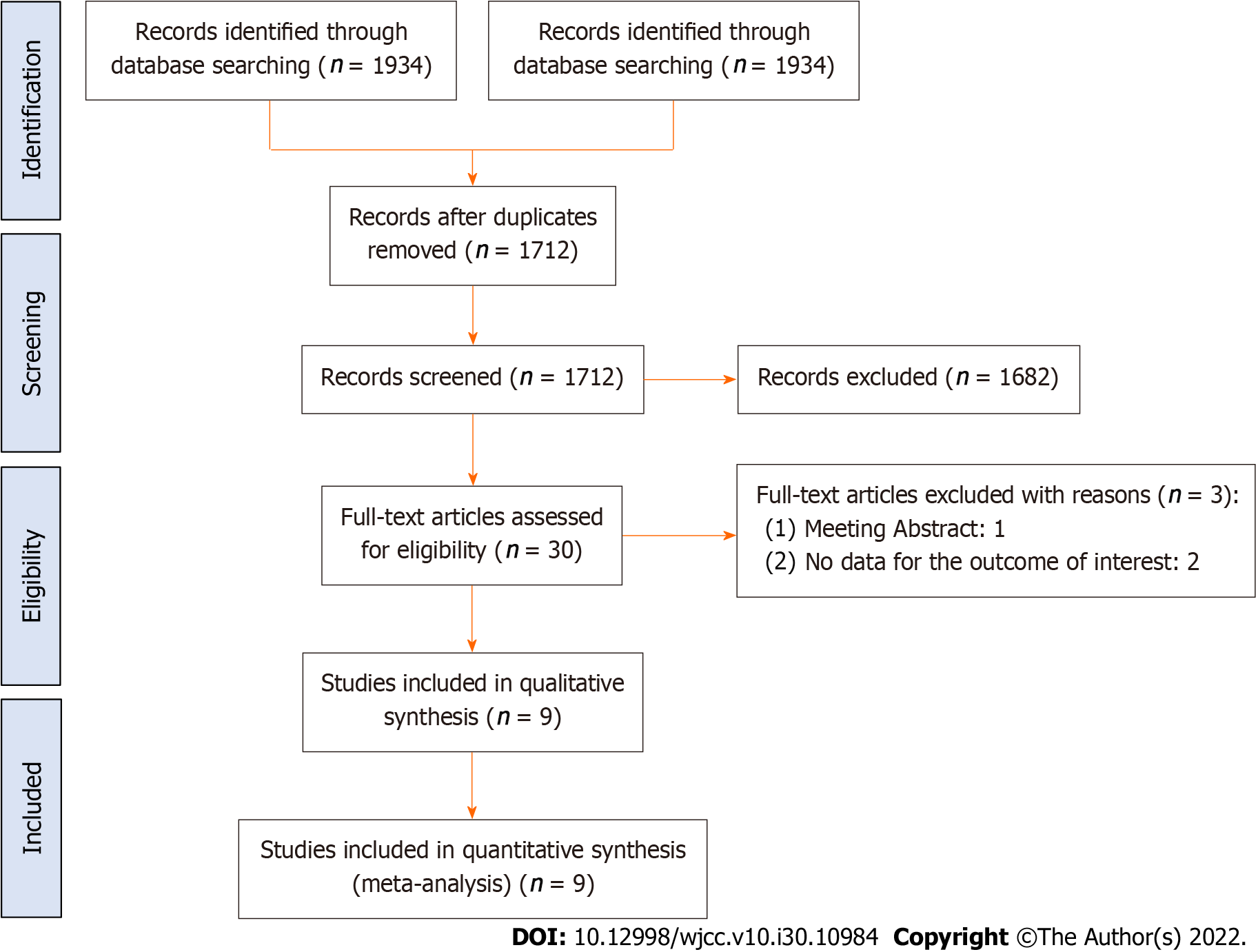
The analysis included nine studies that consisted of 1080 patients (554 in the BCAA groups and 526 in the control groups)[17,19-24,31,32]. The nine studies were randomized control trials (RCTs) (Figure 1). The characteristics of the studies included in the meta-analysis are shown in Table 1. The patient baseline characteristics of the studies included in the meta-analysis are shown in Table 2.
| Trail | Country | Group | n | Treatment time | Child grade | Mean age | M/F | Study type |
| Etsushi Kawamura, 2009 | Japan | BCAA | 27 | 12 mo | A | 62.70 ± 10.08 | 13/14 | RCT |
| Control | 23 | 62.30 ± 7.30 | 12/11 | |||||
| Muto Y, 2005 | Japan | BCAA | 314 | > 5 mo | A/B/C | 62 ± 8 | 147/167 | RCT |
| Control | 308 | 61 ± 9 | 147/161 | |||||
| Yutaka Nakaya, 2007 | Japan | BCAA | 19 | 3 mo | A/B | 67 ± 9 | 13/6 | RCT |
| Control | 19 | 67 ± 8 | 7/12 | |||||
| Les, 2011 | Spain | BCAA | 58 | 56 wk | A/B | 64.1 ± 10.4 | 45/13 | RCT |
| Control | 58 | 62.5 ± 10.4 | 43/15 | |||||
| Tangkijvanich P, 2000 | Thailand | BCAA | 15 | 4 wk | - | 53.07 ± 10.58 | 10/5 | RCT |
| Control | 15 | 53.20 ± 12.74 | 12/3 | |||||
| Marchesini G, 1990 | Italy | BCAA | 29 | 12 mo | - | 60 | 24/6 | RCT |
| Control | 32 | 60 | 27/7 | |||||
| Michel H, 1985 | France | BCAA | 36 | 5 d | A/B/C | 60.5 ± 11.5 | 25/11 | RCT |
| Control | 34 | 59.3 ± 12.8 | 24/10 | |||||
| Ruiz-Margain, A, 2017 | Mexico | BCAA, | 37 | 6 mo | A/B | 54.9 ± 10.3 | 6/31 | RCT |
| Control | 35 | 47.8 ± 14.6 | 8/27 | |||||
| Masahiro Kobayashi, 2008 | Japan | BCAA | 19 | 168 wk | A/B | 62.9 ± 5.7 | 19/0 | RCT |
| Control | 20 | 59.5 ± 7.2 | 20/0 |
| Trail | Group | Albumin in g/dL | Etiology as viral hepatitis/alcoholic/others | Ascites as absent/presence | Hepatic encephalopathy as absent/presence | Esophagogastric varices as absence/presence |
| Etsushi Kawamura, 2009 | BCAA | 3.70 ± 0.38 | 25/2/0 | 27/0 | 27/0 | 27/0 |
| Control | 3.81 ± 0.32 | 21/2/0 | 23/0 | 23/0 | 23/0 | |
| Muto, Y, 2005 | BCAA | 3.3 ± 0.3 | 266/20/28 | 240/74 | 287/27 | 144/170 |
| Control | 3.3 ± 0.3 | 237/32/39 | 241/66 | 295/12 | 121/187 | |
| Yutaka Nakaya, 2007 | BCAA | 3.0 ± 0.4 | - | 16/3 | - | - |
| Control | 3.0 ± 0.3 | - | 15/4 | - | - | |
| Les, 2011 | BCAA | 2.9 ± 0.6 | 24/17/17 | - | - | - |
| Control | 2.9 ± 0.5 | 18/25/15 | - | - | - | |
| Tangkijvanich P, 2000 | BCAA | 3.81 ± 0.86 | 6/6/2 | - | - | - |
| Control | 3.66 ± 0.75 | 7/6/2 | ||||
| Marchesini, G, 1990 | BCAA | 3.41 ± 0.45 | 9/20/1 | - | - | - |
| Control | 3.39 ± 0.43 | 7/16/1 | - | - | - | |
| Michel, H, 1985 | BCAA | 2.61 ± 0.10 | 4/28/4 | 10/26 | 0/36 | - |
| Control | 2.76 ± 0.08 | 4/29/1 | 11/23 | 0/34 | - | |
| Ruiz-Margain, A, 2017 | BCAA | 3.2 ± 0.6 | - | - | - | - |
| Control | 3.2 ± 0.7 | - | - | - | - | |
| Masahiro Kobayashi, 2008 | BCAA | 3.86 ± 0.26 | - | 19/0 | 19/0 | 9/10 |
| Control | 3.90 ± 0.33 | - | 20/0 | 20/0 | 10/10 |
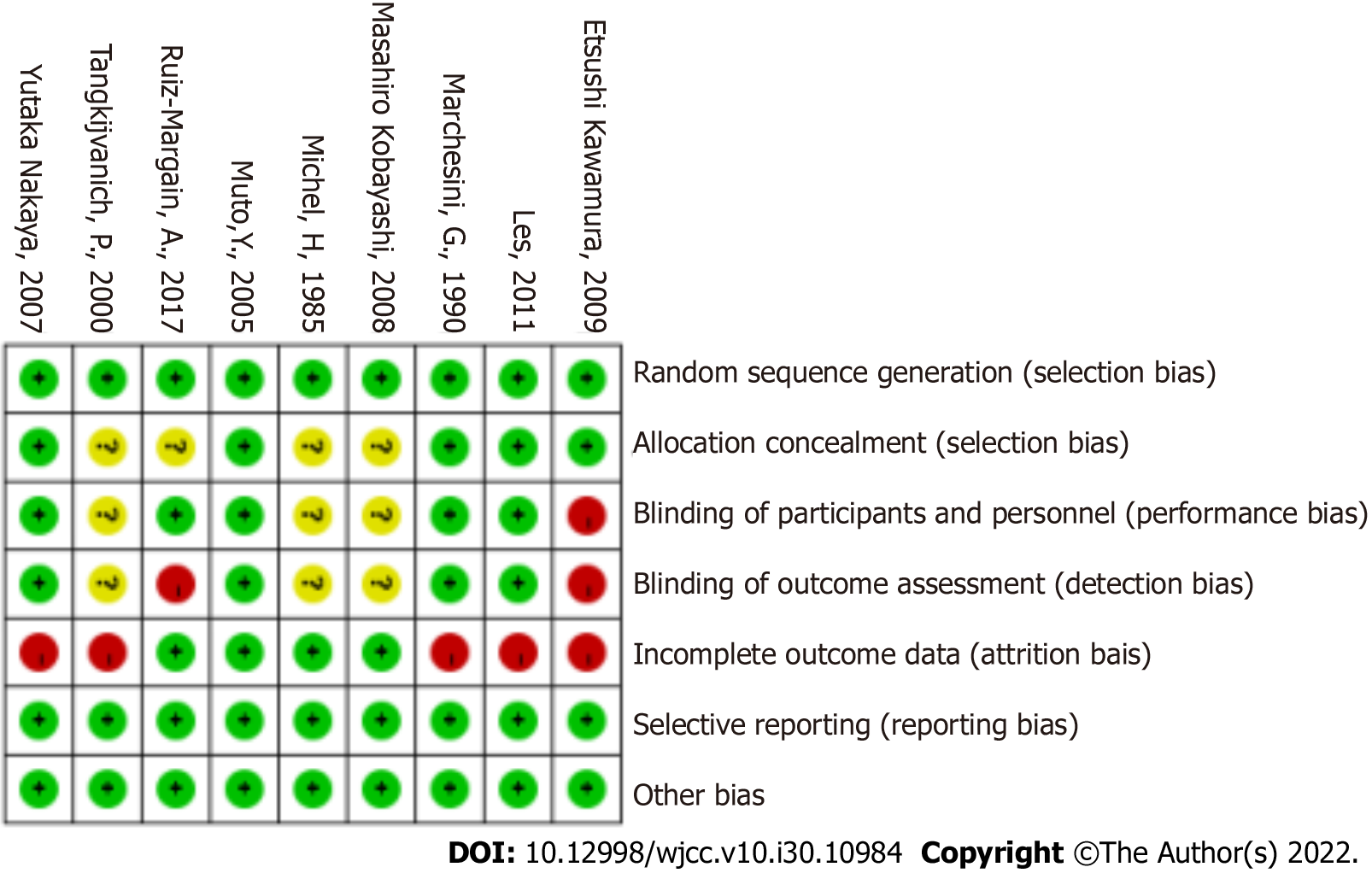
The quality of the studies was assessed using the risk of bias method recommended by the Cochrane Collaboration. Some trials had a high risk of bias (Figure 2)[22]. The main reason is that blind methods are not adopted and the inevitable loss of visits is inevitable.
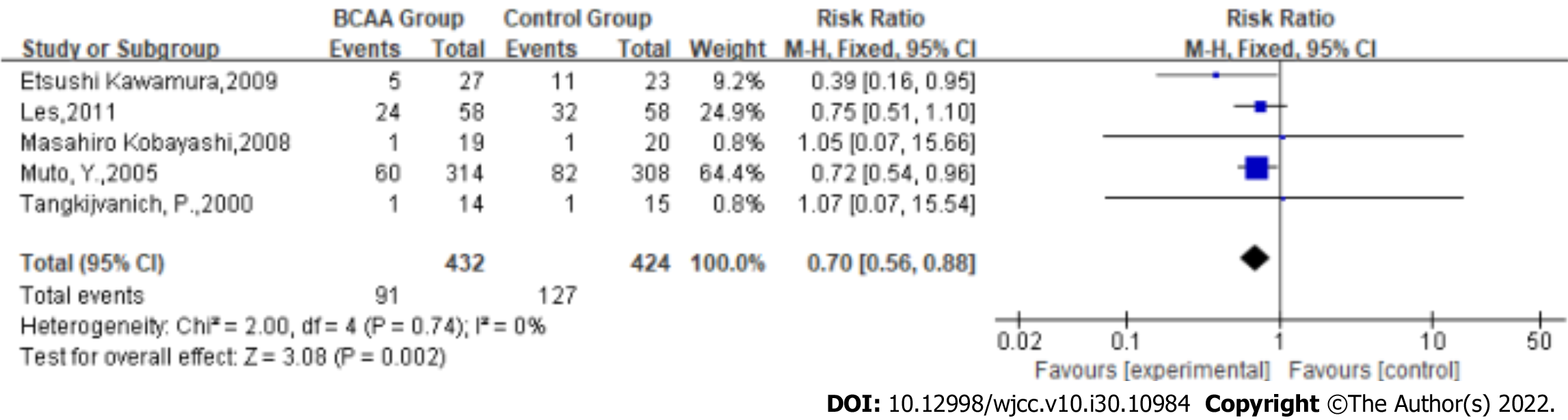
Complications rate: Statistical heterogeneity was low across the studies for the complication rate (Tau2 = 0.00; χ2 = 2.00, df = 4 (P = 0.74); I2 = 0%) by fitting a fixed-effects model. The complication rate of LC was significantly reduced in BCAA-treated patients (RR: 0.70, 95%CI: 0.56-0.88, P = 0.002, Figure 3).
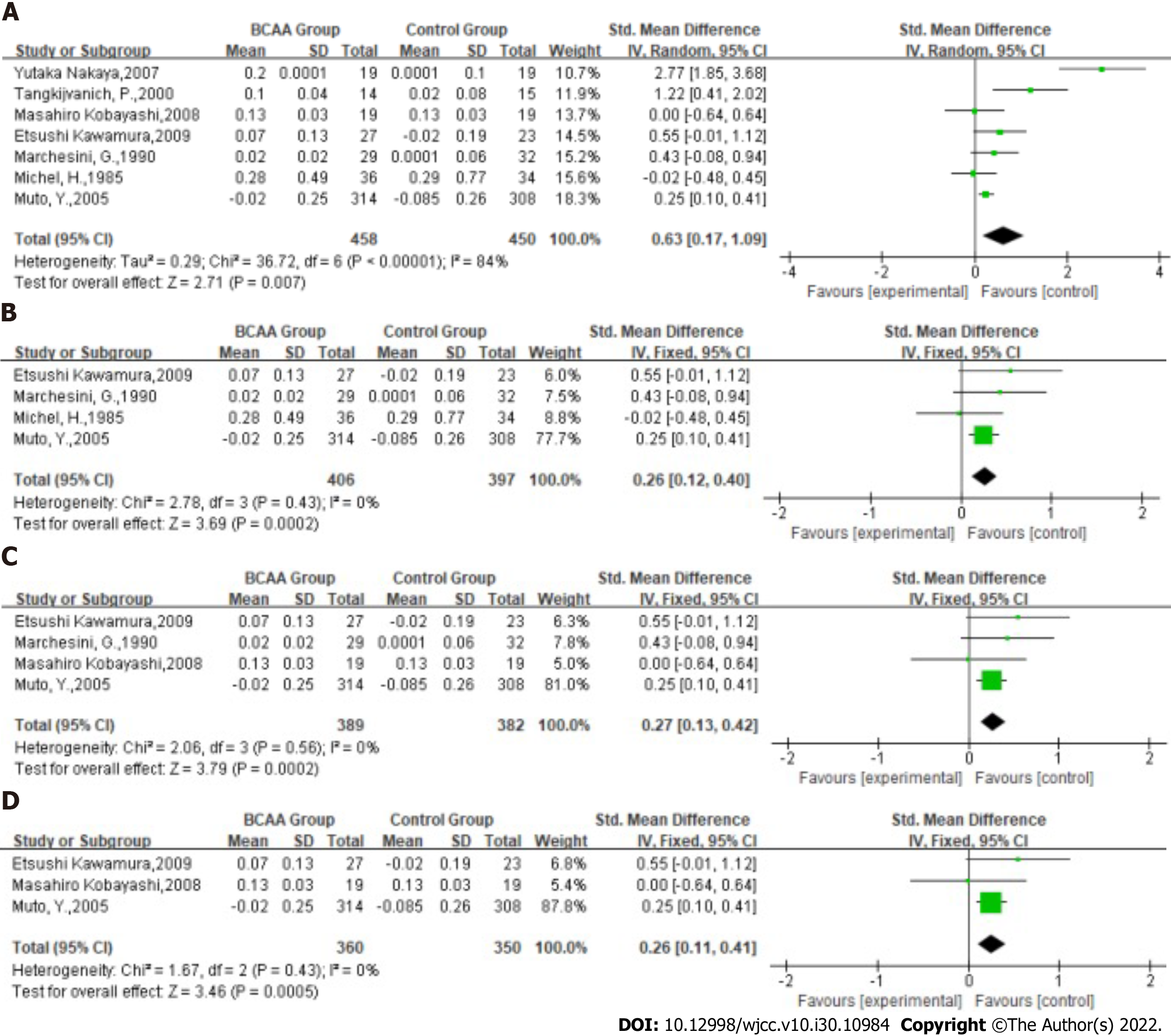
Nutritional status: Statistical heterogeneity was high across the studies for nutritional status [Tau2 = 0.29; χ2 = 36.72, df = 6 (P < 0.00001); I2 = 84%] by fitting a random-effects model. The albumin level of LC was significantly ameliorated in BCAA-treated patients (SMD: 0.63, 95%CI: 0.17-1.09, P = 0.007, Figure 4A). Nevertheless, they have slight heterogeneity.
Subgroup analysis was therefore performed according to the number of included patients and studies with a total number of patients less than 50 were excluded. Statistical heterogeneity was low across the studies for nutritional status [Tau2 = 0.00; χ2 = 2.78, df = 3 (P = 0.43); I2 = 0%] by fitting a fixed-effects model. The SMD of the fixed effect model analysis was 0.26 (95%CI: 0.12-0.40, P = 0.0002, Figure 4B).
Additional subgroup analysis included studies with treatment durations greater than 3 mo. Statistical heterogeneity was low across the studies for nutritional status [Tau2 = 0.00; χ2 = 2.06, df = 3 (P = 0.56); I2 = 0%] by fitting a fixed-effects model. The SMD of the fixed effect model analysis was 0.27 (95%CI: 0.13-0.42, P = 0.0002, Figure 4C).
The last subgroup analysis included studies in which the majority of patients had Child grade A or B and treatment duration was greater than 3 mo. Statistical heterogeneity was low across the studies for nutritional status [Tau2 = 0.00; χ2 = 1.67, df = 2 (P = 0.43); I2 = 0%] by fitting a fixed-effects model. The SMD of the fixed effect model analysis was 0.26 (95%CI: 0.11-0.41, P = 0.0005, Figure 4D).
These results further confirmed that BCAAs significantly ameliorate nutritional status in these patients.
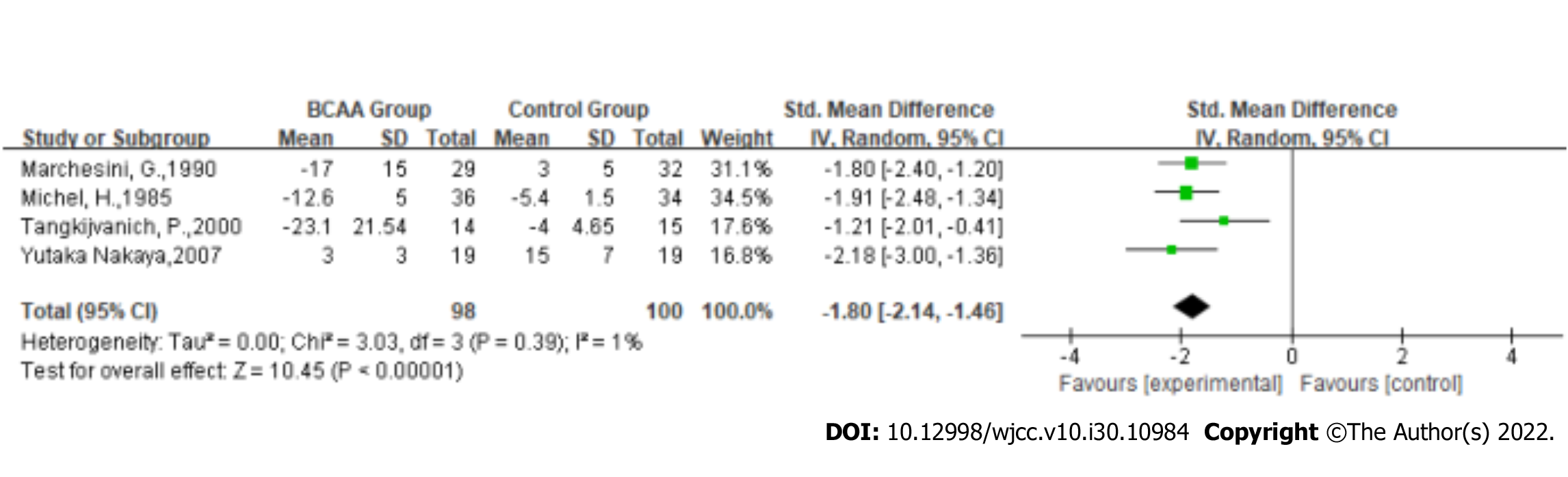
Aspartate aminotransferase (AST): Statistical heterogeneity was low across the studies for AST [Tau2 = 0.00; χ2 = 3.03, df = 3 (P = 0.39); I2 = 1%] by fitting a random-effects model. AST of LC was significantly ameliorated in BCAA treatment patients (SMD: -1.8, 95%CI: -2.14 to -1.46, P < 0.00001, Figure 5).
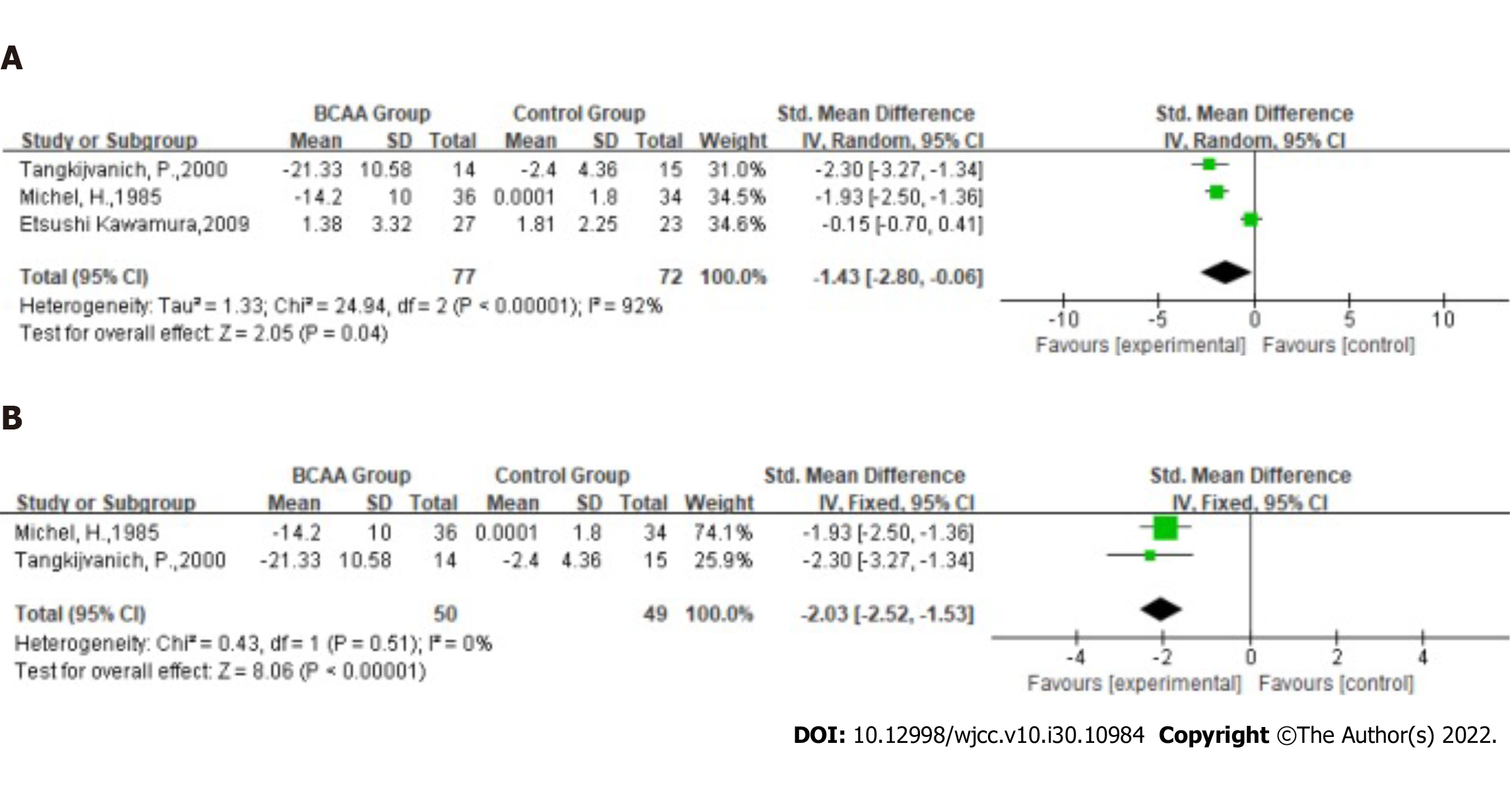
Alanine transaminase (ALT): Statistical heterogeneity was high across the studies for ALT (Tau2 = 1.33; χ2 = 24.94, df = 2 (P < 0.00001); I2 = 92%) by fitting a random-effects model. The ALT level in the LC was significantly ameliorated in BCAA-treated patients (SMD: -1.43, 95%CI: -2.80 to -0.06, P = 0.04, Figure 6A). Nevertheless, they have slight heterogeneity.
In the sensitivity analysis, the study by Kawamura et al[19] was excluded because the disease cause of most patients in this study was found to be a virus. However, the antiviral drugs available in 2009 temporarily failed to achieve good control of viremia, resulting in persistently high serum AST/ALT levels. Statistical heterogeneity was low across the studies for ALT [χ2 = 0.43, df = 1 (P = 0.51); I2 = 0%] by fitting a fixed-effects model. The ALT of LC was significantly ameliorated in BCAA-treated patients (SMD: -2.03, 95%CI: -2.52 to -1.53, P < 0.00001, Figure 6B).
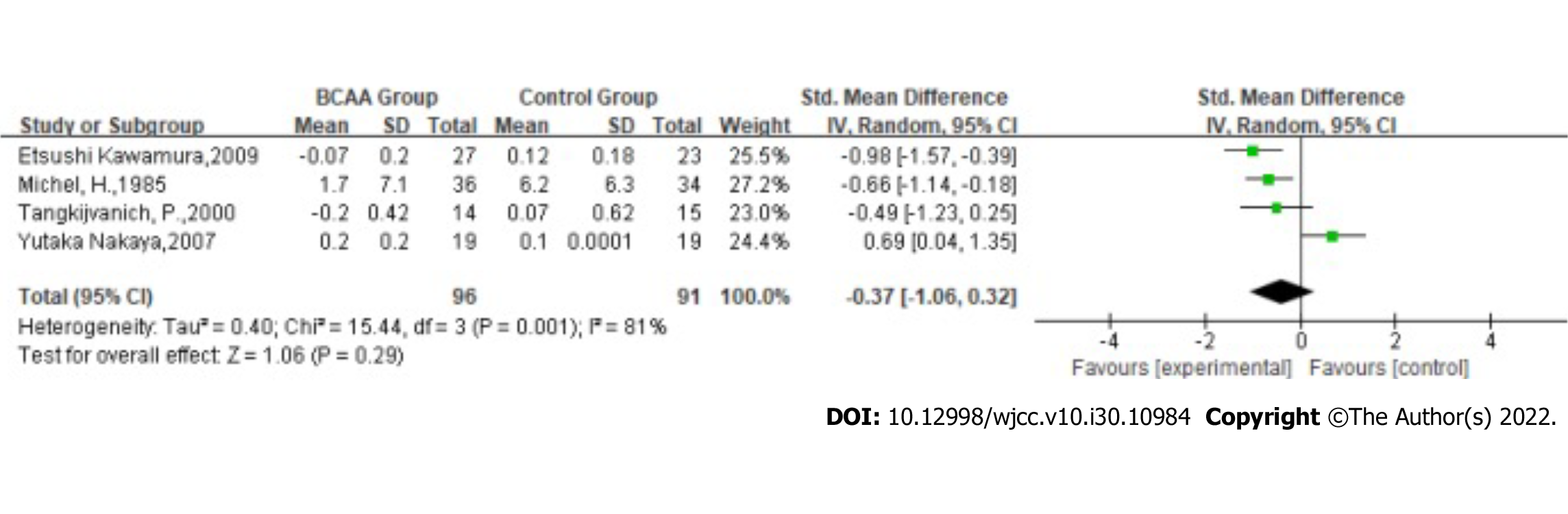
Bilirubin: Statistical heterogeneity was high across the studies for bilirubin [Tau2 = 0.40; χ2 = 15.44, df = 3 (P = 0.001); I2 = 81%] by fitting a random-effects model. The results showed that the effect of BCAAs on bilirubin in patients with LC was not statistically significant (SMD: -0.37, 95%CI: -1.06-0.32, P = 0.29, Figure 7).
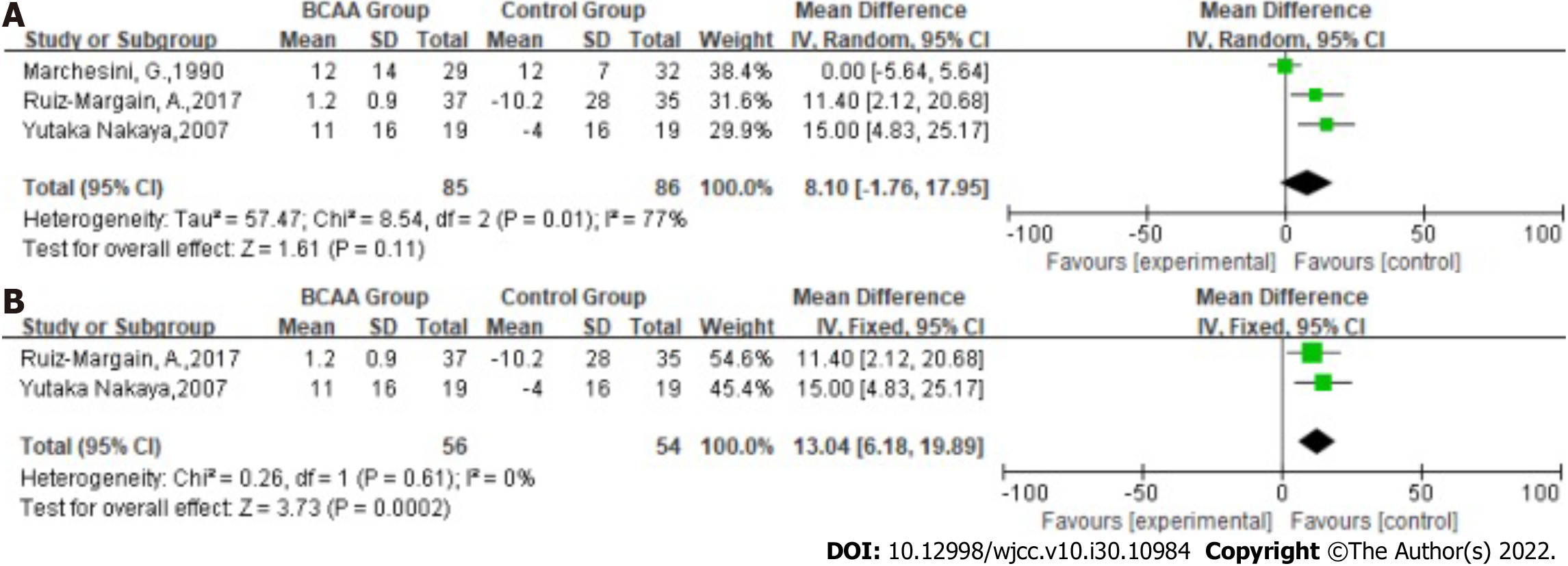
Statistical heterogeneity was high across the studies for glucose [Tau2 = 57.47; χ2 = 8.54, df = 2 (P = 0.01); I2 = 77%] by fitting a random-effects model. The results showed that the effect of BCAAs on glucose in patients with LC was not statistically significant (MD: 8.10, 95%CI: -1.76-17.95, P = 0.11, Figure 8A). Nevertheless, they have slight heterogeneity.
In the sensitivity analysis, the study by Marchesini et al[23]was excluded because the Child grade of patients included in the other two studies was graded A or B. Statistical heterogeneity was low across the studies for glucose [χ2 = 0.26, df = 1 (P = 0.61); I2 = 0%] by fitting a fixed-effects model. The Glucose of the LC was significantly increased in BCAA-treated patients (MD: 13.04, 95%CI: 6.81-19.89, P = 0.0002, Figure 8B).
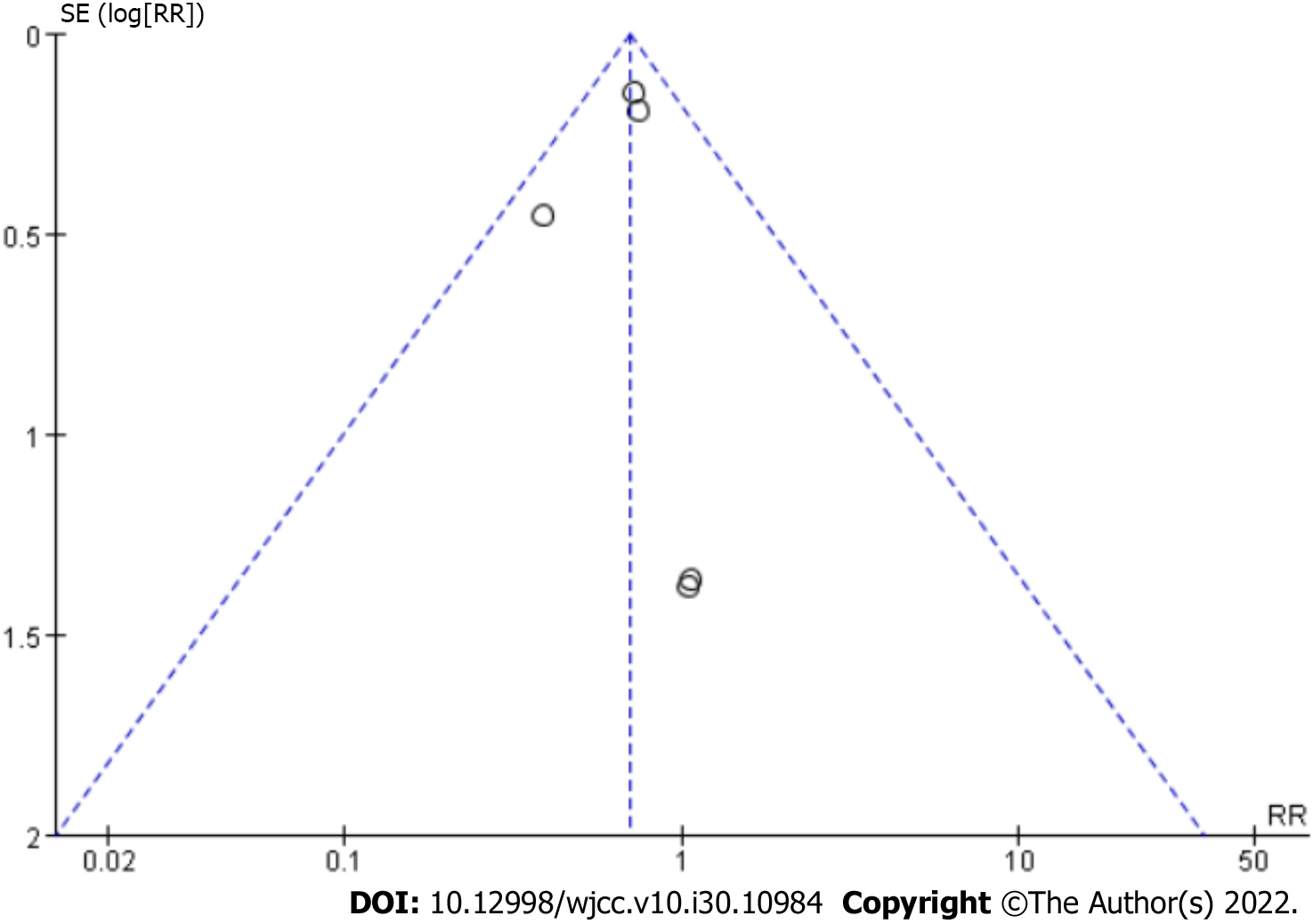
In our meta-analysis, we demonstrated that BCAAs reduce the occurrence of complications in patients with LC. Moreover, nutritional status was improved by BCAA treatment. There was no significant publication bias in the main outcome indicators (Figure 9).
The occurrence of LC complications indicates the decompensated stage of LC, and the prognosis is inferior. It is essential to delay the progression of LC. Most of the complications of LC were hepatic encephalopathy, ascites and esophageal varices in our analysis. Our study showed that BCAAs can significantly reduce the occurrence of complications. In our opinion, the mechanism by which BCAAs ameliorate hepatic encephalopathy mainly includes the following aspects. First, BCAAs can promote the metabolism of ammonia in muscle and reduce the level of blood ammonia in patients with hepatic encephalopathy[33]. Second, BCAAs can ameliorate albumin levels in patients with hepatic encephalopathy[34,35] thus increasing skeletal muscle weight. The increased muscle mass may increase extrahepatic ammonia detoxification[36]. Third, BCAAs may further enhance the detoxification of ammonia in skeletal muscle through the amidation process of glutamine synthesis[37]. Last, the addition of BCAAs reduces the brain efflux of aromatic amino acids across the blood brain barrier and the imbalance of dopamine, norepinephrine and serotonin synthesis[38]. There is a lack of detailed research on the mechanism by which BCAAs prevent other complications. Although many studies have shown that BCAAs are helpful for delaying LC[17,19], Michel et al[32] and Kobayashi et al[31] showed that BCAAs have no pronounced effect on the progression of LC. However, the subgroup analysis showed that BCAAs could inhibit the occurrence of hepatocellular carcinoma (HCC) in patients with compensated cirrhosis whose serum albumin level was less than 4 g/gL[31].
We also showed that BCAAs increased the nutritional status in patients with LC. The albumin level is an important indicator to evaluate the nutritional status of patients with LC. However, there is no further discussion on the correlation between albumin level and BCAA treatment. Some studies have shown that BCAAs can significantly improve the level of albumin[17,19,20]. In addition, many studies used mid-arm muscle circumference (MAMC) and skinfold thickness to determine patients’ nutritional level with LC[24]. These indexes are essential for evaluating the nutritional level of patients with LC. However, there is no meta-analysis on these indexes in this paper due to the lack of several homogeneous studies. Meanwhile, sarcopenia is a complication of LC and an independent risk factor for the disease[39,40]. Qiu et al[41] confirmed that hyperammonemia-induced autophagy is a potential cause of skeletal muscle loss in cirrhosis. The incidence of sarcopenia is increasing year by year. Kitajima et al[42] confirmed that BCAAs could prevent muscle loss. A large number of experiments are needed to explore the effect of BCAAs on patients with LC and sarcopenia.
Meanwhile, the decreases in AST and ALT were investigated after BCAA treatment. ALT and AST are enzymes of hepatic gluconeogenesis. When hepatocytes are damaged, they are released from the cells. The increase in AST and ALT levels can be used as a reference index of liver function damage, but other diseases may increase AST and ALT levels which need to be excluded[43]. The included studies did not adequately report data on INR, creatinine, resolution of ascites or remission of encephalopathy. Therefore, as a meta-analysis, the relationship between BCAAs and liver function could not be determined at this time. Additionally, with regard to bilirubin, the meta-analysis related to bilirubin was not statistically significant due to the heterogeneity of the included studies and inadequate sample size, and it is hoped that more studies with sufficient data size will be discussed further in the future.
The meta-analysis of the two studies included in this paper demonstrated that BCAAs might increase the glucose level of patients. BCAAs have a specific effect on blood glucose, which has been confirmed in many studies. A review has shown that BCAAs may increase insulin resistance. Elevated BCAAs stimulate mTORC1, a nutrient sensing complex, and IRS-1 serine phosphorylation results in insulin resistance and other metabolic disorders[44]. Simultaneously, it has been widely confirmed that BCAAs upregulate glucose transporters and activate insulin secretion[45-47]. Some studies have shown that BCAAs may induce insulin resistance by inhibiting insulin signaling[48,49]. Recently, a clinical trial showed that BCAAs can induce insulin resistance through mTOR activation[50]. In contrast, it is still reported that BCAAs can decrease insulin resistance[51,52]. Despite the controversy, we recommend, based on our results, that we still need to adhere to monitoring the changes in blood glucose and be alert to endocrine disorders when taking BCAAs. In addition, it has been reported that supplementation with BCAAs may lead to an increase in ammonia produced by glutamine decomposition in the intestine and kidney due to the stimulating effect of BCAAs on glutamine synthesis, which may harm the development of hepatic encephalopathy. Therefore, BCAAs and α-ketoglutarate or phenyl butyric acid should be used simultaneously to treat hepatic encephalopathy[53].
Our study has some limitations. First, the article only included RCT research, excluding non-RCT research. Second, the article aims to uneven the population areas and lacks targeted research for a specific area. There may be deviations in treatment. Third, because of the lack of high-quality literature in this area, we only selected the articles that met the requirements after excluding the quality problems and needed large-scale experiments to confirm our ideas further.
Finally, our results provide a reference for the nutritional treatment of patients with LC which is helpful for clinical and nursing applications. We hope that there will be better nutritional support treatment plans for LC patients in the future.
Branched-chain amino acids could reduce the incidence of complications in patients with liver cirrhosis and ameliorate nutritional status.
Liver cirrhosis (LC) mainly includes increasing dietary intake, food intake time and branched-chain amino acids (BCAAs). Despite the recommendation of BCAAs in some guidelines, adverse effects have been reported in studies so the efficacy and safety of BCAAs remain controversial.
We performed a meta-analysis to determine the effects of BCAAs in patients with LC.
To determine the effects of BCAAs in patients with LC.
Nine studies were finally included. The primary outcome was complications of LC. The secondary outcomes were nutritional status and liver function. This meta-analysis used the Review Manager, version 5 statistical package (Cochrane Collaboration, Oxford, England) for analysis.
BCAAs reduced the rate of complications in LC patients (Risk ratio: 0.70, 95% confidence interval (CI): 0.56-0.88, P = 0.002) and improved patients’ albumin levels [std mean difference SMD: 0.26, 95%CI: 0.12-0.40, P = 0.0002]. Meanwhile, BCAAs significantly ameliorated the levels of alanine transaminase (SMD: -2.03, 95%CI: -2.52 to -1.53, P < 0.00001) and aspartate aminotransferase (SMD: -1.8, 95%CI: -2.14 to -1.46, P < 0.00001). Meanwhile, glucose in the LC was significantly increased in BCAA-treated patients (MD: 13.04, 95%CI: 6.81-19.89, P = 0.0002).
Branched-chain amino acids could reduce the incidence of complications in patients with liver cirrhosis and ameliorate nutritional status.
Our results provide a reference for the nutritional treatment of patients with LC which is helpful for clinical and nursing applications. We hope that there will be better nutritional support treatment plans for LC patients in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen GX, United States; Ielasi L, Italy S-Editor: Chen YL L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1310] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 2. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2283] [Article Influence: 380.5] [Reference Citation Analysis (0)] |
| 3. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 807] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 4. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1580] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 5. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5730] [Article Influence: 110.2] [Reference Citation Analysis (2)] |
| 6. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 7. | Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, Wood NL, Gentry SE, Kwong AJ. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021;161:1887-1895.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 337] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 8. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2129] [Article Influence: 112.1] [Reference Citation Analysis (3)] |
| 9. | Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Sarcopenic Obesity in Liver Cirrhosis: Possible Mechanism and Clinical Impact. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, Caregaro L. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 340] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Kalman DR, Saltzman JR. Nutrition status predicts survival in cirrhosis. Nutr Rev. 1996;54:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | McCullough AJ, Mullen KD, Smanik EJ, Tabbaa M, Szauter K. Nutritional therapy and liver disease. Gastroenterol Clin North Am. 1989;18:619-643. [PubMed] |
| 13. | Sam J, Nguyen GC. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009;29:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Romanelli RG, Stasi C. Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr Drug Targets. 2016;17:1804-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Toshikuni N, Arisawa T, Tsutsumi M. Nutrition and exercise in the management of liver cirrhosis. World J Gastroenterol. 2014;20:7286-7297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (6)] |
| 17. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, Kumada H; Long-Term Survival Study Group. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 18. | Nakaya Y, Harada N, Kakui S, Okada K, Takahashi A, Inoi J, Ito S. Severe catabolic state after prolonged fasting in cirrhotic patients: effect of oral branched-chain amino-acid-enriched nutrient mixture. J Gastroenterol. 2002;37:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Kawamura E, Habu D, Morikawa H, Enomoto M, Kawabe J, Tamori A, Sakaguchi H, Saeki S, Kawada N, Shiomi S. A randomized pilot trial of oral branched-chain amino acids in early cirrhosis: validation using prognostic markers for pre-liver transplant status. Liver Transpl. 2009;15:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H, Tsubouchi H, Kato S, Kaito M, Watanabe A, Habu D, Ito S, Ishikawa T, Kawamura N, Arakawa Y; Hepatic Nutritional Therapy (HNT) Study Group. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M, Soriano G, Vila C, Esteban R, Córdoba J. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Tangkijvanich P, Mahachai V, Wittayalertpanya S, Ariyawongsopon V, Isarasena S. Short-term effects of branched-chain amino acids on liver function tests in cirrhotic patients. Southeast Asian J Trop Med Public Health. 2000;31:152-157. [PubMed] |
| 23. | Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, Martines D, Abbiati R. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol. 1990;11:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 127] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Ruiz-Margáin A, Macías-Rodríguez RU, Ríos-Torres SL, Román-Calleja BM, Méndez-Guerrero O, Rodríguez-Córdova P, Torre A. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev Gastroenterol Mex (Engl Ed). 2018;83:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Suzuki K, Suzuki K, Koizumi K, Ichimura H, Oka S, Takada H, Kuwayama H. Measurement of serum branched-chain amino acids to tyrosine ratio level is useful in a prediction of a change of serum albumin level in chronic liver disease. Hepatol Res. 2008;38:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Marchesini G, Forlani G, Zoli M, Angiolini A, Scolari MP, Bianchi FB, Pisi E. Insulin and glucagon levels in liver cirrhosis. Relationship with plasma amino acid imbalance of chronic hepatic encephalopathy. Dig Dis Sci. 1979;24:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Hayashi M, Ohnishi H, Kawade Y, Muto Y, Takahashi Y. Augmented utilization of branched-chain amino acids by skeletal muscle in decompensated liver cirrhosis in special relation to ammonia detoxication. Gastroenterol Jpn. 1981;16:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Holeček M, Mráz J, Tilšer I. Plasma amino acids in four models of experimental liver injury in rats. Amino Acids. 1996;10:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26:1SA-138SA. [PubMed] |
| 30. | Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine), Ferenci P, Holm E, Vom Dahl S, Müller MJ, Nolte W; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 31. | Kobayashi M, Ikeda K, Arase Y, Suzuki Y, Suzuki F, Akuta N, Hosaka T, Murashima N, Saitoh S, Someya T, Tsubota A, Kumada H. Inhibitory effect of branched-chain amino acid granules on progression of compensated liver cirrhosis due to hepatitis C virus. J Gastroenterol. 2008;43:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Michel H, Bories P, Aubin JP, Pomier-Layrargues G, Bauret P, Bellet-Herman H. Treatment of acute hepatic encephalopathy in cirrhotics with a branched-chain amino acids enriched versus a conventional amino acids mixture. A controlled study of 70 patients. Liver. 1985;5:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Dam G, Keiding S, Munk OL, Ott P, Buhl M, Vilstrup H, Bak LK, Waagepetersen HS, Schousboe A, Møller N, Sørensen M. Branched-chain amino acids increase arterial blood ammonia in spite of enhanced intrinsic muscle ammonia metabolism in patients with cirrhosis and healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2011;301:G269-G277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 35. | Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013;28:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. 2002;36:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 810] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 38. | Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173, 173.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 40. | Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 41. | Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, McDonald C, Almasan A, Hazen SL, Naga Prasad SV, Dasarathy S. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983-E993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Kitajima Y, Takahashi H, Akiyama T, Murayama K, Iwane S, Kuwashiro T, Tanaka K, Kawazoe S, Ono N, Eguchi T, Anzai K, Eguchi Y. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol. 2018;53:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 43. | Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Yoon MS. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 45. | Floyd JC Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 469] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Nishitani S, Takehana K, Fujitani S, Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1292-G1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Zhang S, Zeng X, Ren M, Mao X, Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. 2017;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 396] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 48. | Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 539] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 49. | Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 50. | White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, Trimmer JK, Brosnan MJ, Rolph TP, Newgard CB. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab. 2016;5:538-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 51. | Tabaru A, Shirohara H, Moriyama A, Otsuki M. Effects of branched-chain-enriched amino acid solution on insulin and glucagon secretion and blood glucose level in liver cirrhosis. Scand J Gastroenterol. 1998;33:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med. 2008;22:105-112. [PubMed] |
| 53. | Holecek M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition. 2013;29:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |