Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.10956
Peer-review started: May 5, 2022
First decision: July 29, 2022
Revised: August 9, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: October 26, 2022
Processing time: 168 Days and 6.5 Hours
Cardiogenic shock continues to be a highly morbid complication that affects around 7%-10% of patients with acute myocardial infarction or heart failure. Similarly, obesity has become a worldwide epidemic.
To analyze the impact of higher body mass index (BMI) on outcomes of patients with cardiogenic shock.
A systematic and comprehensive search was undertaken on the electronic databases of PubMed, Embase, ScienceDirect, CENTRAL, and Google Scholar for all types of studies comparing mortality outcomes of patients with cardiogenic shock based on BMI. All studies defined overweight or obese patients based on the World Health Organization BMI criteria. The data were then extracted and assessed on the basis of the Reference Citation Analysis (https://www.referencecitationanalysis.com/).
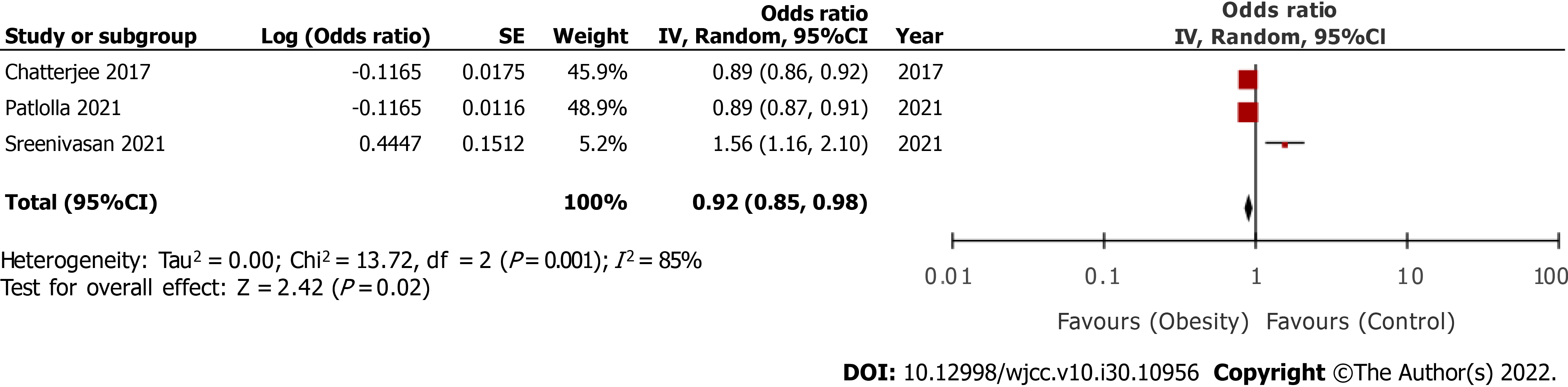
Five studies were included. On pooled analysis of multivariable-adjusted ratios, we noted a statistically significantly reduced risk of mortality in overweight/ obese vs normal patients (three studies; odds ratio [OR] = 0.92, 95% confidence interval [CI]: 0.85-0.98, I2 = 85%). On meta-analysis, we noted that crude mortality rates did not significantly differ between overweight/obese and normal patients after cardiogenic shock (OR = 0.95, 95%CI: 0.79-1.15, I2 = 99%). The results were not stable on sensitivity analysis and were associated with substantial heterogeneity.
Current evidence on the association between overweight/obesity and mortality after cardiogenic shock is scarce and conflicting. The obesity paradox might exist in patients with cardiogenic shock but could be confounded by the use of mechanical circulatory support. There is a need for further studies to clarify this relationship.
Core Tip: Cardiogenic shock continues to be a highly morbid complication that affects around 7%-10% of patients and similarly, obesity is now prevalent around the globe. We reviewed data from five studies to assess the impact of obesity on outcomes of cardiogenic shock. Pooled analysis of adjusted data indicated that overweight/obese was associated with a reduced risk of mortality vs normal patients but the same relationship was not noted in the analysis of crude mortality rates. Thus, current evidence on the association between overweight/obesity and mortality after cardiogenic shock is scarce and conflicting and there is a need for further studies.
- Citation: Tao WX, Qian GY, Li HD, Su F, Wang Z. Body mass index and outcomes of patients with cardiogenic shock: A systematic review and meta-analysis. World J Clin Cases 2022; 10(30): 10956-10966
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/10956.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.10956
Obesity is a recognized global health problem that has significantly burdened the entire healthcare system[1]. The epidemic of obesity has touched countries across the globe and more than 2 billion people are affected by it[2]. According to estimates, the prevalence of obesity has tripled since 1975 and more than 39% of adults older than 18 years were overweight in 2016[3]. The World Health Or
The methodology of our review was based on reporting guidelines of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses)[14]. The protocol of the review was prospectively registered on PROSPERO (No. CRD42021274841).
A systematic and comprehensive search was undertaken on the electronic databases of PubMed, Embase, ScienceDirect, and CENTRAL. Google Scholar was used to search the gray literature, but only for the first 200 results of each search query. To minimize single reviewer bias, two authors separately explored the databases. The search limits were set from the time of inception of databases up to 25th August 2021. Search terms included were: "obese", “obesity”, "overweight", "body mass index", and “cardiogenic shock”. Further details of the search strategy which was common for all databases are presented in Supplementary Table 1. Reference Citation Analysis (https://www.referencecitationanalysis.com/) was used to supplement the search. After the initial search, the results were deduplicated and the remaining articles were assessed by their titles and abstracts. We identified studies relevant to the review and extracted their full texts. The two reviewers independently evaluated these studies for final inclusion in the review. Any discrepancies in study selection were resolved by consensus. In the end, manual scoping of the reference list of included studies was carried out for any missed references.
The inclusion criteria were: (1) All types of studies comparing mortality rates of patients with cardiogenic shock based on BMI; (2) Studies that clearly defined overweight or obese patients based on the WHO BMI criteria (i.e., overweight > 25 kg/m2 and obese > 30 kg/m2) and compared outcomes with normal BMI patients; and (3) Language of publication should have been English. We excluded the following studies: (1) Studies including less than 50 patients; (2) Studies not reporting mortality outcomes; (3) Non-comparative studies; and (4) Studies reporting duplicate data. If the same database was used by two studies, we judged the period of overlap. In case of partial overlap, the study was included and the strength of the results was analyzed by a sensitivity analysis.
Two authors independently extracted the following data: Author details, publication year, study type, study location, BMI definition, primary diagnosis, sample size, demographic details, comorbidities (diabetes mellitus, hypertension, chronic kidney disease, dyslipidemia, and cardiovascular disease), revascularization details, use of mechanical circulatory support (MCS), and study outcomes. The primary outcome of the study was early mortality defined as in-hospital or 30-d mortality. The methodological quality of studies was assessed using the Newcastle-Ottawa scale[15]. It was conducted by two authors independent of each other. Any disagreements were solved by a discussion. Studies were assessed for selection of study population, comparability, and outcomes, with each domain being awarded a maximum of four, two, and three points, respectively. The maximum score which can be awarded was nine. Studies with a score of 9 points, 7-8 points, and 6 points and below were considered to have a low, moderate, and high risk of bias, respectively.
The meta-analysis was performed using “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre [Cochrane Collaboration], Copenhagen, Denmark; 2014). We extracted multivariable-adjusted odds ratios (ORs), risk ratios (RRs), or hazard ratios (HRs) on mortality rates and pooled them using the generic inverse variance function of RevMan. The final effect size was calculated as OR with 95% confidence interval (CI). Crude mortality rates were also extracted from the included studies and pooled OR was generated. All meta-analyses were conducted using the random-effects model. Heterogeneity was assessed using the I2 statistic. I2 values of 25%-50% represented low, values of 50%-75% medium, and more than 75% represented substantial heterogeneity. Funnel plots were not used to assess publication bias as less than ten studies were available for each meta-analysis. A sensitivity analysis was carried out to assess the contribution of each study to the pooled estimate by removing one study at a time and recalculating the pooled effect estimates for the remaining studies.
The search strategy and the number of records at each stage are presented in Figure 1. Based on the screening criteria, a total of five studies were included in this systematic review and meta-analysis[16-20]. Details of included studies are presented in Table 1. Three studies[16,17,20] were conducted in the United States, one in Denmark[18], and one in Pakistan[19]. All, except for one[19], were retrospective cohort studies. The primary diagnosis was AMI in all studies but the study of Sreenivasan et al[16] also included patients with heart failure. Two studies[17,20] used the same “National Inpatient database” from the United States with a partial overlap of data. Patlolla et al[17] and Chatterjee et al[20] used the database from 2008 to 2017 and 2004 to 2013, respectively. Thus, an overlap of six years was noted in these studies, albeit with a minor difference. Patlolla et al[17] reported combined data of overweight and obese patients whereas Chatterjee et al[20] classified their sample as obese and non-obese only. All the studies used the WHO classification of overweight and obesity. Two studies[16,17] additionally classified obesity as mild, moderate, and severe. However, for the meta-analysis, all groups were combined into a single group of obese patients. The mean age of the patients was above 55 years in the majority of studies. The percentage of patients undergoing revascularization varied across the included studies. In the study of Hermansen et al[18], all patients underwent percutaneous coronary intervention and none underwent coronary artery bypass grafting (CABG). In general, fewer patients underwent CABG as compared to percutaneous interventions in the remaining studies across obese and non-obese groups. Two studies did not report data on the percentage of patients receiving MCS[19,20]. In the study of Sreenivasan et al[16], all patients received MCS while in the remaining two studies, the percentage varied from 15% to 49% across the study sub-groups. Two studies reported mortality outcomes within 30 d while the remaining reported in-hospital outcomes[16,18].
| Ref. | Location | Type | Primary diagnosis | Groups | Definition as per BMI (kg/m2) | Sample size | Age (yr) | Male gender (%) | Smokers (%) | DM (%) | HTN (%) | CKD (%) | DL (%) | PCI (%) | CABG (%) | MCS (%) | Follow-up |
| Sreenivasan et al[16], 2021 | United States | R | AMI or HF | Severe obesity | > 40 | 8782 | 59.9 | 52.3 | NR | 64.4 | 72.2 | 41.3 | NR | 53.2 | 25.8 | 100 | 30-d |
| Moderate obesity | 35-39.9 | 6862 | 60.9 | 68.9 | 66 | 77.6 | 38.4 | 47.1 | 31 | 100 | |||||||
| Mild obesity | 30-34.9 | 10880 | 62.9 | 71.2 | 59.1 | 75.4 | 33.1 | 54.4 | 32.2 | 100 | |||||||
| Normal | 20-29.9 | 7111 | 65.9 | 71.6 | 45.4 | 65.5 | 39.8 | 47 | 27.5 | 100 | |||||||
| Underweight | < 19.9 | 1920 | 65.6 | 67.9 | 30.9 | 54 | 30.9 | 37.3 | 14.7 | 100 | |||||||
| Patlolla et al[17], 2021 | United States | R | AMI | Overweight/Obese | > 24.9 | 46675 | 63.8 | 60.3 | NR | NR | NR | NR | NR | 53.6 | 24.9 | 49 | In-hospital |
| Normal | 19.9-24.9 | 290333 | 69 | 64.5 | 53.6 | 16.3 | 45.7 | ||||||||||
| Underweight | < 19.9 | 2356 | 73.7 | 49.4 | 39.8 | 12.2 | 27.8 | ||||||||||
| Hermansen et al[18], 2021 | Denmark | R | AMI | Moderate/Severe | ≥ 35 | 42 | 63 | 69 | 68 | 43 | 75 | NR | 55 | 100 | 0 | 17 | 30-d |
| Obesity | 30-34.9 | 131 | 64 | 80 | 82 | 21 | 54 | 34 | 100 | 0 | 15 | ||||||
| Mild obesity | 25-29.9 | 391 | 65.2 | 82 | 79 | 21 | 55 | 33 | 100 | 0 | 21 | ||||||
| Overweight | < 25 | 453 | 66.1 | 75 | 74 | 13 | 42 | 29 | 100 | 0 | 16 | ||||||
| Hashmi et al[19], 2018 | Pakistan | P | AMI | Obese | ≥ 30 | 137 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | In-hospital |
| Normal | < 30 | 214 | |||||||||||||||
| Chatterjee et al[20], 2017 | United States | R | AMI | Obese | ≥ 30 | 25835 | 63.1 | 58.2 | 34.3 | 45.2 | 68.8 | 23.5 | 54.8 | 50.9 | 19.6 | NR | In-hospital |
| Normal | < 30 | 265059 | 69.4 | 62.3 | 24 | 24.4 | 50.6 | 18.9 | 33.8 | 47.9 | 13.6 |
Amongst the included studies, three[16,17,20] reported multivariable-adjusted ratios on the relationship between overweight/obesity and early mortality. On pooled analysis, we noted ae statistically significantly reduced risk of early mortality after cardiogenic shock in overweight/obese vs normal patients (OR = 0.92, 95%CI: 0.85-0.98) (Figure 2). There was significantly high heterogeneity in the meta-analysis (I2 = 85%). Given the high heterogeneity, we conducted a sensitivity analysis by excluding one study at a time and recalculating the effect size. Results are presented in Table 2. In the exclusion of the study of Patlolla et al[17] and Chatterjee et al[20], the results indicated no difference in the risk of mortality in overweight/obese vs normal patients. Second, we also extracted crude early mortality rates and pooled them in a meta-analysis. Including data from all five studies[16-20], we noted that crude mortality rates did not significantly differ between overweight/obese and normal patients after cardiogenic shock (OR = 0.95, 95%CI: 0.79-1.15) (Figure 3). There was significantly high heterogeneity in the meta-analysis (I2 = 99%). On sensitivity analysis (Table 2), we noted that the exclusion of the study of Sreenivasan et al[16] changed the significance of the results with a reduced risk of mortality in overweight/obese patients as compared to normal patients. A similar tendency was noted in the exclusion of the study of Hashmi et al[19].
| Excluded study | Odds ratio |
| Adjusted mortality rates | |
| Sreenivasan et al[16], 2021 | 0.89 95%CI: 0.87, 0.91 I2 = 0% |
| Patlolla et al[17], 2021 | 1.15 95%CI: 0.67, 2.00 I2 = 93% |
| Chatterjee et al[20], 2017 | 1.15 95%CI: 0.67, 2.00 I2 = 93% |
| Crude mortality rates | |
| Sreenivasan et al[16], 2021 | 0.79 95%CI: 0.70, 0.89 I2 = 95% |
| Patlolla et al[17], 2021 | 1.12 95%CI: 0.76, 1.67 I2 = 99% |
| Hermansen et al[18], 2021 | 0.98 95%CI: 0.80, 1.20 I2 = 99% |
| Hashmi et al[19], 2018 | 0.83 95%CI: 0.69, 1.00 I2 = 99% |
| Chatterjee et al[20], 2017 | 1.13 95%CI: 0.80, 1.60 I2 = 99% |
We were unable to conduct any subgroup analysis to explore the source of high heterogeneity in the included studies due to the limited number of the included studies. However, a few studies conducted subgroup analysis in their respective cohorts and their results are descriptively presented in Table 3. Sreenivasan et al[16] further compared outcomes of obese and non-obese patients based on the primary diagnosis (acute AMI or heart failure) and age (< 60 years and ≥ 60 years). On the other hand, Chatterjee et al[20] conducted a subgroup analysis based on the type of AMI (ST-elevated and non-ST elevated) and the use of revascularization.
| Ref. | Subgroups | Result |
| Sreenivasan et al[16], 2021 | Acute MI only | Significantly higher mortality in severely obese patients as compared to normal patients |
| Acute HF only | Significantly higher mortality in severely obese patients as compared to normal patients | |
| Age < 60 years | Significantly higher mortality in severely obese patients as compared to normal patients | |
| Age ≥ 60 years | Significantly higher mortality in severely obese patients as compared to normal patients | |
| Chatterjee et al[20], 2017 | ST-elevated MI | No statistically significant difference in mortality between obese and normal patients |
| Non-ST elevated MI | Significantly lower morality in obese as compared to normal patients | |
| Revascularization group | Significantly lower morality in obese as compared to normal patients | |
| Non-revascularization group | No statistically significant difference in mortality between obese and normal patients |
The risk of bias analysis of included studies is presented in Table 4. Four studies[16,17,19,20] received a score of 7 while one study[18] received a score of 5.
| Ref. | Selection | Comparability | Outcome | Total | |||||
| Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest | Basis of the design or analysis | Assessment of outcome | Follow-up long enough for outcomes | Adequate follow up | ||
| Sreenivasan et al[16], 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 7 |
| Patlolla et al[17], 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 7 |
| Hermansen et al[18], 2021 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Hashmi et al[19], 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 7 |
| Chatterjee et al[20], 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 7 |
Obesity has been a well-recognized risk factor for a wide spectrum of cerebrovascular and cardiovascular diseases. Higher body fat increases the bulk of atherosclerotic plaques, which leads to plaque instability. It also generates a low-grade generalized inflammatory state which increases pro-inflammatory cytokines like C-reactive protein and interleukins[21]. Indeed, recent research suggests that anti-inflammatory therapies may reduce the risk of adverse cardiovascular events in patients with CAD, lending support to the inflammation hypothesis[22]. These proinflammatory cytokines have also been implicated in the pathophysiology of heart failure due to their cardio-depressant properties[23]. Despite being associated with the etiology of both CAD and heart failure, the mechanism by which high BMI is associated with better outcomes in these patients, i.e., the obesity paradox, is still incompletely understood. Lavie et al[24] have pointed out that BMI per se does not describe the body composition and they found that patients with higher lean mass along with higher body fat had lower mortality due to CAD as compared to those with lower lean mass and lower body fat. Another aspect to consider is the cardiorespiratory fitness of the individual as poor fitness levels are associated with a poorer prognosis in CAD, independent of adiposity[25]. While the obesity paradox is firmly established in several cardiovascular diseases, its association with outcomes of patients with cardiogenic shock is still unclear. In the previous meta-analysis of three studies, Meng et al[13] noted no difference in all-cause mortality between obese and non-obese patients with cardiogenic shock (OR = 0.88, 95%CI: 0.71-1.08, I2 = 96%). In a sub-group analysis, they found that cardiogenic shock mortality was lower in developed countries (United States), but higher in developing countries (Pakistan). In addition to the lower number of studies in this meta-analysis, several other errors make this previous review unreliable. Foremost is that the two included studies in their review used the same United States database from 2005-2014 and 2004-2013, which is a considerable overlap. Second, in their multivariable analysis, the authors included the trial of Hashmi et al[19] which only reported unadjusted ORs.
In our updated meta-analysis of five studies, we noted that overweight/obese patients did not have an increased risk of early mortality after cardiogenic shock as compared to normal BMI patients when only crude mortality rates were pooled. However, it is important to note that the significant heterogeneity in the meta-analyses reduces the confidence of our results. Assessing the included studies individually, we noted extremely divergent results amongst the studies. The studies of Sreenivasan et al[16] and Hashmi et al[19] demonstrated that obese patients had significantly higher mortality as compared to normal patients after cardiogenic shock. On the other hand, Patlolla et al[17] and Chatterjee et al[20] who used the same United States database with a partial overlap noted that an obesity paradox existed with cardiogenic shock as they found significantly lower mortality in higher BMI patients. The lone study of Hermansen et al[18] was neutral and they found no impact of obesity on outcomes of cardiogenic shock in a contemporary cohort of Danish patients. Furthermore, it needs to be pointed out that several confounders can also influence outcomes of cardiogenic shock in addition to obesity. Hence, to establish the independent role of overweight/obesity on mortality rates, a multivariable-adjusted analysis is needed. A limitation of our review is that only three studies reported such data and their results were similar to the crude mortality data, with Patlolla et al[17] and Chatterjee et al[20] reporting better outcomes in overweight/obese patients and Sreenivasan et al[16] reporting worse outcomes in such individuals. On meta-analysis of these three studies, we noted a reduced risk of mortality in overweight/obese patients but again with high heterogeneity.
One cause of the divergent results amongst the studies could be related to the use of MCS. In the study of Sreenivasan et al[16], 100% of patients received MCS while the number was much lower in the remaining studies. In a separate cohort (for which details were unavailable), Sreenivasan et al[16] noted that amongst individuals not receiving MCS, patients with mild obesity had significantly lower mortality compared with the non-obese patients (OR = 0.8, 95%CI: 0.6–0.9), but this difference was non-significant for moderately and severely obese patients. These results conform to the obesity paradox found by Patlolla et al[17] and Chatterjee et al[20]. Higher mortality in patients receiving MCS could be due to the increased morbidity and complications like major bleeding, thrombosis, and vascular complications associated with the invasive procedure and MCS devices[16]. The study of Sreenivasan et al[16] also had a significant proportion of patients with severe obesity. It is plausible that higher grades of obesity are associated with severe comorbidities like diabetes, end-organ damage, and worse hemodynamic function which requires more robust MCS support like Impella or/Tandem Heart and extracorporeal membrane oxygenation as compared to intra-aortic balloon pump required for patients with mild obesity[16]. This may also have contributed to the opposing results of Sreenivasan et al[16]. Furthermore, the contradictory results of Hashmi et al[19] and the neutral results of Hermansen et al[18] need to be interpreted with caution considering the small sample size of obese patients in their cohorts.
Several diverse mechanisms have also been put forward that may explain better or even worse outcomes in obese patients with cardiogenic shock. Higher lean and fat mass in obese patients may contribute to the higher metabolic reserve in such individuals and guard them against the inflammatory cascade of cardiogenic shock[26]. Lower levels of tumor necrosis factor-alpha and monocyte chemoattractant protein-1 in obese patients may attenuate the inflammatory damage associated with cardiogenic shock[27]. Adipose cells secrete adiponectin which has anti-inflammatory properties. Obese patients may also have a better neurohormonal profile and reduced B-type natriuretic peptide (BNP). BNP is associated with adverse outcomes in cardiogenic shock[28]. Larger coronary arteries in obese patients may also lower the extent of CAD and improve outcomes[29].
Contrastingly, obesity augments the metabolic demand of the body which requires greater blood volume and increased cardiac output. High volumes increase venous return and subsequently myocardial wall tension and cause ventricular dilation. While initial ventricular hypertrophy overcomes this process, with further increase in volume, the ventricles no longer adapt and systolic dysfunction occurs. Hypertension, arrhythmias, and CAD associated with obesity can cause several functional and structural alterations which could lead to worse outcomes in obese patients[16]. Our meta-analysis has some limitations. First, only a small number of predominantly retrospective studies were available for meta-analysis. Selection bias is an important limitation of these studies which can skew the results. Furthermore, databases are also prone to errors in record keeping. Second, the sample size of the included studies varied widely with two studies including a small cohort of obese patients. As mentioned earlier, there was a partial overlap of data in another two studies. Third, overweight patients were also merged into the obese group of one study which may have influenced the results. Since separate analyses for different grades of obesity were not available from all included studies, subgroup analysis for the same could not be carried out. Fourth, the treatment modality varied across the studies and obese and non-obese groups. While we used adjusted mortality data for the pooled analysis, it was not reported by all studies. A meta-regression based on treatment modality could not be conducted due to a scarcity of data. Fifth, BMI is not the sole indicator of obesity and may not correctly represent the relationship between obesity and outcomes. Several other factors like cardiorespiratory fitness, lean mass, and fat mass could also influence the relationship between the two entities. Lastly, data in our meta-analysis were from a limited number of countries and hence not generalizable to the world population.
Current evidence on the association between overweight/obesity and mortality after cardiogenic shock is scarce and conflicting. The obesity paradox might exist in patients with cardiogenic shock but could be confounded by the use of MCS. There is a need for further studies to clarify this relationship.
Cardiogenic shock continues to be a highly morbid complication that affects around 7%-10% of patients with acute myocardial infarction or heart failure. Similarly, obesity has become a worldwide epidemic.
Despite intense research on the outcomes of cardiogenic shock, it is still unclear how obesity affects the outcomes of patients with cardiogenic shock.
We aimed to compare mortality outcomes of patients with cardiogenic shock based on body mass index (BMI).
A systematic search of the literature was conducted on the databases of PubMed, Embase, ScienceDirect, CENTRAL, and Google Scholar for all types of studies comparing mortality outcomes of patients with cardiogenic shock based on BMI.
Five studies were eligible for inclusion. On pooled analysis of multivariable-adjusted ratios, we noted a statistically significantly reduced risk of mortality in overweight/obese vs normal patients with cardiogenic shock (three studies; OR = 0.92, 95%CI: 0.85-0.98, I2 = 85%). In meta-analysis, we also noted that crude mortality rates did not significantly differ between overweight/obese and normal patients after cardiogenic shock (OR = 0.95, 95%CI: 0.79-1.15, I2 = 99%). The results were not stable on sensitivity analysis and were associated with substantial heterogeneity.
Based on the current review, we found that the association between overweight/obesity and mortality after cardiogenic shock is scarce and conflicting. The obesity paradox might exist in patients with cardiogenic shock but could be confounded by the use of mechanical circulatory support.
Given the scarce number of studies available, there is a need for further research on the impact of obesity on outcomes of cardiogenic shock. Future studies should be prospective with a large sample size and also assess the impact of mechanical circulatory support on the outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Apiratwarakul K, Thailand; Tangsuwanaruk T, Thailand S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:s176-s185. [PubMed] |
| 2. | Caballero B. Humans against Obesity: Who Will Win? Adv Nutr. 2019;10:S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 378] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 4. | Kushner RF, Ryan DH. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. JAMA. 2014;312:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | González-Gross M, Meléndez A. Sedentarism, active lifestyle and sport: Impact on health and obesity prevention. Nutr Hosp. 2013;28 Suppl 5:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 6. | Neeland IJ, Poirier P, Després JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137:1391-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 7. | De Schutter A, Lavie CJ, Milani RV. The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease-the obesity paradox. Prog Cardiovasc Dis. 2014;56:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Niedziela J, Hudzik B, Niedziela N, Gąsior M, Gierlotka M, Wasilewski J, Myrda K, Lekston A, Poloński L, Rozentryt P. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol. 2014;29:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 10. | Ho KS, Wu L, Sheehan J, Salonia J. Obesity paradox? vs. 156:A1141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e232-e268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 1161] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 12. | Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis AP, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Park JG, Phreaner N, Roswell RO, Schulman SP, Jeffrey Snell R, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ Cardiovasc Qual Outcomes. 2019;12:e005618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 298] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 13. | Meng F, Guo F, Abulimiti B, Zhao K, Dong Y, Ma X, Fu Z, Ma Y. Body mass index and all-cause mortality in patients with cardiogenic shock: A systematic review and meta-analysis. Am J Emerg Med. 2021;43:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 4621] [Article Influence: 1155.3] [Reference Citation Analysis (0)] |
| 15. | Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 16. | Sreenivasan J, Khan MS, Sharedalal P, Hooda U, Fudim M, Demmer RT, Yuzefpolskaya M, Ahmad H, Khan SS, Lanier GM, Colombo PC, Rich JD. Obesity and Outcomes Following Cardiogenic Shock Requiring Acute Mechanical Circulatory Support. Circ Heart Fail. 2021;14:e007937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Patlolla SH, Ponamgi SP, Sundaragiri PR, Cheungpasitporn W, Doshi RP, Alla VM, Nicholson WJ, Jaber WA, Vallabhajosyula S. Influence of Body Mass Index on the Management and Outcomes of Acute Myocardial Infarction-Cardiogenic Shock in the United States, 2008-2017. Cardiovasc Revasc Med. 2022;36:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Hermansen GF, Junker Udesen NL, Josiassen J, Lerche Helgestad OK, Møller EE, Povlsen AL, Ravn HB, Jensen LO, Holmvang L, Schmidt H, Hassager C, Møller JE. Association of Body Mass Index with Mortality in Patients with Cardiogenic Shock following Acute Myocardial Infarction: A Contemporary Danish Cohort Analysis. Cardiology. 2021;146:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Hashmi KA, Abbas K, Hashmi AA, Irfan M, Edhi MM, Ali N, Khan A. In-hospital mortality of patients with cardiogenic shock after acute myocardial infarction; impact of early revascularization. BMC Res Notes. 2018;11:721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Chatterjee K, Gupta T, Goyal A, Kolte D, Khera S, Shanbhag A, Patel K, Villablanca P, Agarwal N, Aronow WS, Menegus MA, Fonarow GC, Bhatt DL, Garcia MJ, Meena NK. Association of Obesity With In-Hospital Mortality of Cardiogenic Shock Complicating Acute Myocardial Infarction. Am J Cardiol. 2017;119:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol. 2015;31:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4997] [Cited by in RCA: 6603] [Article Influence: 825.4] [Reference Citation Analysis (0)] |
| 23. | Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, Oddi-Erdle C, Abouzaki NA, Dixon D, Biondi-Zoccai G, Arena R, Abbate A. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2018;11:e005036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 24. | Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the "obesity paradox". J Am Coll Cardiol. 2012;60:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 25. | McAuley PA, Artero EG, Sui X, Lee DC, Church TS, Lavie CJ, Myers JN, España-Romero V, Blair SN. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012;87:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Horwich TB, Fonarow GC. Measures of obesity and outcomes after myocardial infarction. Circulation. 2008;118:469-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Martí A, Marcos A, Martínez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Kellett J. Prediction of mortality of patients with suspected heart failure by brain natriuretic peptide concentrations > 100 pg/mL: comparison of a clinical model with brain natriuretic peptide concentrations. Heart. 2006;92:1512-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |