Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.966
Peer-review started: August 30, 2021
First decision: October 27, 2021
Revised: November 5, 2021
Accepted: December 23, 2021
Article in press: December 23, 2021
Published online: January 21, 2022
Processing time: 137 Days and 21.6 Hours
Longstanding intestinal inflammation increases the risk of colorectal neoplasia in patients with inflammatory bowel disease (IBD). Accurately predicting the risk of colorectal neoplasia in the early stage is still challenging. Therefore, identifying visible warning markers of colorectal neoplasia in IBD patients is the focus of the current research. Post-inflammatory polyps (PIPs) are visible markers of severe inflammation under endoscopy. To date, there is controversy regarding the necessity of strengthened surveillance strategies for IBD patients with PIPs.
To determine whether IBD patients with PIPs carryan increased risk of colorectal neoplasia.
Researchers searched the following databases up to July 31, 2021: MEDLINE (PubMed), MEDLINE (Ovid), EMBASE, Cochrane Library, China National Knowledge Infrastructure, Wan-Fang Data, China Science and Technology Journal Database and Chinese BioMedical Literature Database. Cohort and case-control studies that compared the risk of colorectal neoplasia between IBD patients with or without PIPs and published in English or Chinese were included. Methodological quality was assessed using the Risk of Bias in Nonrandomized Studies-of Interventions assessment tool. The outcomes of interest were the rates of various grades of colorectal neoplasia. The pooled risk ratio (RR) and 95% confidence interval (95%CI) were calculated using the random-effects model. Begg’s test and Egger’s test were used to calculate the publication bias. Sensitivity and subgroup analyses were performed to verify the robustness of the results. The Grading of Recommendations, Assessment, Development and Evaluation approach was used to assess the overall quality of evidence supporting the outcomes of interest.
Nine studies involving 5424 IBD patients (1944 with PIPs vs 3480 without PIPs) were included. The overall bias in each included study ranged from moderate to serious. Compared with nonconcurrent PIPs, patients with PIPs had a higher risk of colorectal neoplasia (RR = 1.74, 95%CI: 1.35-2.24, P < 0.001, I2 = 81.4%; aHR = 1.31, 95%CI: 1.01-1.70, P = 0.04, I2 = 26.2%; aOR = 2.62, 95%CI: 1.77-3.88, P < 0.001, I2 = 0%), advanced colorectal neoplasia (RR = 2.07, 95%CI: 1.49-2.87, P < 0.001, I2 = 77.4%; aHR = 1.63, 95%CI: 1.05-2.53, P = 0.03, I2 = 10.1%) and colorectal cancer (RR = 1.93, 95%CI: 1.32-2.82, P = 0.001, I2 = 83.0%). Publication bias was not observed in Begg’s test or Egger’s test. Sensitivity and subgroup analyses showed that the results are robust. The overall quality of evidence was assessed as moderate to low.
IBD patients with PIPs may have an increased incidence of colorectal neoplasia.
Core Tip: This study is the first systematic review and meta-analysis to separately evaluate the potential risk between post-inflammatory polyps (PIPs) and colorectal neoplasia, advanced colorectal neoplasia, and colorectal cancer. Interestingly, we found that although malignant transformation from PIPs is rare, inflammatory bowel disease (IBD) patients with PIPs still bear an increased incidence of various grades of colorectal neoplasia. As an early warning of the increasing risk of colorectal neoplasia, IBD patients with PIPs should undergo strengthened surveillance to detect early dysplastic changes to allow for appropriate management so that there are improvements in both quality of life and survival rates.
- Citation: Shi JL, Lv YH, Huang J, Huang X, Liu Y. Patients with inflammatory bowel disease and post-inflammatory polyps have an increased risk of colorectal neoplasia: A meta-analysis. World J Clin Cases 2022; 10(3): 966-984
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/966.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.966
Longstanding intestinal inflammation increases the risk of colorectal neoplasia in patients with inflammatory bowel disease (IBD)[1,2]. Unlike sporadic colorectal neoplasms, IBD-related colorectal neoplasms are usually characterized by a younger onset age, more malignant behavior and a poorer prognosis[3-5]. Therefore, clinical guidelines recommend regular endoscopic surveillance for IBD patients to enable the early detection of colorectal neoplasms. Furthermore, patients with certain risk factors need to undergo an intensified surveillance strategy; these risk factors include extensive colitis, family history of colorectal cancer, concurrent primary sclerosing cholangitis or post-inflammatory polyps (PIPs)[6-9].
Post-inflammatory polyps (PIPs) are usually formed from the alternating cycling of intestinal inflammation and mucous epithelial cell regeneration. According to published data, PIPs are not rare in IBD patients, with their prevalence ranging from 4% to 74%[10,11]. To date, there is controversy in the literature regarding the necessity of a strengthened surveillance strategy for IBD patients with PIPs. Some earlier case-control studies showed an increased risk of colorectal neoplasia in patients with PIPs[12,13]. For this reason, clinical guidelines suggest a strengthened surveillance strategy for IBD patients with previous or present PIPs in endoscopy. However, the recommended endoscopic surveillance intervals for IBD patients with PIPs vary considerably from country to country. In addition, some recent multicenter cohort studies showed no significant correlation between PIPs and colorectal neoplasia in IBD patients, in contrast to prior views and clinical guidelines[14,15]. Unnecessary and frequent endoscopic surveillance not only decreases the quality of life of IBD patients but also increases the burdens of health care and resource stewardship. Therefore, it is crucial to explore the potential risk association between PIPs and colorectal neoplasia and to clarify the safe and reasonable endoscopic surveillance intervals for IBD patients with PIPs.
Because of the lack of large, randomized trials and meta-analyses specifically focused on the risk of PIPs and colorectal neoplasia, most of the current data are from small-scale, observational, nonrandomized studies. Therefore, researchers systematically identified and analyzed data from observed trials and evaluated the association between PIPs and colorectal neoplasia, advanced colorectal neoplasia, and colorectal cancer in IBD patients separately. This study aimed to determine whether IBD patients with PIPs bear an increased risk of various grades of colorectal neoplasia.
This meta-analysis was conducted and presented according to the PRISMA and MOOSE guidelines. The methods were established prior to the conduct of the review. The protocol of this study was registered in PROSPERO (CRD42020172539).
The following databases were searched systematically from inception up to July 31, 2021: MEDLINE (PubMed), MEDLINE (Ovid), EMBASE, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wan-Fang Data, China Science and Technology Journal Database (VIP) and Chinese BioMedical Literature Database (CBM). The search items included “post-inflammatory polyps”, “colorectal neoplasms”, “inflammatory bowel diseases” and their associated words. The search strategy is detailed in the Supplementary data. Additional records were identified through hand searches of reference lists in clinical guidelines and relevant articles.
PIPs were defined as nonneoplastic lesions originating from the mucosa after the alternating cycling of intestinal inflammation and mucous epithelial cell regeneration and were proposed to be related to excessive healing processes. PIPs are usually diagnosed by endoscopists and pathologists and have been described as inflammatory polyps, pseudopolyps or post-inflammatory polyps in the literature[10].
The inclusion criteria were as follows: (1) Participants with confirmed IBD (including ulcerative colitis, Crohn’s disease and unclassified IBD); (2) Comparison of the colorectal neoplasia burden and prognosis between patients with PIPs and patients without PIPs; (3) Reported outcomes of interest (such as colorectal neoplasia, advanced colorectal neoplasia, colorectal cancer); and (4) Cohort study or case-control study published in English or Chinese. The exclusion criteria were as follows: (1) Participants with a known history of colorectal neoplasm before IBD diagnosis; (2) Participants with synchronous diagnoses of IBD and colorectal neoplasm; (3) Full-text versions were not available for assessing risk of bias; and (4) Reviews, case reports, or poster abstracts. Two researchers (Lv YH and Huang J) applied eligibility criteria and selected studies for inclusion in the systematic review independently. Disagreements between individual judgments were resolved by discussion and consultation with a third researcher (Jialing Shi) until a consensus was reached.
The methodological quality of each included study was assessed using the Risk of Bias in Nonrandomized Studies-of Interventions (ROBINS-I) assessment tool[16]. Two researchers (Yehong Lv, Jun Huang) assessed the methodological quality of each included study independently. Researchers were blinded to each other’s decisions. Disagreements between individual judgments were resolved by discussion and consultation with a third researcher (Jialing Shi) until a consensus was reached. The final score was listed in a homemade Excel form.
The outcomes of interest were the related variables of IBD-associated colorectal neoplasia, including dysplastic number, pathologic grading, cytologic type, and time from diagnosis to dysplastic change. However, many published studies reported only 1-2 relevant indices, and most of them focused on tumor incidence. This aspect made it difficult to synthesize and analyze many other useful outcome variables for colorectal neoplasia. Because the incidence of colorectal neoplasia (including the number of cases and its effect size) well reflected the potential associations between risk factors and tumorigenesis, the researchers ultimately chose the incidence of various grades of colorectal neoplasia (including colorectal neoplasia, advanced colorectal neoplasia and colorectal cancer) as the outcome of interest in this review. Neoplasia in this review was defined as not only the malignant transformation of PIPs but also the malignant transformation of colorectal mucosa. All cases of neoplasia were diagnosed by pathological examination. Colorectal neoplasia was defined as low-grade dysplasia, high-grade dysplasia and colorectal cancer. Advanced colorectal neoplasia was defined as high-grade dysplasia and colorectal cancer. All relevant dysplasia data were extracted from final pathology reports or electronic medical records. Relevant clinical data for cases were extracted from electronic medical records.
The following data were collected: study characteristics (first author, publication year, study design, follow-up time, study conclusions), participant characteristics (numbers of PIPs and control group, IBD phenotypes, country of origin, primary sclerosing cholangitis (PSC), family history of colon cancer, extensive colitis), andoutcome assessment (occurrence of various grades of colorectal neoplasia, including the numbers of colorectal neoplasia and its specific effective size). If the data were not reported in texts or tables, researchers contacted the corresponding author of the eligible study for additional information when necessary. Two researchers (Yehong Lv, Jun Huang) performed data extraction independently. Disagreements between individual judgments were resolved by discussion and consultation with a third researcher (Xue Huang until consensus was reached). The extracted data were listed in a homemade Excel form.
Data synthesis was performed using STATA 15.0. The random-effects model was used for all data synthesis and statistical analysis. The pooled risk ratio (RR) and 95% confidence interval (95%CI:) were calculated to evaluate the potential risk between PIPs and colorectal neoplasia. When adjusted ratios were available, pooled adjusted ratios, such as the pooled adjusted hazard ratio (aHR), the pooled adjusted relative risk (aRR), or the pooled adjusted odds ratio (aOR), and their 95%CI:s were also calculated.
Researchers used the I2 statistic to quantify statistical heterogeneity. An I² less than 25% was considered low-level heterogeneity, 25% to 50% was considered moderate-level heterogeneity, and more than 50% was considered high-level heterogeneity. Because the number of included studies was less than ten, funnel plots for evaluating the potential publication bias were not constructed. Instead, Begg’s test and Egger’s test were used to calculate the publication bias.
In the sensitivity analysis, the following two methods were performed to verify the robustness of the results: (1) The use of the fixed-effects model; and (2) The exclusion of outliers or studies with significant clinical heterogeneity.
For further analysis, subgroup analysis was performed according to study design (cohort vs case-control study) and methodological quality (serious/critical vs low/moderate/unclear risk of bias) for screening the heterogeneous origin. Because geography plays a role in IBD-associated colorectal cancer, the recommended endoscopic surveillance intervals vary considerably in different countries and societies. The geographic heterogeneity between PIPs and colorectal neoplasia was investigated in further analysis. The potential risk between PIPs and colorectal neoplasia in different IBD phenotypes (ulcerative colitis, Crohn’s disease, unclassified IBD) was also investigated in further analysis. A P value less than 0.05 was considered significant.
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to assess the overall quality of evidence supporting the outcomes of interest[17]. The final quality of evidence was classified as high, moderate, low or very low. The quality of evidence was assessed using GRADE profiler 3.6.
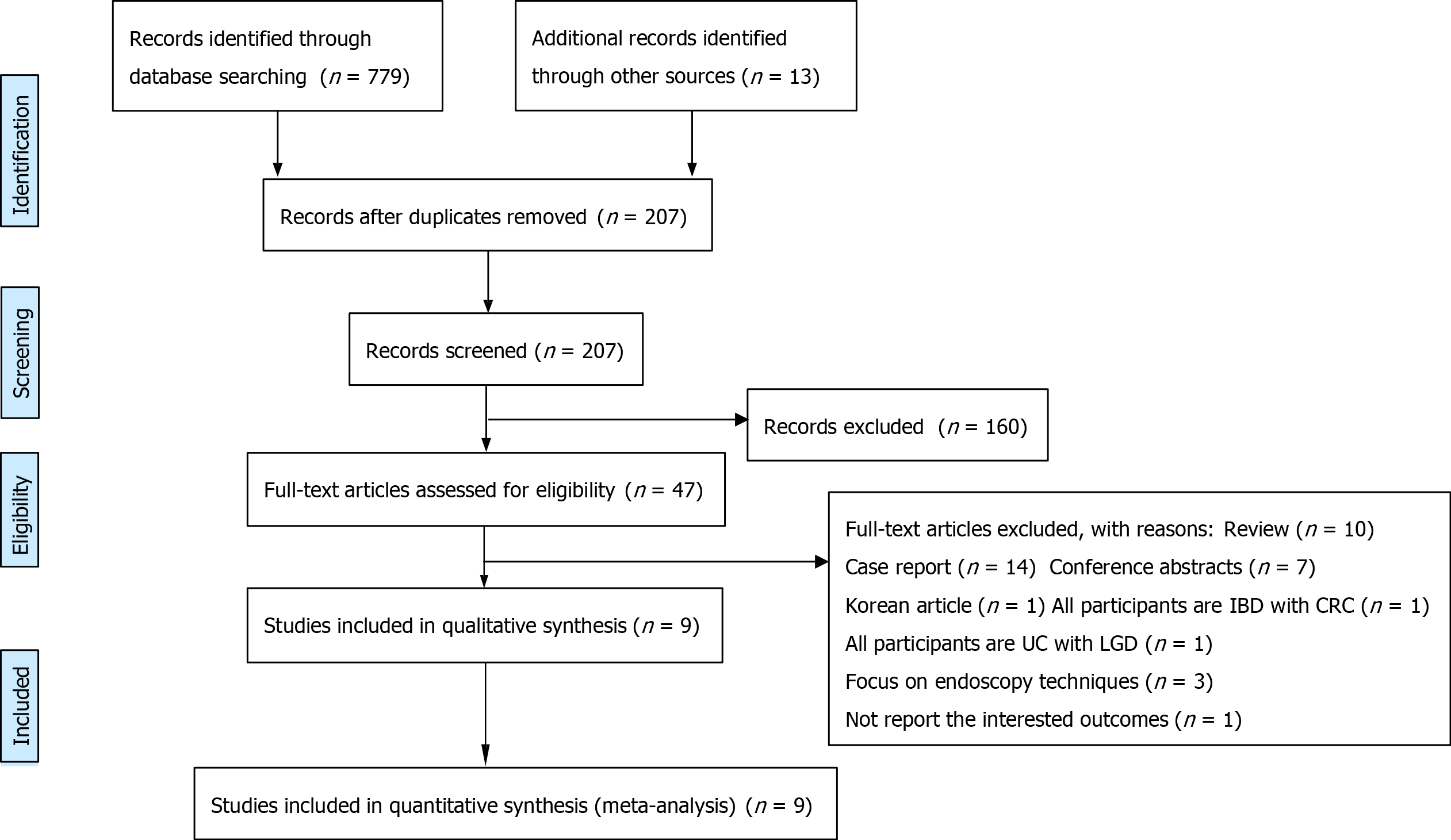
A literature search was conducted up to July 31, 2021, with 779 records identified through database searching and 13 additional records identified through other sources. After removing duplicates, 207 articles were eligible for screening. Researchers excluded 160 articles after screening the titles and abstracts, and 47 articles remained. In the full-text articles assessed, 38 articles were excluded for the following reasons: review (n = 10), case report (n = 14), conference abstracts (n = 7), and paper written in Korean (n = 1). All participants were IBD patients with colorectal cancer (n = 1). All participants were ulcerative colitis patients with low-grade dysplasia (n = 1), interventions focused on endoscopy techniques (n = 3), and there were no reports of the outcomes of interest (n = 1). Ultimately, 9 studies met the inclusion criteria and were all included in the qualitative and quantitative synthesis[12-15,18-22] (Figure 1).
Four cohort studies and five case-control studies were included in this study. The sample sizes of participants ranged from 204 to 1582. PIPs were present in 1944/5424 (35.8%) IBD patients (median prevalence, 29.7%). The median follow-up durations ranged from 3.0 to 22.9 years (median follow-up, 13.0 years). In different IBD phenotypes, five studies exclusively focused on ulcerative colitis (UC), and four remaining studies focused on mixed IBD phenotypes. In different cohort geographies, the included studies were conducted in the Netherlands (n = 4), the United States of America (n = 3), the United Kingdom (n = 2), Belgium (n = 1) and China (n = 1). The summarized characteristics from the included studies are presented in Table 1.
| Included studies | Study design | IBD phenotypes | Country | Median disease duration(yr) | PSC (n, %) | Family history of CRC (n, %) | Extensive colitis (n, %) | Median follow-up time (yr) | The risk of various grades of colorectal neoplasia (PIPs vs nonPIPs) | Conclusion |
| Jong MEd 2019[21] | Cohort Study | UC, CD, UNCLASSIFIED IBD | Netherlands | ≥ 8.0 | 27 (5.2%) | 74 (14.3%) | 345 (66.5%) | 21.6 years in PIPs, 22.9 yr in nonPIPs | CRN 36/154 vs 65/365 (aHR = 1.08, 95%CI: 0.66-1.75a); ACRN 9/154 vs 10/365 (aHR = 1.38, 95%CI: 0.52-3.68b); CRC 6/154 vs 7/365 | PIPs did not increase the risk of CRN, ACRN or CRC |
| Mahmoud R 2019[15] | Cohort Study | UC, CD, UNCLASSIFIED IBD | NetherlandsAmerica | ≥ 8.0 | 234 (14.8%) | 93 (5.9%) | 1275 (80.6%) | 5.4 years in PIPs, 4.5 years in nonPIPs | CRN 64/462 vs 124/1120 (aHR = 1.25, 95%CI: 0.88-1.77c); ACRN 17/462 vs 24/1120 (aHR = 1.17, 95%CI: 0.59-2.31d) | PIPs did not increase the risk of CRN or ACRN |
| Xu W 2020[22] | Cohort Study | UC | China | 6.0 | 10 (4.1%) | NR | 116 (47.2%) | 13.0 | ACRN 11/57 vs 8/189 (aOR = 5.46, 95%CI: 1.69-17.638e) | PIPs increased the risk of ACRN |
| Choi C-HR 2017[14] | Cohort Study | UC | United Kingdom | ≥ 8.0 | 42 (4.3%) | 48 (4.9%) | 987 (100%) | 13.0 | CRN 66/447 vs 31/540 (aHR = 1.20, 95%CI: 0.80-1.80f) | PIPs did not increase the risk of CRC |
| Jegadeesan R 2016[20] | Case-Control Study | UC | American | 12.5 | 47 (10.1%) | 65 (13.1%) | 457 (97.9%) | 3.0 | CRN 32/138 vs 79/329 | PIPs did not increase the risk of CRN |
| Lutgens M 2015[19] | Case-Control Study | UC, CD, UNCLASSIFIED IBD | Netherlands Belgium | NR | 30 (5.7%) | 33 (6.2%) | 349 (65.7%) | NR | CRC 126/260 vs 62/270 (aHR = 2.30, 95%CI: 1.20-4.10g) | PIPs increased the risk of CRC |
| Baars JE 2011[13] | Case-Control Study | UC, CD, UNCLASSIFIED IBD | Netherlands | 9.0 | 22 (4.3%) | 34 (6.6%) | 156 (30.4%) | 15.5 | CRC 71/147 vs 68/366 (aRR = 1.92, 95%CI: 1.28-2.88h) | PIPs increased the risk of CRC |
| Velayos FS 2006[12] | Case-Control Study | UC | American | 17.0 | 50 (13.3%) | 24 (6.4%) | 318 (84.6%) | NR | CRC 105/184 vs 83/192 (aOR = 2.50, 95%CI: 1.40-4.60i) | PIPs increased the risk of CRC |
| Rutter MD 2004[18] | Case-Control Study | UC | United Kingdom | 22.0 | NR | NR | 204 (100%) | NR | CRN 42/95 vs 26/109 (aOR = 2.29, 95%CI: 1.28-4.11j) | PIPs increased the risk of CRN |
Methodological quality was assessed using the ROBINS-I. The overall bias in each included study ranged from moderate to serious. Overall, five studies had a moderate risk of bias, three studies had a serious risk of bias, and one study had an unknown risk of bias. Because of the lack of information on missing data, the study by M D Rutter had unknown risks of missing data and overall bias. The outcomes of interest in our research were not the main outcomes in some studies, which may have led to the lack of detailed data and processing methods. For this reason, studies commonly have a moderate or serious risk in the sections of “bias due to confounding”, “bias in the selection of participants for the study”, and “bias in classification of interventions”. The risk of bias assessment from each included study is presented in Table 2.
| Included study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Jong et al[21], 2019 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Mahmoud et al[15], 2019 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Xu et al[22], 2020 | Moderate | Moderate | Moderate | Low | Low | Low | Moderate | Moderate |
| Choi et al[14], 2017 | Serious | Moderate | Moderate | Low | Low | Low | Moderate | Serious |
| Jegadeesan et al[20], 2016 | Serious | Moderate | Moderate | Low | Low | Low | Moderate | Serious |
| Lutgens et al[19], 2015 | Serious | Moderate | Moderate | Low | Low | Low | Low | Serious |
| Baars et al[13], 2011 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Velayos et al[12], 2006 | Moderate | Moderate | Moderate | Low | Low | Low | Moderate | Moderate |
| Rutter et al[18], 2004 | Moderate | Moderate | Moderate | Low | No information | Low | Moderate | No information |
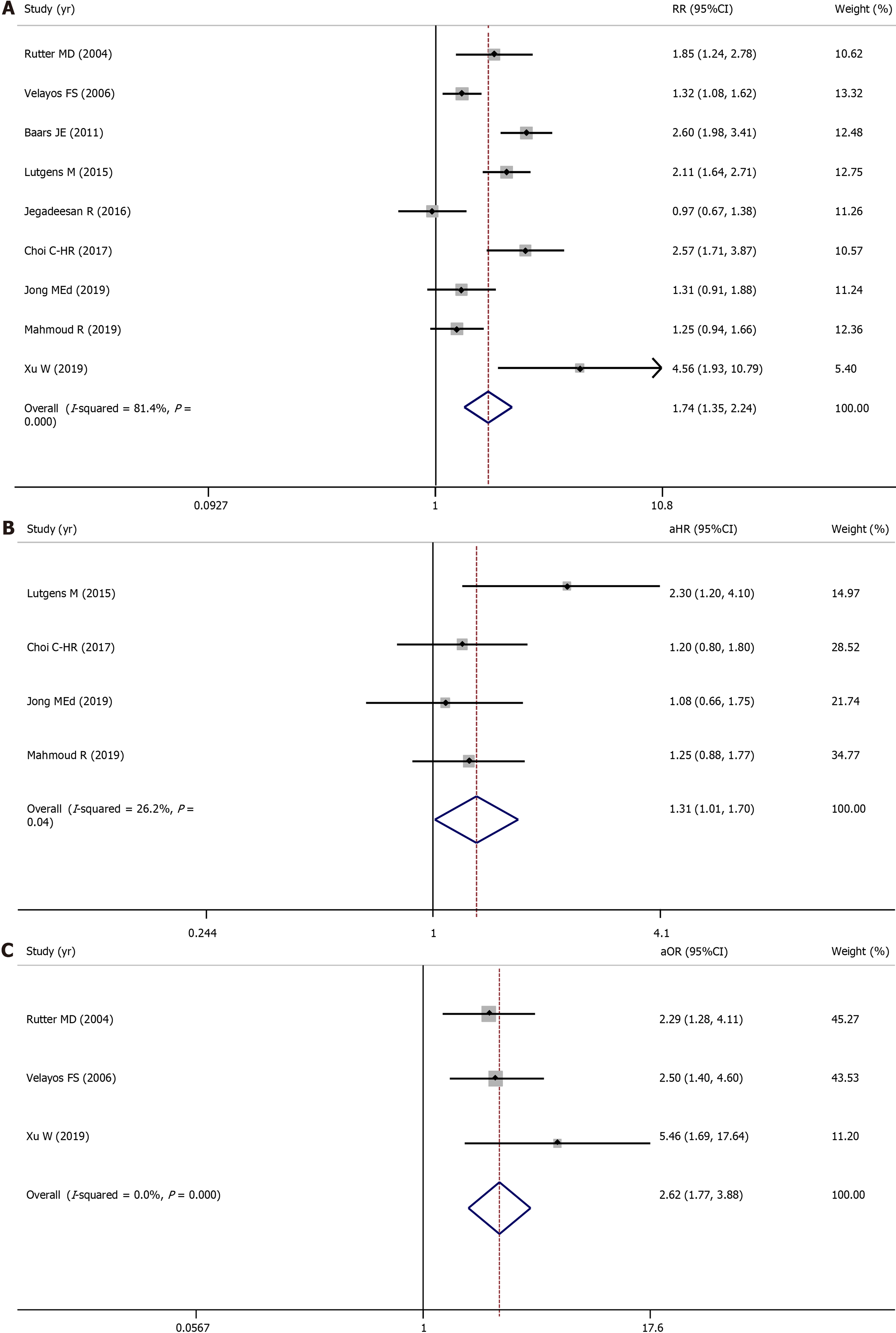
All nine included studies evaluated the association between PIPs and colorectal neoplasia and involved 5424 IBD patients (1944 with PIPs vs 3480 without PIPs). A total of 553 (28.4%) IBD patients with PIPs were diagnosed with colorectal neoplasia, compared with 546 (15.7%) IBD patients without PIPs. Using a random-effects model, IBD patients with PIPs were significantly associated with a higher risk of colorectal neoplasia than IBD patients without PIPs (RR = 1.74, 95%CI: 1.35-2.24, P < 0.001, I2 = 81.4%) (Figure 2A). Four studies reported the adjusted aHR ratio, three studies reported the adjusted aOR ratio, and one study reported the adjusted aRR ratio. When pooling the aHR and aOR, significant differences between these two groups were still observed (pooled aHR = 1.31, 95%CI: 1.01-1.70, P = 0.04, I2 = 26.2%; pooled aOR = 2.62, 95%CI: 1.77-3.88, P < 0.001, I2 = 0%) (Figure 2B, 2C). Publication bias was not observed in Begg’s test or Egger’s test.
In the sensitivity analysis, IBD patients with PIPs were still significantly associated with a higher risk of colorectal neoplasia than IBD patients without PIPs when researchers used the fixed-effects model (RR = 1.67, 95%CI: 1.50-1.85, P < 0.001, I2 = 81.4%). The results did not change after excluding outliers or studies with significant clinical heterogeneity.
In the subgroup analysis, different study designs and methodological qualities did not change the results or heterogeneity of each group. In different IBD phenotypes, five studies exclusively focused on UC and involved 2280 patients (921 with PIPs vs 1359 without PIPs). PIPs were also significantly associated with a higher risk of colorectal neoplasia in UC patients (RR = 1.76, 95%CI: 1.18-2.63, P = 0.006, I2 = 81.6%). Because of the lack of CD and UNCLASSIFIED IBD data, the effects of PIPs on colorectal neoplasia in CD and UNCLASSIFIED IBD patients are not available. In different cohort geographies, patients with PIPs had an increased risk of colorectal neoplasia in Europe (RR = 2.05, 95%CI: 1.62-2.59, P < 0.001, I2 = 60.7%) and Asia (RR = 4.56, 95%CI: 1.93-10.79, P < 0.001, I2 not available). No association was observed in the US (RR = 1.17, 95%CI: 0.86-1.59, P = 0.314, I2 = 56.1%) (Table 3).
| Subgroup | Study | Pooled RR (95%CI) | P value | I2 value,% |
| Colorectal neoplasia | ||||
| Study design | ||||
| Cohort study | 4 | 1.88 (1.18-3.00) | 0.008 | 80.0 |
| Case-control study | 5 | 1.68 (1.20-2.35) | 0.002 | 85.6 |
| Methodological quality | ||||
| Serious/Critical risk of bias | 3 | 1.74 (1.00-3.01) | 0.049 | 87.5 |
| Low/Moderate/Unclear risk of bias | 6 | 1.74 (1.28-2.36) | 0.000 | 80.7 |
| IBD phenotypes | ||||
| UC | 5 | 1.76 (1.18-2.63) | 0.006 | 81.6 |
| CD | NA | NA | NA | NA |
| UNCLASSIFIED IBD | NA | NA | NA | NA |
| Geographic regions | ||||
| Europe | 5 | 2.05 (1.62-2.59) | 0.000 | 60.7 |
| America | 2 | 1.17 (0.86,1.59) | 0.314 | 56.1 |
| Asia | 1 | 4.56 (1.93,10.79) | 0.000 | NA |
| Advanced colorectal neoplasia (ACRN) | ||||
| Study design | ||||
| Cohort study | 3 | 2.42 (1.36-4.32) | 0.003 | 40.1 |
| Case-control study | 3 | 1.92 (1.27-2.90) | 0.002 | 88.6 |
| Methodological quality | ||||
| Serious/Critical risk of bias | 1 | 2.11 (1.64-2.71) | 0.000 | NA |
| Low/Moderate/Unclear risk of bias | 5 | 2.1 (1.35-3.27) | 0.001 | 80.6 |
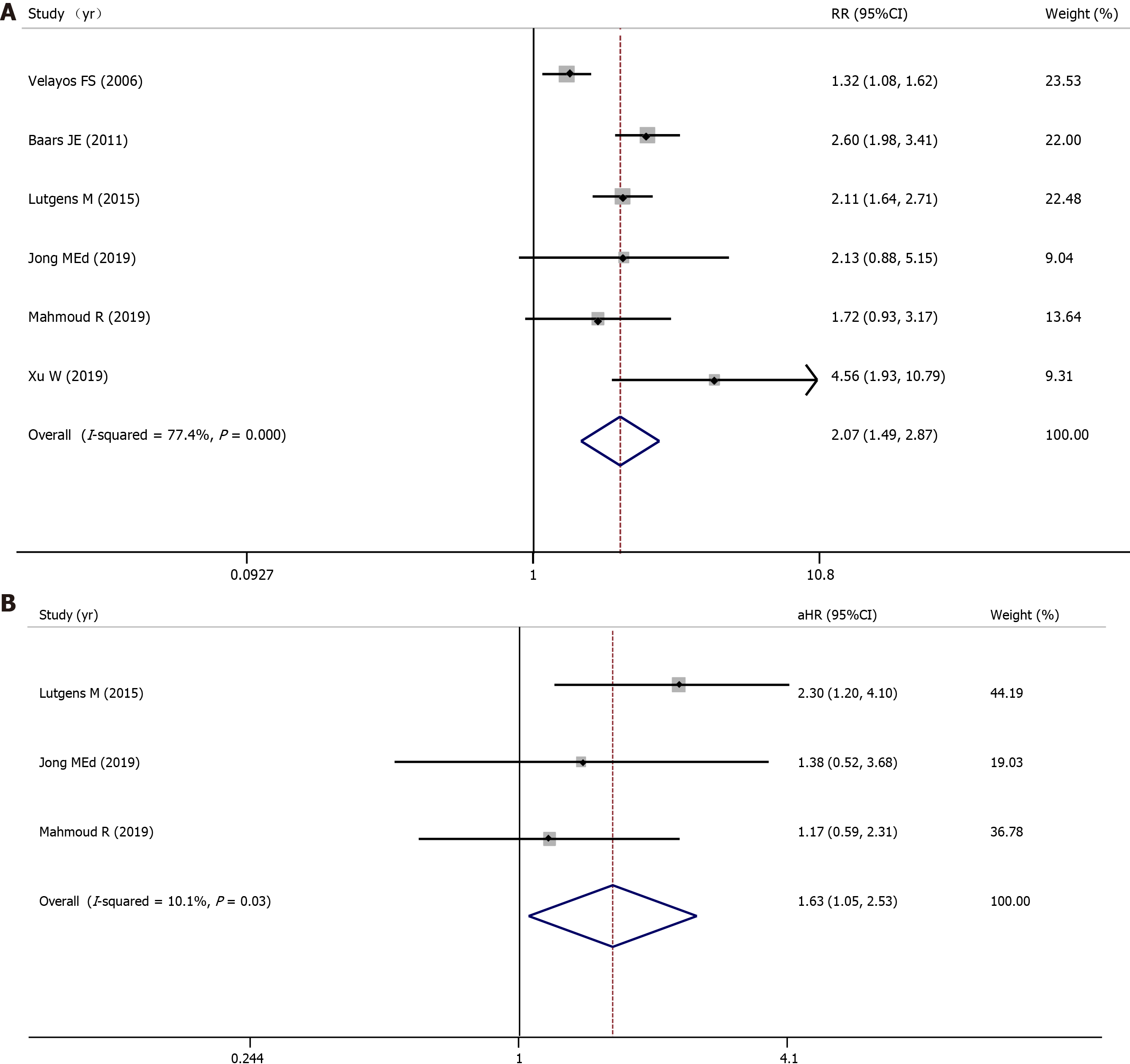
Three cohort studies and three case-control studies evaluated the association between PIPs and advanced colorectal neoplasia and involved 3766 IBD patients (1264 with PIPs vs 2502 without PIPs). A total of 339 (26.8%) IBD patients with PIPs were diagnosed with advanced colorectal neoplasia, compared with 255 (10.2%) IBD patients without PIPs. Using a random-effects model, IBD patients with PIPs were significantly associated with a higher risk of advanced colorectal neoplasia than IBD patients without PIPs (RR = 2.07, 95%CI: 1.49-2.87, P < 0.001, I2 = 77.4%) (Figure 3A).
Three studies reported the adjusted aHR ratio, two studies reported the adjusted aOR ratio, and one study reported the adjusted aRR ratio. When pooling the aHR, significant differences between these two groups were still observed (pooled aHR = 1.63, 95%CI: 1.05-2.53, P = 0.03, I2 = 10.1%) (Figure 3B). Publication bias was not observed in Begg’s test or Egger’s test.
In the sensitivity analysis, IBD patients with PIPs were still significantly associated with a higher risk of advanced colorectal neoplasia than IBD patients without PIPs when researchers used the fixed-effects model (RR = 1.91, 95%CI: 1.67-2.18, P < 0.001,
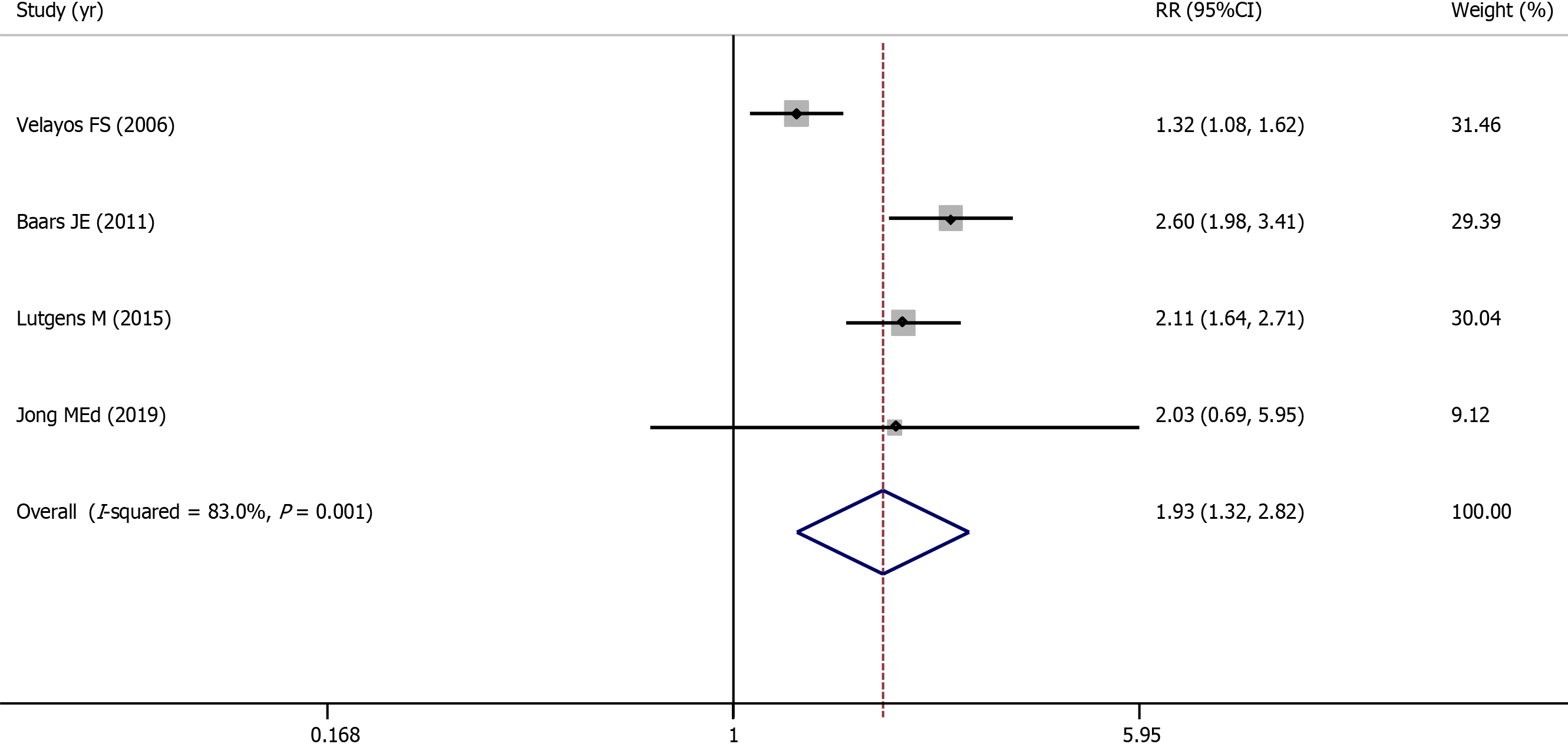
One cohort study and three case-control studies evaluated the association between PIPs and colorectal cancer and involved 1938 IBD patients (745 with PIPs vs 1193 without PIPs). A total of 308 (41.3%) IBD patients with PIPs were diagnosed with colorectal cancer, compared with 220 (18.4%) IBD patients without PIPs. Using a random-effects model, IBD patients with PIPs were significantly associated with a higher risk of developing colorectal cancer than IBD patients without PIPs (RR = 1.93, 95%CI: 1.32-2.82, P = 0.001, I2 = 83.0%) (Figure 4). Publication bias was not observed in Begg’s test or Egger’s test. Because the adjusted ratios were not available, the pooled adjusted ratio was not calculated. Because few studies were included in this section, sensitivity analysis and subgroup analysis were not performed.
The GRADE approach was used to assess the overall quality of evidence. There is low-quality evidence to support that IBD patients with PIPs bear an increased risk of colorectal neoplasia and colorectal cancer. There is moderate-quality evidence to support that IBD patients with PIPs bear an increased risk of advanced colorectal neoplasia. A summary of the assessment is presented in Table 4.
| Outcomes | Illustrative comparative risksa (95%CI) | Relative effect (95%CI) | No of Participants (studies) | Quality of the evidence(GRADE) | |
| Assumed risk | Corresponding risk | ||||
| NonPIPs | PIPs | ||||
| Association of PIPs with colorectal neoplasia; Follow-up: 3.0-22.9 yr | Study populationb | ||||
| 157 per 1000 | 273 per 1000(212 to 351) | RR 1.74 (1.35 to 2.24) | 5424 (9 studies) | Low | |
| Association of PIPs with advanced colorectal neoplasia; Follow-up: 3.0-22.9 yr | Study populationb | ||||
| 102 per 1000 | 211 per 1000(151 to 293) | RR 2.07 (1.48 to 2.87) | 3766 (6 studies) | Moderate due to large effect | |
| Association of PIPs with colorectal cancer; Follow-up: 3.0-22.9 yr | Study populationb | ||||
| 184 per 1000 | 356 per 1000(243 to 520) | RR 1.93 (1.32 to 2.82) | 1938 (4 studies) | Low |
This study aimed to explore the potential association between PIPs and colorectal neoplasia in IBD patients. The results indicated that IBD patients with PIPs bear an increased risk of colorectal neoplasia, advanced colorectal neoplasia, and colorectal cancer.
In contrast to sporadic colorectal cancer, IBD-related colorectal cancer follows a sequence of “inflammation-dysplasia-carcinoma”. In IBD patients, recurrent mucosal inflammation is the primary risk factor for intestinal neoplasia. The alternating cycling of intestinal inflammation and mucous epithelial cell regeneration provides more opportunities for transcription errors and the subsequent development of neoplasia by activating procarcinogenic genes and inhibiting tumor suppressor genes. The development of colorectal neoplasia is frequently associated with mutations, methylation and dysregulation of genes. It induces microsatellite instability, telomere shortening, and chromosomal instability and further induces tumor progression[23-26]. The related genes and molecules involve the adenomatous polyposis coli (APC) gene, k-ras, deleted in colorectal cancer (DCC) genes, deleted in pancreatic cancer-4 (DPC4) genes, and tumor protein 53 (p53), among others[27-31]. Meanwhile, the inflammatory microenvironment of IBD, which consists of a variety of immune cells, epithelial cells, stromal cells, cytokines and chemokines, has many similarities to the microenvironment of cancer[32]. The innate and adaptive immune systems are involved in tumor development by the release of reactive oxygen species, nitrogen species and cytokines[25]. The use of immunosuppression may also allow neoplasia to progress at a faster rate. Moreover, intestinal dysbacteriosis also appears to play a role in IBD-related colorectal neoplasia, such as Escherichia coli, Bacteroides fragilis, Enterococcus faecalis and Fusobacterium nucleatum[24,33,34].
These changes were detectable not only in dysplastic mucosa but also in morphologically normal intestinal mucosa. Their accumulation will lead to extensive genomic and epigenomic alterations and then create a favorable microenvironment for tumor progression. This phenomenon is called field cancerization[35-37]. In theory, the earlier the field cancerization can be detected, the earlier the interventions will be to slow or stop tumor progression. Unfortunately, the above changes are invisible under endoscopy. Accurately predicting the risk of colorectal neoplasia in IBD patients in the early stage is still challenging. Therefore, looking for visible warning markers of colorectal neoplasia in IBD patients is the focus of current research.
PIPs are formed as a consequence of repeated cycles of active inflammation and regeneration of the intestinal epithelium. Under endoscopy, PIPs look like polyps or loose mucosal tags[10,38]. Although malignant transformation from PIPs is rare, IBD patients with PIPs are at an increased risk of various grades of colorectal neoplasia. Previous studies have shown that PIPs positively correlate with the severity of inflammation and are considered surrogate markers of significant cumulative inflammatory burden[26,39,40]. Given this finding, researchers have proposed that PIPs are visible markers of severe inflammation under endoscopy and an early warning of an increased risk of colorectal neoplasia in IBD patients.
In different IBD phenotypes, the colorectal neoplasia burden of UC patients with PIPs is also increased, which is consistent with the burden of IBD patients. Thus, compared with UC patients without PIPs, a strengthened surveillance strategy is preferable for UC patients with PIPs. Meanwhile, because of the lack of data on Crohn’s colitis patients, there is still doubt whether surveillance intervals should be independent of IBD phenotypes. Additional well-designed trials are needed for further research.
Geographic heterogeneity exists in the incidence of IBD and IBD-associated colorectal cancer[41-43]. Currently, there is controversy regarding reasonable endoscopic surveillance intervals for patients with PIPs. The recommended intervals vary considerably from country to country. Therefore, what actual role does geography play in PIPs and colorectal neoplasia? In this study, compared with patients without PIPs, patients with PIPs had an increased risk of colorectal neoplasia in Europe and Asia. Conversely, no association between PIPs and colorectal neoplasia has been observed in the United States. The reason for this geographic heterogeneity is multifactorial and includes genetics, diet, IBD phenotype, inflammation burden, treatment options, and differences in endoscopic surveillance. However, it is important to note that this result should be interpreted and applied cautiously because of the small numbers of included studies on certain national cohorts. More well-designed trials are needed to verify this variation in future research. In contrast to these results, the American Society of Gastrointestinal Endoscopy (ASGE) recommends annual endoscopic surveillance for IBD patients with PIPs, which is more frequent than the every 2-3 years that is recommended by the European Crohn’s and Colitis Organization (ECCO), the British Society of Gastroenterology (BSG), the Association of Coloproctology for Great Britain and Ireland (ACPGBI) and the National Institute for Clinical Excellence (NICE)[6,8,44,45].
When an endoscopist identifies an IBD patient with concurrent PIPs, what should they do? Because IBD patients with PIPs bear an increased risk of colorectal neoplasia, it is necessary for them to enroll in a rigorous treatment program that includes strengthened endoscopic surveillance strategies to achieve complete histological mucosal healing and identify colorectal neoplasia in an early stage. The purpose of endoscopic surveillance is to detect early dysplastic changes to allow for appropriate management so that there are improvements in quality of life and survival rates. To reduce the rate of missing dysplasia, surveillance should be performed by an experienced gastroenterologist in IBD when the disease is in remission. Adequate bowel preparation, meticulous inspection with slow withdrawal, and the application of advanced endoscopic equipment are key for high-quality surveillance. Detailed recommendations of various societies for IBD patients with PIPs are summarized in Table 5.
| Society | Surveillance intervals | Surveillance techniques |
| AGA 2010 | More frequent surveillance (No specific interval recommended) | Chromoendoscopy with targeted biopsies OR Standard or high-definition colonoscopy along with random biopsies |
| ASGE 2015 | Every year | Chromoendoscopy with targeted biopsies OR Random biopsies (2-4 biopsies every from 10 cm) and targeted biopsies if chromoendoscopy is not available or the yield of chromoendoscopy is reduced |
| Cancer Council Australian 2019 | Every year | Chromoendoscopy with targeted biopsies |
| BSG/ACPGBI 2010 | Every 3 yr | Chromoendoscopy with targeted biopsies OR Random biopsies (2-4 biopsies every from 10 cm) and targeted biopsies if chromoendoscopy is not available |
| NICE 2011 | Every 3 yr | Chromoendoscopy with targeted biopsies |
| ECCO 2013/2017 | Every 2-3 yr | Chromoendoscopy with targeted biopsies OR White light endoscopy with random biopsies (4 biopsies every from 10 cm) and targeted biopsies |
| JSGE 2018/2020 | Not mention the definite interval (Every 1-2 yr for patients with left-sided colitis or extensive colitis) | Chromoendoscopy with targeted biopsies OR Available endoscopic technology with targeted biopsies to increase the neoplasia detection rate |
| Chinese Society of Gastroenterology 2018/2020 | Not mention the definite interval (Every 1-2 yr for patients with left-sided colitis or extensive colitis) | Chromoendoscopy/magnifying endoscopy with targeted biopsies |
When considering endoscopic surveillance intervals, societies recommend different intervals that range from one to three years. European societies suggest that PIPs are an intermediate risk factor for developing colorectal cancer in IBD patients and that IBD patients with PIPs should undergo endoscopic surveillance every 2-3 years[6,44,45]. Nevertheless, US and Australian societies suggest shortening the surveillance interval to every year because they believe that IBD patients with PIPs are at high risk of colorectal cancer[8,46]. In China and Japan, current guidelines and specifications do not mention a definite interval for patients with PIPs. Correspondingly, these Asian societies advocate initiating endoscopic surveillance from 8-10 years after disease onset and recommend annual or biennial endoscopic surveillance for patients with left-sided colitis or extensive colitis[47-49]. To summarize, the optimal interval of endoscopic surveillance for IBD patients with PIPs has not been established, and additional well-designed trials are needed for further research.
How can colonoscopy screening be performed for IBD-associated colorectal cancer? During recent decades, new technology has improved in terms of endoscopic devices, including white light endoscopy (WLE), chromoendoscopy, magnifying endoscopy, endomicroscopy, narrow band imaging (NBI), and endoscopic molecular imaging. Among them, the majority of clinical guidelines recommend methylene blue or indigo carmine chromoendoscopy with targeted biopsies for surveillance colonoscopy. Under chromoendoscopy, the visualization of the colonic epithelium is improved by highlighting the areas of mucosal irregularities and delineating the borders of suspected lesions. Studies have shown that 61%-84% of neoplastic lesions could be visualized by recent endoscopy[50-53]. In this context, targeted biopsies have the advantage of fewer samples. Therefore, although chromoendoscopy takes a longer time and may be more cumbersome, chromoendoscopy with targeted biopsies has a higher dysplasia detection rate and is more cost-effective than conventional colonoscopy[54-58]. However, random biopsies are beneficial for monitoring disease progression, evaluating histologic stage and assessing treatment efficacy. In special circumstances, such as a known history of dysplasia, concomitant PSC or a foreshortened colon, random biopsies are still recommended regardless of the screening method. With advances in optical imaging techniques, it is unclear whether chromoendoscopy should still be used when surveillance is performed with high-definition colonoscopy or new endoscopic imaging. Additional well-designed trials are needed for further research.
The increased risk of colorectal neoplasia in IBD patients with PIPs probably reflects the increased risk of previous severe inflammation rather than the PIPs themselves having malignant potential. In a multicenter cohort study, researchers found that most patients with PIPs undergo colectomy due to uncontrolled inflammation but not colorectal neoplasia[15]. Therefore, it is not necessary to remove PIPs conventionally unless there is diagnostic uncertainty or concerning malignant features or clinical symptoms, such as bleeding or intussusception. Features of underlying malignancy include uneven redness, nodularity, villous texture, slight elevation or depression, friability, obscured vascular pattern, ulcerated or velvety surface, disruption of innominate lines, and inability to lift with submucosal injection[57,59,60]. In patients with multiple PIPs or uncontrolled inflammation, a terrible intestinal mucosal environment makes it difficult for endoscopists to identify abnormal lesions, and prophylactic colectomy should be considered[18]. To summarize, the management of IBD patients with PIPs, including prophylactic colectomy and enhanced endoscopic surveillance, requires careful consideration of the individual patient, their disease, and endoscopic and histologic factors and involves a multidisciplinary team discussion that should include gastroenterologists, surgeons and pathologists.
In this study, the overall quality of evidence was assessed as moderate to low. There are several obstacles to designing and performing randomized controlled trials for endoscopic surveillance of IBD patients, such as ethical issues and the relatively low incidence of colorectal neoplasia. Thus, robust and available evidence usually comes from well-designed multicenter observational trials. Having recognized these limitations, we systematically searched several databases, undertook a meta-analysis of the latest and most favorable evidence, and used multiple methods to verify the robustness of the potential risk between PIPs and colorectal neoplasia. In the three outcomes of interest, the results did not change when researchers excluded outliers or studies with significant clinical heterogeneity. This result indicated that based on the current studies, the results of this meta-analysis are robust and that individual studies have less influence.
A meta-analysis that focused on the prognostic factors for ACRN in IBD patients was published in 2021[61]. Similar to our study, the researchers found that patients with PIPs were at higher risk for ACRN based on three cohort studies and two case-control studies (OR = 3.29, 95%CI: 2.41-4.48, P < 0.001, I2 = 0%). However, this association was not confirmed in the pooled HR analysis (univariable HR = 1.67, 95%CI: 0.99-2.82, P = 0.05, I2 = 0%; multivariable HR = 1.73, 95%CI: 0.88-3.40, P = 0.11, I2 = 56%). A probable reason for this result was that the number of available studies and patients included was too small for an accurate performance assessment. In contrast, we extended the search cutoff time to July 31, 2021 to include additional literature and participants. Finally, three cohort studies and three case-control studies involving 3766 IBD patients (1264 with PIPs vs 2502 without PIPs) were included. The results showed that patients with PIPs were at higher risk for ACRN, which was confirmed in both pooled RR analysis and pooled HR analysis.
This study is the first meta-analysis to separately assess the relationship between PIP and CRN, ACRN and CRC. This study has several strengths. First, this study evaluated the association between PIPs and colorectal neoplasia, advanced colorectal neoplasia, and colorectal cancer separately. Disparity in the risk stratification of different grades of colorectal neoplasia can provide bases for surveillance strategy, treatment options and prognosis judgment. Second, this study used a new tool (ROBINS-I) to assess the methodological quality of each included study. Third, this study used multiple methods to identify the robustness of the results.
This study also has some limitations. First, the heterogeneity of outcomes is high. Therefore, researchers used multiple methods to identify the robustness of the results and conducted subgroup analyses to search for the source of heterogeneity. Second, a family history of colon cancer and concurrent primary sclerosing cholangitis have been reported as risk factors for colorectal neoplasia in several studies. However, because of missing data in the target population, no high-quality evidence could be obtained.
IBD patients with PIPs may have an increased incidence of various grades of colorectal neoplasia. Due to the lower rate of malignant transformation, PIPs do not need to be removed conventionally. However, due to the increased risk of colorectal neoplasia, IBD patients with PIPs should undergo strengthened surveillance to detect early dysplastic changes to allow for appropriate management to improve quality of life and survival rates. Meanwhile, there are still many gaps in this field of research, such as information on safe and reasonable endoscopic surveillance intervals for patients with PIPs and the pathogenic process of PIPs in colorectal neoplasia. Therefore, additional well-designed multicenter trials are needed.
Longstanding intestinal inflammation increases the risk of colorectal neoplasia in patients with inflammatory bowel disease (IBD). Accurately predicting the risk of colorectal neoplasia in IBD patients in the early stage is still challenging. Post-inflammatory polyps (PIPs) are visible markers of severe inflammation under endoscopy. To date, there is controversy in the literature regarding the necessity of a strengthened surveillance strategy for IBD patients with PIPs.
Unnecessary and frequent endoscopic surveillance not only decreases the quality of life of IBD patients but also increases the burdens of health care and resource stewardship. Therefore, it is crucial to explore the potential risk association between PIPs and colorectal neoplasia. A better insight into this topic would help physicians to clarify the safe and reasonable endoscopic surveillance intervals for IBD patients with PIPs.
To determine whether IBD patients with PIPs bear an increased risk of various grades of colorectal neoplasia.
Researchers systematically searched eight databases up to July 31, 2021. Cohort and case-control studies that compared the risk of colorectal neoplasia between IBD patients with or without PIPs and published in English or Chinese were included. Methodological quality was assessed using the Risk of Bias in Nonrandomized Studies-of Interventions (ROBINS-I) assessment tool. The outcomes of interest were the rates of various grades of colorectal neoplasia. The pooled risk ratio (RR) and 95% confidence interval (95%CI:) were calculated using the random-effects model. Begg’s test and Egger’s test were used to calculate the publication bias. Sensitivity and subgroup analyses were performed to verify the robustness of the results. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to assess the overall quality of evidence supporting the outcomes of interest.
Of 792 records, four cohort studies and five case-control studies involving 5424 IBD patients (1944 with PIPs vs 3480 without PIPs) were included in this study. The overall bias in each included study ranged from moderate to serious. After meta-analyses, IBD patients with PIPs were significantly associated with a higher risk of colorectal neoplasia than IBD patients without PIPs (RR = 1.74, 95%CI: 1.35-2.24, P < 0.001, I2 = 81.4%). Meanwhile, patients with PIPs also had a higher risk of advanced colorectal neoplasia (RR = 2.07, 95%CI: 1.49-2.87, P < 0.001, I2 = 77.4%) and colorectal cancer (RR = 1.93, 95%CI: 1.32-2.82, P = 0.001, I2 = 83.0%). Publication bias was not observed. And Sensitivity and subgroup analyses showed that the results are robust. The overall quality of evidence was assessed as moderate to low.
IBD patients with PIPs may have an increased incidence of various grades of colorectal neoplasia. Due to the lower rate of malignant transformation, PIPs do not need to be removed conventionally. However, due to the increased risk of colorectal neoplasia, IBD patients with PIPs should undergo strengthened surveillance to detect early dysplastic changes to allow for appropriate management to improve quality of life and survival rates.
There are still many gaps in this field of research, such as information on safe and reasonable endoscopic surveillance intervals for patients with PIPs and the pathogenic process of PIPs in colorectal neoplasia. Therefore, additional well-designed multicenter trials are needed.
The authors would like to thank Dr. Long JX from the Department of Epidemiology and Biostatistics (School of Public Health, Guangxi Medical University) for his kind help in reviewing the statistical methods and techniques mentioned in the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manrai M, Sitkin S S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-105; quiz 1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 2. | Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 907] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 4. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 451] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 5. | Burke KE, Nayor J, Campbell EJ, Ananthakrishnan AN, Khalili H, Richter JM. Interval Colorectal Cancer in Inflammatory Bowel Disease: The Role of Guideline Adherence. Dig Dis Sci. 2020;65:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR; British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 7. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, Ordás I, Repici A, Rosa B, Sebastian S, Kucharzik T, Eliakim R; European Crohn's and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 8. | American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Foley K, Hwang JH, Jue TL, Khashab MA, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD, DeWitt JM. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81:1101-1121.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 9. | Adami HO, Bretthauer M, Emilsson L, Hernán MA, Kalager M, Ludvigsson JF, Ekbom A. The continuing uncertainty about cancer risk in inflammatory bowel disease. Gut. 2016;65:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Politis DS, Katsanos KH, Tsianos EV, Christodoulou DK. Pseudopolyps in inflammatory bowel diseases: Have we learned enough? World J Gastroenterol. 2017;23:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (3)] |
| 11. | Ashktorab H, Brim H, Hassan S, Nouraie M, Gebreselassie A, Laiyemo AO, Kibreab A, Aduli F, Latella G, Brant SR, Sherif Z, Habtezion A. Inflammatory polyps occur more frequently in inflammatory bowel disease than other colitis patients. BMC Gastroenterol. 2020;20:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Velayos FS, Loftus EV Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Baars JE, Looman CW, Steyerberg EW, Beukers R, Tan AC, Weusten BL, Kuipers EJ, van der Woude CJ. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011;106:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Choi CR, Al Bakir I, Ding NJ, Lee GH, Askari A, Warusavitarne J, Moorghen M, Humphries A, Ignjatovic-Wilson A, Thomas-Gibson S, Saunders BP, Rutter MD, Graham TA, Hart AL. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2019;68:414-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Mahmoud R, Shah SC, Ten Hove JR, Torres J, Mooiweer E, Castaneda D, Glass J, Elman J, Kumar A, Axelrad J, Ullman T, Colombel JF, Oldenburg B, Itzkowitz SH; Dutch Initiative on Crohn and Colitis. No Association Between Pseudopolyps and Colorectal Neoplasia in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2019;156:1333-1344.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10881] [Article Influence: 1209.0] [Reference Citation Analysis (2)] |
| 17. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14931] [Article Influence: 878.3] [Reference Citation Analysis (0)] |
| 18. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Lutgens M, Vermeire S, Van Oijen M, Vleggaar F, Siersema P, van Assche G, Rutgeerts P, Oldenburg B; Dutch Initiative on Crohn and Colitis. A rule for determining risk of colorectal cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:148-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Jegadeesan R, Navaneethan U, Gutierrez NG, Venkatesh PG, Hammel JP, Sanaka MR, Shen B. Pattern of Inflammation on Surveillance Colonoscopy Does Not Predict Development of Colitis-associated Neoplasia. Inflamm Bowel Dis. 2016;22:2221-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | de Jong ME, Gillis VELM, Derikx LAAP, Hoentjen F. No Increased Risk of Colorectal Neoplasia in Patients With Inflammatory Bowel Disease and Postinflammatory Polyps. Inflamm Bowel Dis. 2020;26:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Xu W, Ding W, Gu Y, Cui L, Zhong J, Du P. Risk Factors of Colorectal Stricture Associated with Developing High-Grade Dysplasia or Cancer in Ulcerative Colitis: A Multicenter Long-term Follow-up Study. Gut Liver. 2020;14:601-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Manne U, Shanmugam C, Katkoori VR, Bumpers HL, Grizzle WE. Development and progression of colorectal neoplasia. Cancer Biomark. 2010;9:235-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Cannon J. Colorectal Neoplasia and Inflammatory Bowel Disease. Surg Clin North Am. 2015;95:1261-1269, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Driessen A, Macken E, Moreels T, Jouret-Mourin A. Dysplasia in inflammatory bowel disease. Acta Gastroenterol Belg. 2017;80:299-308. [PubMed] |
| 26. | Pulusu SSR, Lawrance IC. Dysplasia and colorectal cancer surveillance in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2017;11:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Shanahan F. Relation between colitis and colon cancer. Lancet. 2001;357:246-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 389] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 29. | Baek SJ, Kim SH. Colitis-associated colorectal cancer in patients with inflammatory bowel disease. Minerva Chir. 2017;72:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Cohen-Mekelburg S, Schneider Y, Gold S, Scherl E, Steinlauf A. Advances in the Diagnosis and Management of Colonic Dysplasia in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2017;13:357-362. [PubMed] |
| 31. | Axelrad JE, Shah SC. Diagnosis and management of inflammatory bowel disease-associated neoplasia: considerations in the modern era. Therap Adv Gastroenterol. 2020;13:1756284820920779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 33. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1670] [Article Influence: 128.5] [Reference Citation Analysis (1)] |
| 34. | Keller DS, Windsor A, Cohen R, Chand M. Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech Coloproctol. 2019;23:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 35. | Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22:4794-4801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 285] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (4)] |
| 36. | Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Bye WA, Ma C, Nguyen TM, Parker CE, Jairath V, East JE. Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease: A Cochrane Systematic Review and Meta-Analysis. Am J Gastroenterol. 2018;113:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Lim YJ, Choi JH, Yang CH. What is the Clinical Relevance of Filiform Polyposis? Gut Liver. 2012;6:524-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 39. | Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 40. | Shah SC, Itzkowitz SH. Management of Inflammatory Bowel Disease-Associated Dysplasia in the Modern Era. Gastrointest Endosc Clin N Am. 2019;29:531-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007;50:839-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 42. | Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53-61. [PubMed] |
| 43. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1455] [Article Influence: 291.0] [Reference Citation Analysis (0)] |
| 44. | Colonoscopic Surveillance for Prevention of Colorectal Cancer in People with Ulcerative Colitis, Crohn's Disease or Adenomas. London: National Institute for Health and Clinical Excellence (UK), 2011. [PubMed] |
| 45. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1299] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 46. | Cancer Council Australia Surveillance Colonoscopy Guidelines Working Party. Clinical practice guidelines for surveillance colonoscopy, 2018. |
| 47. | Wu K, Liang J, Ran Z, Qian J, Yang H, Chen M, He Y. Clinical practice guidelines and consensus on diagnosis and management of inflammatory bowel disease(2018, beijing)[in chinese]. Zhonghua Shiyong Neikexue Zazhi. 2018;38:796-813. [DOI] [Full Text] |
| 48. | Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinded Medical Association. Experts guideline on digestive endoscopy techniques in the diagnosis and management of inflammatory bowel disease in china [in chinese]. Zhongguo Yanzhengxing Changbing Zazhi. 2020;4:283-291. [DOI] [Full Text] |
| 49. | Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, Saruta M, Hirai F, Hata K, Hiraoka S, Esaki M, Sugimoto K, Fuji T, Watanabe K, Nakamura S, Inoue N, Itoh T, Naganuma M, Hisamatsu T, Watanabe M, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 311] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 50. | Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | van den Broek FJ, Stokkers PC, Reitsma JB, Boltjes RP, Ponsioen CY, Fockens P, Dekker E. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: low yield and absence of clinical consequences. Am J Gastroenterol. 2014;109:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 53. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489-501.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 54. | Subramanian V, Mannath J, Ragunath K, Hawkey CJ. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Wu L, Li P, Wu J, Cao Y, Gao F. The diagnostic accuracy of chromoendoscopy for dysplasia in ulcerative colitis: meta-analysis of six randomized controlled trials. Colorectal Dis. 2012;14:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Picco MF, Pasha S, Leighton JA, Bruining D, Loftus EV Jr, Thomas CS, Crook JE, Krishna M, Wallace M. Procedure time and the determination of polypoid abnormalities with experience: implementation of a chromoendoscopy program for surveillance colonoscopy for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Soetikno R, Subramanian V, Kaltenbach T, Rouse RV, Sanduleanu S, Suzuki N, Tanaka S, McQuaid K. The detection of nonpolypoid (flat and depressed) colorectal neoplasms in patients with inflammatory bowel disease. Gastroenterology. 2013;144:1349-1352, 1352.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 58. | Watanabe T, Ajioka Y, Mitsuyama K, Watanabe K, Hanai H, Nakase H, Kunisaki R, Matsuda K, Iwakiri R, Hida N, Tanaka S, Takeuchi Y, Ohtsuka K, Murakami K, Kobayashi K, Iwao Y, Nagahori M, Iizuka B, Hata K, Igarashi M, Hirata I, Kudo SE, Matsumoto T, Ueno F, Watanabe G, Ikegami M, Ito Y, Oba K, Inoue E, Tomotsugu N, Takebayashi T, Sugihara K, Suzuki Y, Watanabe M, Hibi T. Comparison of Targeted vs Random Biopsies for Surveillance of Ulcerative Colitis-Associated Colorectal Cancer. Gastroenterology. 2016;151:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 59. | Flynn AD, Valentine JF. Chromoendoscopy for Dysplasia Surveillance in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24:1440-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol. 2019;25:4148-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 61. | Wijnands AM, de Jong ME, Lutgens MWMD, Hoentjen F, Elias SG, Oldenburg B; Dutch Initiative on Crohn and Colitis (ICC). Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology. 2021;160:1584-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |