Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.10852
Peer-review started: January 13, 2022
First decision: June 15, 2022
Revised: June 23, 2022
Accepted: August 13, 2022
Article in press: August 13, 2022
Published online: October 26, 2022
Processing time: 280 Days and 11.2 Hours
The pursuit of this paper is to collect principal reviews and systematic reviews about hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) used in colorectal cancer (CRC). We focus on principal biological aspects of CRC, hyperthermia effects, and surgical procedures. We searched PubMed/MEDLINE for the principal reviews and systematic reviews published from 2010 to 2021 regarding the bimodal treatment (CRS + HIPEC) against local and advanced CRC. In the literature, from several studies, it seems that the efficacy of bimodal treatment with an accurate CRS can extend overall survival. Despite these studies, there are not still any straight guidelines more detailed and scheduled about the use of combined treatment in patients with CRC. Even if the concept is still not very clear and shared, after a careful evaluation of the published data, and after some technical and pathophysiological descriptions, we concluded that it is possible to improve the overall survival and quality of life and to reduce the tumor relapse in patients affected by locally advanced (pT4) CRC with peritoneal metastases.
Core Tip: The purpose of this review is to summarize the most relevant evidence on the use of hyper
- Citation: Ammerata G, Filippo R, Laface C, Memeo R, Solaini L, Cavaliere D, Navarra G, Ranieri G, Currò G, Ammendola M. Hyperthermic intraperitoneal chemotherapy and colorectal cancer: From physiology to surgery. World J Clin Cases 2022; 10(30): 10852-10861
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/10852.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.10852
The synergistic anti-tumor effects of heat and intraperitoneal chemotherapy, have been known to be effective since decades and is now well known as hyperthermic intraperitoneal chemotherapy (HIPEC); this strategy is based on both hyperthermia and high intraperitoneal concentration of chemotherapy (IP).
Originally, hyperthermia was introduced by Spratt et al[1] who demonstrated the benefit of heated IP perfusion in canine models; furthermore, the first prototype IP therapy filtration system was designed and tested by Palta et al[2].
HIPEC is based on the physiological effect of the "peritoneal-plasma barrier": While peritoneal surface malignancies (PSM) cannot be effectively reached by intravenous chemotherapy[3], these tumors can benefit from intraperitoneal administration of high-dose cytotoxic drugs in direct contact with tumor cells, combining the effect of hyperthermia and minimizing the systemic toxic effects of drug reabsorption.
The first use of HIPEC was described in 1979 for the treatment of recurrent peritoneal pseudo
Later, HIPEC was also applied in the treatment of PSM from GC and OC: In 1988, Fujimoto et al[9] reported the effects of HIPEC in patients with PMs from GC and in 1996, Yonemura et al[13] showed a 5-year survival of 11% in a cohort of 83 patients who underwent CRS and HIPEC for GC; moreover, in 1989, HIPEC was used for peritoneal lesions from OC[14].
Since the 1990s, CRS combined with HIPEC has been a treatment option for PSM; today, CRS and HIPEC represent the standard of care for PMP and peritoneal mesothelioma[15]. In particular, regarding the combination of CRS plus HIPEC in CRC, there were several clinical trials about this specific combined treatment; nevertheless, this option of treatment remains a debated topic. Ceelen[16] first described the efficacy of CRS + HIPEC in peritoneal carcinomatosis arising from CRC. Later, numerous studies have been conducted until nowadays, reaching a better use of the bimodal treatment. For instance, Birgisson et al[17] studied the use of the peritoneal cancer index (PCI) as a prognostic factor in patients affected by PMs from CRC and treated with CRS + HIPEC.
Rosa et al[18] showed the clinical outcomes in 67 patients affected by CRC, focusing on the complete cytoreduction of PMs and calculating the median overall and disease-free survival.
Moreover, Elias et al[19] showed the results of HIPEC plus second look surgery in selected patients, increasing their 5-year overall survival to 90%.
However, despite several studies on this topic, there is still no consensus on the indication of CRS combined with HIPEC in CRC.
The purpose of this review is to summarize the most relevant evidence on the use of HIPEC in CRC and to specify the main properties of HIPEC and its application.
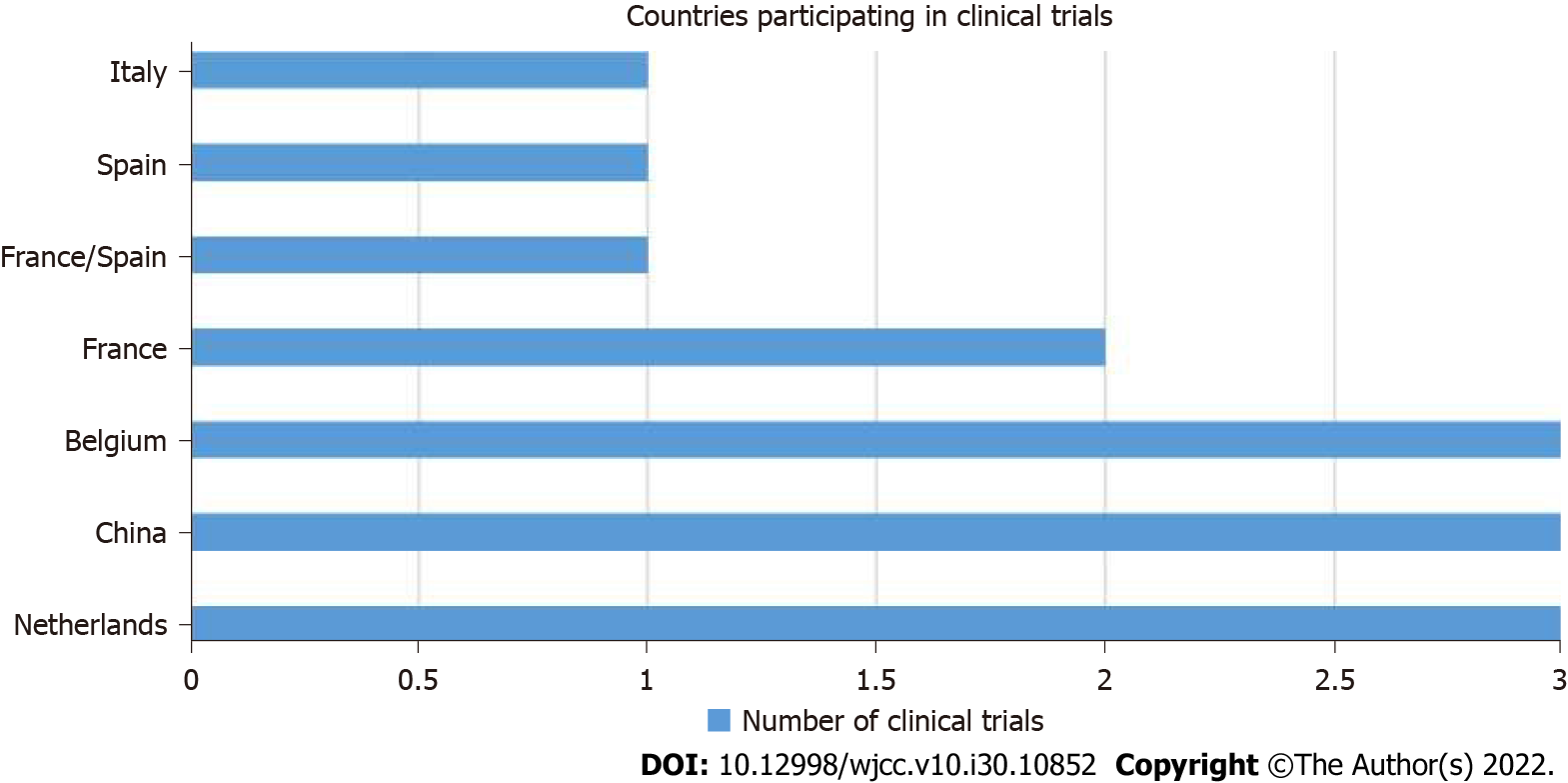
In this narrative review, we searched PubMed, a free online biomedical database developed by the National Center for Biotechnology Information at the National Library of Medicine, using the following keywords: “Colorectal Tumor” and “HIPEC”, “Colorectal Cancer” and “HIPEC”, “HIPEC”, “Hyperthermic Intraperitoneal Chemotherapy”, “Hyperthermia and HSPs”, “Hyperthermia and Tumor”, and “Hyperthermia and Cancer” (Figure 1). We collected the reviews and systematic reviews published from 2010 to 2021 and analyzed the principal clinical studies about the management of PMs arising from CRC (Table 1; Figure 2). This current work focuses the attention on HIPEC, from its first uses to now, looking at principal uses in different tumors like gastric, ovarian, and colorectal. Moreover, the review explains in detail the biologic effects of HIPEC on tumoral cells and the latest clinical trials regarding locally advanced colorectal cancer by describing CRS technique step by step.
| Phase | Institution/Group | Country | Author/ClinicalTrials.gov ID | Year beginning |
| II | Fudan University | China | Guoxiang Cai/NCT02965248 | November 2016 |
| III | Zhejiang University | China | Ding Ke-Feng/NCT02179489 | October 2014 |
| II | Wuhan University | China | Bin Xiong/NCT028301391 | July 2016 |
| II/III | Catharina, Ziekenhuis, Eindhoven | Netherlands | Koen Rovers/NCT02758951 | June 2017 |
| III | Academisch Medisch Centrum-Universiteit van Amsterdam | Netherlands | P.J. Tanis/NCT02231086 | March 2015 |
| III | Universitair Medisch Centrum Groningen | Netherlands | NACHO trial /2010-020787-37 | January 2013 |
| II | University Hospital, Ghent | Belgium | Wim P Ceelen/NCT02399410 | December 2015 |
| II | University Hospital, Ghent | Belgium | Trial Bureau/2012-000701-772 | May 2012 |
| II | University Hospital, Ghent | Belgium | Bimetra Clinics/2014-000882-343 | June 2014 |
| III | Gustav Roussy, Cancer Campus, Grand Paris | France | Diane GOERE/NCT01226394 | April 2010 |
| I/II | Hospices Civils de Lyon | France | Benoit You/NCT028669034 | May 2017 |
| III | UNICANCER | France/Spain | BEATA JUZYNA/2006-006175-20 | December 2012 |
| III | University of Roma La Sapienza | Italy | P.Sammartino/NCT02974556 | March 2019 |
| III | Maimonides Biomedical Research Institute of Cordoba | Spain | Alvaro Arjona Sanchez /NCT02614534 | November 2015 |
CRC is a big killer, representing the third most commonly diagnosed malignancy worldwide; it represents the third most common cancer in men (746000 cases; 10 % of the total) and the second in women (614000 cases; 9.2 % of the total)[20]. Surgery represents the elective therapy for resectable CRC (to gain R0 resection); instead, chemotherapy, radiotherapy, and the combination of both have indications for neoadjuvant and adjuvant purposes[21].
CRC most frequently metastasizes to the liver and peritoneum. PMs from CRC are tumoral deposits on the peritoneal surface, originating from the primitive cancer. PMs originated from CRC can cause several and severe complications like bowel and ureteral obstruction, and malignant ascites[22-24].
There are a variety of chemohyperthermia protocols in the literature, but in general they consist of intraperitoneal infusion of different drugs at a particular range of temperature and pression for a definite time at the end of surgery to eradicate the residual microscopic tumor tissue[25,26].
Today, HIPEC after CRS is still considered an investigational treatment for CRC-originated PMs, but its role is not yet defined.
HIPEC for PMs from CRC has some technical specific parameters like temperature, drugs, and pressure.
Mitomycin-C (MMC) is administered as monotherapy in a large majority of protocols[11,27-29] or in combination with cisplatin[30-34]. The second most commonly used drug in monotherapy is oxaliplatin or also in combination with irinotecan. MMC gives higher efficacy as a single agent administered at a dose of 35 mg/m2[35-40], although the dose of MMC can be modified from 10 to 40 mg/m2[41].
Establishing the drug dose is the cornerstone for even distribution of chemotherapy; some institutions use an approach called body surface area-based and others use a concentration-based approach. These approaches have some limitations such as gender and the presence of malignant ascites, but both attempt to find a conventional dose[42].
High temperature is a key issue; there are three temperature ranges that classify the type of hyperthermia: Fever hyperthermia (39-40 °C), mild hyperthermia (heat shock temperature 41-43 °C), and thermal ablation (cytotoxic temperature, > 43 °C).
Focusing attention on biological aspects, it is necessary to discuss about hyperthermia effects on tumor tissue. Hyperthermia has an important role in different paths like apoptosis regulation, neoangiogenesis, and immune status of the tumor. For example, hyperthermia induces DNA damage response by activating single strand break, double strand break, histone H2AX with phosphorylated C-terminal serine (γ-H2AX) site formation, and ataxia-telangiectasia mutated protein phosphorylation, and by decreasing DNA replication and repair.
Indirectly, hyperthermia activates DNA damage response and induces tumor suppressor alternative reading frame by starting reactive oxygen species (ROS) production, cell cycle arrest, cell cycle checkpoint arrest, and cell death; in addition, hyperthermia decelerates DNA replication.
Hyperthermia can also damage cancer stem cells exceeding conventional therapeutic regimens, so it is used in combination with chemotherapy or radiation to result in non-reversible damage to tumor cellular DNA[43].
Specifically, fever range hyperthermia can alter cell membrane fluidity and stability, changing cell shape and affecting intracellular sodium-calcium levels[44]; moreover, it recruits heat shock proteins (HSPs) and endoplasmic reticulum (ER) stress (type II) at the same time[45].
ER stress and ROS (type I) activate immunogenic cell death (ICD) and promote, through specific signals “eat me” and “enabler”, the recruitment of immune cells. Definitively, hyperthermia can be considered an inducer of ICD and a powerful change of tumor microenvironment (TME)[46-48].
A range of heat shock temperature recruits different molecules like L-selectin, P-selectin, and intracellular cell adhesion molecule-1 in the vessel wall, and causes the production of pro-inflammatory cytokines and chemokines [interleukin (IL)-1β, IL-6, IL-8, IL-10, and C-C class chemokines 22][49-52]. These cytokines “storm” facilitates the infiltration of lymphocytes in the TME and triggers an immune cascade against solid tumor.
Many HSPs are expressed on the surface of different types of solid and haematological tumors, a high concentration of mHSPs is associated with tumor progression and resistance to anti-tumor therapies, but under stress (for example anoxia and hyperthermia), HSPs can modify their relationship with tumor. Hyperthermia, in this sense, becomes an inducer of HSPs; it rapidly regulates different heat shock factors in tumor cells and activates HSPs, and in particular leads the translocation of HSPs to the nucleus and their synthesis by an autocrine loop[53].
HSPs constitute a large family of proteins, acting as molecular chaperones; they reside in three intracellular compartments such as the cytosol, nucleus, and plasma membrane or in the extracellular space. For example, HSP70, localized on the plasma membrane of tumor cells in CRC[54], facilitates cross-presentation of antigenic peptides via major histocompatibility complex class I molecules and determines the consecutive induction of a CD8+ T cell-mediated immune response. CD8+ T cell differentiation and their cytotoxicity against pathogens are both temperature sensitive, in fact hyperthermia can promote antigen-specific naïve CD8+ T cell differentiation and increase cytotoxic potential of T cells and memory stem T cell generation. Moreover, chaperones of HSP70 family can activate the proliferation of natural killer cells even without immunogenic peptides[55].
There is no univocal standard on the temperature to be used during HIPEC: A range from 38.5 °C to 44 °C is reported in the abdomen with an average value that is usually considered close to 42 °C. However, it is essential that the temperature does not exceed thermal ablation to avoid systemic toxic effects.
CRS has a curative intent and consists of a complex series of steps, aiming to achieve a radical visceral and parietal peritonectomy to eliminate the macroscopic visible tumor nodules, leaving a most microscopic residual tumor tissue. It is important to consider cancer histopathology, radiological imaging, and PCI to plan the best surgery[56-58].
The patient is placed in the lithotomic position, supine with legs extended and laid on St. Mark’s holders and arms beneath the torso.
The tools used in CRS are based on electrosurgery and electro-evaporation, specifically it is usual to use ball-tipped electrosurgical handpiece to minimize blood lost.
The median incision is the from the xiphi-sternal junction to the pubis; at this point, if tumor nodules are visible, the surgeon can start with parietal peritonectomy or rather separating parietal peritoneum from the inferior surface of the anterior abdominal wall. The dissection of visceral peritoneum starts generally from bottom to top.
Omentectomy is often necessary and sometimes mandatory in order to better explore the peritoneal cavity.
CRS continues with dissection of the left hypochondrium: We start by dissecting the peritoneum behind the rectus muscle to the left diaphragmatic dome and mobilizing the left colic flexure, thus exposing the diaphragm with its vessels and exposing the spleen, which can be removed, if it is infiltrated by neoplastic tissue.
The approach to the right side is mirrored by the contralateral; it is essential to totally mobilize the liver from its peritoneal attachment, particularly the right and left triangular ligament, the falciform ligament, and the teres hepatic ligament; sometimes, the Glisson's capsule must be fulgurated if cancerous deposits are present.
As for the small intestine and its mesentery, it is important to free them and completely inspect them because if they are involved by the tumor, the surgeon will remove the affected part. The colon is another elective situs of peritoneal carcinomatosis, particularly the fusion layer between the parietal and visceral peritoneum of the right colon. Sometimes, it is necessary to proceed to partial or total colectomy.
The pelvic peritonectomy is another important step in CRS. Two anatomic structures represent the polar star in this part of pelvic dissection: Posteriorly the ureters and anteriorly the muscular portion of bladder. The right and left ureters are identified and preserved, in the woman the ovarian vessels are ligated at the lower pole of the kidney, otherwise in men, the testicular vessels are avoided from the surgical field. After these procedures, it is usual to execute in woman hysterectomy with colic resection and in men an anterior resection of the rectum. If there are some visceral resection, the surgeon will restore intestinal functions with anastomoses or stomas[59].
Nowadays there are two attitudes to execute HIPEC: In closed or in open abdominal cavity (also defined coliseum technique)[60,61].
Close technique implies the insertion of two inflow drains under the left and right diaphragmatic cupola and an outflow drain in the pouch of Douglas. Temperature probes are also inserted within the abdominal cavity (behind the liver pedicle and near the first jejunal loop). Other temperature probes are set up outside the abdominal cavity on the inflow and outflow drains and inside the bladder within a Foley catheter. As a final step, there is a closure of laparotomy incision and the inflow and outflow drains are connected to a closed sterile circuit. Also, in this case there is no specific evidence that suggests which technique is better than other, and probably closed technique reduces risks of exposure of drugs to the personnel[62].
The intraperitoneal chemotherapy after cytoreductive surgery trial compared the benefit of HIPEC with early, postoperative, normothermic IP chemotherapy (EPIC) in patients affected by colorectal cancer and undergoing optimal CRS.
Enrolment began in March 2013 with completion date of March 2018 at Memorial Sloan Kattering Cancer Center and was given MMC in HIPEC and floxuridine in IPEC.
The outcome measure of the study is disease-free survival, within 3 years, though secondary measures were used to monitor surgical and chemotherapy–related toxicities up to 60 d postoperatively[63].
The PRODIGE7 study, a randomized, multicentre, phase III trial at the Institut di Cancer Val d’ Aurelle (Monpellier, France), started to evaluate the benefit of HIPEC to complete CRS.
The recruitment of 270 patients with CRC and limited peritoneal dissemination was completed in 2013. Patients undergoing CRS were randomized intraoperatively to receive HIPEC or saline lavage only. In this study, oxaliplatin (460 mg/m2) in 2 L/m2 of dextrose 5% over 30 min at a temperature of 42 °C was used. One hour before the HIPEC, 20 mg/m2 of leucovarin and 400 mg/m2 of 5-fluorouracil were given intravenously in the HIPEC arm[64].
The CAIRO-6 study, similarly to the previous study, focused on the role of perioperative systemic therapy on survival in patients undergoing CRS and HIPEC for CRC. This phase II/III study randomized patients to undergo neoadjuvant therapy intravenously with 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CAPOX) with bevacizumab followed by CRS and HIPEC, then adjuvant systemic therapy with FOLFOX and CAPOX[65].
The control arm is represented by CRS and HIPEC only. Both the studies confirm the importance of full CRS in patients that received systemic chemotherapy.
The ProphyloCHIP-PRODIGE 15 (France) study started the recruitment of 150 patients in 2010. This is a multicentre, randomized, phase III study to demonstrate the role of HIPEC as a prophylactic measure in initial treatment and in the adjuvant setting in patients with high risk of developing colorectal peritoneal metastases[66].
The COLOPEC (Netherlands) study, enrolled 204 patients, aged from 18 to 75 years, between 2014 and 2017. The patients had CRC at T4N0-2M0 stage, and the treatment consisted of intravenous injection of fluorouracil (400 mg/m2) and leucovarin (20 mg/m2), then, during the surgery, the use of oxaliplatin (460 mg/m2) for 30 min. COLOPEC demonstrated an absolute risk reduction of 15% in PMs[67].
In CRCs, PMs are underestimated and they are correlated with a poor prognosis. Despite the fact that the secondary localizations of tumor are visible in the chest or in the liver, during the execution of full-body computed tomography for tumor staging, PMs cannot be identified easily. Moreover, a failed treatment of PMs determines a median survival of 5 mo; on the contrary, a palliative systematic therapy increases the median survival from 5 to 15 mo. Unfortunately, the survival remains worse in respect to non-peritoneal metastases.
Furthermore, CRC affects in higher percentage the young ages, so it becomes necessary to discuss about a protocol of treatment to prevent the principal complications (malignant ascites and obstruction) of colorectal tumors, eliminate macroscopic malignancies on peritoneal surface, and increase the median survival.
In light of the main studies collected regarding CRS plus HIPEC in CRC, it is still no very clear and shared the indications and technique used in PMs arising from CRC.
However, looking at the clinical trials and physiologic principles, CRS (open or laparoscopic) plus HIPEC can be a valid treatment especially in young patients (< 50 years) affected by locally advanced (pT4) CRC with PMs. In this way, it is possible to improve the overall survival and quality of life and reduce the tumor relapse.
This work was supported by Dr. Thom Douglas for the English language review.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ding L, China; Farouk S, Egypt; Serban ED, Romania S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
| 1. | Spratt JS, Adcock RA, Sherrill W, Travathen S. Hyperthermic peritoneal perfusion system in canines. Cancer Res. 1980;40:253-255. [PubMed] |
| 2. | Palta JR. Design and Testing of a Therapeutic Infusion Filtration System. Columbia: University of Missouri, 1977. |
| 3. | Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 189] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256-260. [PubMed] |
| 5. | Sugarbaker PH, Gianola FJ, Speyer JC, Wesley R, Barofsky I, Meyers CE. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery. 1985;98:414-422. [PubMed] |
| 6. | Sugarbaker PH. Treatment of peritoneal carcinomatosis from colon or appendiceal cancer with induction intraperitoneal chemotherapy. Cancer Treat Res. 1996;82:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Koga S, Hamazoe R, Maeta M, Shimizu N, Kanayama H, Osaki Y. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion in combination with an anticancer drug. Cancer Res. 1984;44:1840-1842. [PubMed] |
| 8. | Koga S. [Prophylactic and therapeutic continuous hyperthermic peritoneal perfusion for peritoneal metastases of gastric cancer]. Gan No Rinsho. 1985;31:1103-1105. [PubMed] |
| 9. | Fujimoto S, Shrestha RD, Kokubun M, Ohta M, Takahashi M, Kobayashi K, Kiuchi S, Okui K, Miyoshi T, Arimizu N. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Toi M, Shiramizu T, Yonemura T, Ezaki T, Oka N, Yoshida T, Tsurumaru H. [Intraperitoneal cisplatin in peritoneal carcinomatosis patients]. Gan No Rinsho. 1985;31:522-526. [PubMed] |
| 11. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1511] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 12. | National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 2. 2016. Available from: https://www2.tri-kobe.org/nccn/guideline/archive/colorectal2016/english/colon.pdf. |
| 13. | Yonemura Y, Fujimura T, Nishimura G, FallaR, Sawa T, Katayama K, Tsugawa K, Fushida S, Miyazaki I, Tanaka M, Endou Y, Sasaki T. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery. 1996;119:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Tsuyoshi H, Inoue D, Kurokawa T, Yoshida Y. Hyperthermic intraperitoneal chemotherapy (HIPEC) for gynecological cancer. J Obstet Gynaecol Res. 2020;46:1661-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Morano WF, Aggarwal A, Love P, Richard SD, Esquivel J, Bowne WB. Intraperitoneal immunotherapy: historical perspectives and modern therapy. Cancer Gene Ther. 2016;23:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Ceelen WP. Use of hyperthermic intraperitoneal chemotherapy (HIPEC) in management of peritoneal carcinomatosis from colorectal origin. Surg Technol Int. 2005;14:125-130. [PubMed] |
| 17. | Birgisson H, Enblad M, Artursson S, Ghanipour L, Cashin P, Graf W. Patients with colorectal peritoneal metastases and high peritoneal cancer index may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2020;46:2283-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Rosa F, Galiandro F, Ricci R, Di Miceli D, Quero G, Fiorillo C, Cina C, Alfieri S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal metastases: analysis of short- and long-term outcomes. Langenbecks Arch Surg. 2021;406:2797-2805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Elias D, Honoré C, Dumont F, Ducreux M, Boige V, Malka D, Burtin P, Dromain C, Goéré D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20499] [Article Influence: 2049.9] [Reference Citation Analysis (20)] |
| 21. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 683] [Article Influence: 97.6] [Reference Citation Analysis (1)] |
| 22. | Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 593] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 23. | Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 24. | Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 518] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 25. | Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1185] [Article Influence: 39.5] [Reference Citation Analysis (33)] |
| 26. | Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2016;7:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 27. | Kuijpers AM, Aalbers AG, Nienhuijs SW, de Hingh IH, Wiezer MJ, van Ramshorst B, van Ginkel RJ, Havenga K, Heemsbergen WD, Hauptmann M, Verwaal VJ. Implementation of a standardized HIPEC protocol improves outcome for peritoneal malignancy. World J Surg. 2015;39:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Witkamp AJ, de Bree E, Kaag MM, Boot H, Beijnen JH, van Slooten GW, van Coevorden F, Zoetmulder FA. Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer. 2001;37:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Zanon C, Bortolini M, Chiappino I, Simone P, Bruno F, Gaglia P, Airoldi M, Deriu L, Mashiah A. Cytoreductive surgery combined with intraperitoneal chemohyperthermia for the treatment of advanced colon cancer. World J Surg. 2006;30:2025-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Elias D, Antoun S, Goharin A, Otmany AE, Puizillout JM, Lasser P. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig. 2000;1:431-439. [PubMed] |
| 31. | Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, Rougier P, Ducreux M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Vaira M, Cioppa T, D'Amico S, de Marco G, D'Alessandro M, Fiorentini G, De Simone M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo. 2010;24:79-84. [PubMed] |
| 33. | Asero S, Caruso M, Vallone N, Luciani AG, Lombardo V, Terranova G, Ettore G, Giannone G. Cytoreductive surgery (cs) and hyperthermic intraperitoneal chemotherapy (hipec) in treatment of peritoneal surface malignances: report of a phase II clinical study. In Vivo. 2009;23:645-647. [PubMed] |
| 34. | Yonemura Y, Canbay E, Ishibashi H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. ScientificWorldJournal. 2013;2013:978394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Braam HJ, Boerma D, Wiezer MJ, van Ramshorst B. Hyperthermic intraperitoneal chemotherapy during primary tumour resection limits extent of bowel resection compared to two-stage treatment. Eur J Surg Oncol. 2013;39:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Cashin PH, Dranichnikov F, Mahteme H. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy treatment of colorectal peritoneal metastases: cohort analysis of high volume disease and cure rate. J Surg Oncol. 2014;110:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Ceelen W, Van Nieuwenhove Y, Putte DV, Pattyn P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann Surg Oncol. 2014;21:3023-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Hagendoorn J, van Lammeren G, Boerma D, van der Beek E, Wiezer MJ, van Ramshorst B. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal and gastrointestinal origin shows acceptable morbidity and high survival. Eur J Surg Oncol. 2009;35:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Hompes D, D'Hoore A, Wolthuis A, Fieuws S, Mirck B, Bruin S, Verwaal V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol. 2014;109:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Simkens GA, van Oudheusden TR, Braam HJ, Wiezer MJ, Nienhuijs SW, Rutten HJ, van Ramshorst B, de Hingh IH. Cytoreductive surgery and HIPEC offers similar outcomes in patients with rectal peritoneal metastases compared to colon cancer patients: a matched case control study. J Surg Oncol. 2016;113:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Morano WF, Khalili M, Chi DS, Bowne WB, Esquivel J. Clinical studies in CRS and HIPEC: Trials, tribulations, and future directions-A systematic review. J Surg Oncol. 2018;117:245-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1016] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 43. | Crezee J, Franken NAP, Oei AL. Hyperthermia-Based Anti-Cancer Treatments. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | de Andrade Mello P, Bian S, Savio LEB, Zhang H, Zhang J, Junger W, Wink MR, Lenz G, Buffon A, Wu Y, Robson SC. Hyperthermia and associated changes in membrane fluidity potentiate P2X7 activation to promote tumor cell death. Oncotarget. 2017;8:67254-67268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182:1449-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, de Witte P, Vandenabeele P, Agostinis P. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 618] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 47. | Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 672] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 48. | Nagata S, Tanaka M. Programmed cell death and the immune system. Nat Rev Immunol. 2017;17:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 49. | Newton JM, Flores-Arredondo JH, Suki S, Ware MJ, Krzykawska-Serda M, Agha M, Law JJ, Sikora AG, Curley SA, Corr SJ. Non-Invasive Radiofrequency Field Treatment of 4T1 Breast Tumors Induces T-cell Dependent Inflammatory Response. Sci Rep. 2018;8:3474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Chen Q, Fisher DT, Kucinska SA, Wang WC, Evans SS. Dynamic control of lymphocyte trafficking by fever-range thermal stress. Cancer Immunol Immunother. 2006;55:299-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Chen Q, Appenheimer MM, Muhitch JB, Fisher DT, Clancy KA, Miecznikowski JC, Wang WC, Evans SS. Thermal facilitation of lymphocyte trafficking involves temporal induction of intravascular ICAM-1. Microcirculation. 2009;16:143-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97:2727-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Kodiha M, Chu A, Lazrak O, Stochaj U. Stress inhibits nucleocytoplasmic shuttling of heat shock protein hsc70. Am J Physiol Cell Physiol. 2005;289:C1034-C1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Pfister K, Radons J, Busch R, Tidball JG, Pfeifer M, Freitag L, Feldmann HJ, Milani V, Issels R, Multhoff G. Patient survival by Hsp70 membrane phenotype: association with different routes of metastasis. Cancer. 2007;110:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Shevtsov M, Multhoff G. Heat Shock Protein-Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Front Immunol. 2016;7:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 56. | Pelz JO, Stojadinovic A, Nissan A, Hohenberger W, Esquivel J. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol. 2009;99:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 57. | Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 58. | Kusamura S, Dominique E, Baratti D, Younan R, Deraco M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Mohamed F, Cecil T, Moran B, Sugarbaker P. A new standard of care for the management of peritoneal surface malignancy. Curr Oncol. 2011;18:e84-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Glehen O, Cotte E, Kusamura S, Deraco M, Baratti D, Passot G, Beaujard AC, Noel GF. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Ceelen W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur J Surg Oncol. 2019;45:400-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 62. | Esquivel J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: survival outcomes and patient selection. J Gastrointest Oncol. 2016;7:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 63. | Rossi AJ, Khan TM, Rehman SU, Nash GM, Hernandez JM. Early Postoperative Intraperitoneal Versus Hyperthermic Intraperitoneal Chemotherapy After Optimal Cytoreductive Surgery for Colorectal Cancer with Isolated Peritoneal Metastasis (ICARuS). Ann Surg Oncol. 2021;28:4100-4101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | van de Vlasakker VCJ, Lurvink RJ, Cashin PH, Ceelen W, Deraco M, Goéré D, González-Moreno S, Lehmann K, Li Y, Moran B, Morris DL, Piso P, Quadros CA, Rau B, Somashekhar SP, Sommariva A, van der Speeten K, Spiliotis J, Sugarbaker PH, Teo MCC, Verwaal VJ, Yonemura Y, Glehen O, de Hingh IHJT. The impact of PRODIGE 7 on the current worldwide practice of CRS-HIPEC for colorectal peritoneal metastases: A web-based survey and 2021 statement by Peritoneal Surface Oncology Group International (PSOGI). Eur J Surg Oncol. 2021;47:2888-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Rovers KP, Bakkers C, Simkens GAAM, Burger JWA, Nienhuijs SW, Creemers GM, Thijs AMJ, Brandt-Kerkhof ARM, Madsen EVE, Ayez N, de Boer NL, van Meerten E, Tuynman JB, Kusters M, Sluiter NR, Verheul HMW, van der Vliet HJ, Wiezer MJ, Boerma D, Wassenaar ECE, Los M, Hunting CB, Aalbers AGJ, Kok NFM, Kuhlmann KFD, Boot H, Chalabi M, Kruijff S, Been LB, van Ginkel RJ, de Groot DJA, Fehrmann RSN, de Wilt JHW, Bremers AJA, de Reuver PR, Radema SA, Herbschleb KH, van Grevenstein WMU, Witkamp AJ, Koopman M, Haj Mohammad N, van Duyn EB, Mastboom WJB, Mekenkamp LJM, Nederend J, Lahaye MJ, Snaebjornsson P, Verhoef C, van Laarhoven HWM, Zwinderman AH, Bouma JM, Kranenburg O, van 't Erve I, Fijneman RJA, Dijkgraaf MGW, Hemmer PHJ, Punt CJA, Tanis PJ, de Hingh IHJT; Dutch Peritoneal Oncology Group (DPOG); Dutch Colorectal Cancer Group (DCCG). Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer. 2019;19:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 66. | Goéré D, Glehen O, Quenet F, Guilloit JM, Bereder JM, Lorimier G, Thibaudeau E, Ghouti L, Pinto A, Tuech JJ, Kianmanesh R, Carretier M, Marchal F, Arvieux C, Brigand C, Meeus P, Rat P, Durand-Fontanier S, Mariani P, Lakkis Z, Loi V, Pirro N, Sabbagh C, Texier M, Elias D; BIG-RENAPE group. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020;21:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 67. | Klaver CE, Musters GD, Bemelman WA, Punt CJ, Verwaal VJ, Dijkgraaf MG, Aalbers AG, van der Bilt JD, Boerma D, Bremers AJ, Burger JW, Buskens CJ, Evers P, van Ginkel RJ, van Grevenstein WM, Hemmer PH, de Hingh IH, Lammers LA, van Leeuwen BL, Meijerink WJ, Nienhuijs SW, Pon J, Radema SA, van Ramshorst B, Snaebjornsson P, Tuynman JB, Te Velde EA, Wiezer MJ, de Wilt JH, Tanis PJ. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |