Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.753
Peer-review started: August 10, 2021
First decision: October 20, 2021
Revised: October 29, 2021
Accepted: December 23, 2021
Article in press: December 23, 2021
Published online: January 21, 2022
Processing time: 158 Days and 1.8 Hours
Although the lung injury caused by cardiopulmonary bypass (CPB) has been extensively investigated, the incidence and mortality of lung injury after CPB remain a prominent clinical problem. The poor outcome has been attributed to multifactorial etiology, including the systemic inflammatory response and ischemia reperfusion (I/R) injury during CPB. Lung injury after CPB is a complex pathophysiological process and has many clinical manifestations of mild to severe disease. Which is associated with prognosis. To alleviate this lung injury, interventions that address the pathogenesis are particularly important. This review summarizes the pathogenesis, mechanism and treatment options of lung injury after CPB, such as lung protection with intralipid.
Core Tip: Respiratory dysfunction is a well-recognized side effect of cardiac surgery combined with cardiopulmonary bypass (CPB). The mechanism of lung injury after CPB is unclear, and the lack of effective treatment results in poor prognosis. This review summarizes the mechanisms of lung injury and proposes a new treatment option.
- Citation: Zheng XM, Yang Z, Yang GL, Huang Y, Peng JR, Wu MJ. Lung injury after cardiopulmonary bypass: Alternative treatment prospects. World J Clin Cases 2022; 10(3): 753-761
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/753.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.753
More than 2 million patients worldwide undergo cardiac surgery annually, and most procedures use cardiopulmonary bypass (CPB)[1]. With the advent of extracorporeal circulation and improvement in surgical techniques, the incidence of postoperative complications after cardiac surgery should have been minimal. However, the incidence of pulmonary complications is 20%–35%, which is significant compared with complications after other types of surgery, partly due to systemic inflammatory response syndrome (SIRS) and ischemia reperfusion (I/R) injury during CPB[2-4]. Postoperative pulmonary complications after cardiac surgery with CPB, such as hypoxemia and acute respiratory distress syndrome, are thought to be significant, with poor prognosis and mortality up to 37.5%[4,5]. Survivors may have persistent physical, neuropsychiatric and neurocognitive disorders, which seriously affects quality of life and increases the medical burden.
In an attempt to minimize the deleterious effects of CPB, investigators have explored various strategies including improved CPB devices and methods[6-9] and pharmacological agents to reduce the systemic response. None of these interventions is, however, known to improve clinical outcomes. Steroids have been used for nearly 30 years as a basic treatment strategy for postoperative lung protection after CPB. However, there is conflicting evidence that steroids improve postoperative complications or reduce postoperative mortality in CPB patients[10-13].
Intralipid is a safe emulsion for intravenous application and is widely used in clinical settings[14]. Byrne et al[15] demonstrated that pretreatment with intralipid attenuated intestinal I/R injury in rats. It is not known whether intralipid has a protective effect in the prevention and treatment of lung injury after CPB. Review discusses the pathogenesis and treatment of lung injury caused by CPB, and explores the effects of intralipid on mitochondrial function of pulmonary vascular endothelial cells from three aspects: Mitochondrial respiratory chain, mitochondrial permeability transition pore (mPTP), and mitochondrial membrane potential (ΔΨm). This provides an additional view of the pulmonary protective mechanism of intralipid.
We conducted a narrative review of the mechanism and treatment of lung injury after CPB. PubMed was searched for articles published from December 1983 to July 2021. We carried out the search with the following MeSH or free-text terms: CPB, lung injury, fat emulsion, intralipid, lipid emulsion, coronary heart disease, ischemia reperfusion, on-pump coronary artery bypass graft (CABG), SIRS, reactive oxygen species (ROS), mPTP, and mitochondrial membrane potential. The search was limited to papers written in English, with no restrictions on the type of article. Two independent reviewers (XM and YZ) evaluated the articles for potential inclusion by screening titles and abstracts. The senior author (MJ) further evaluated the full text of articles with any disagreement.
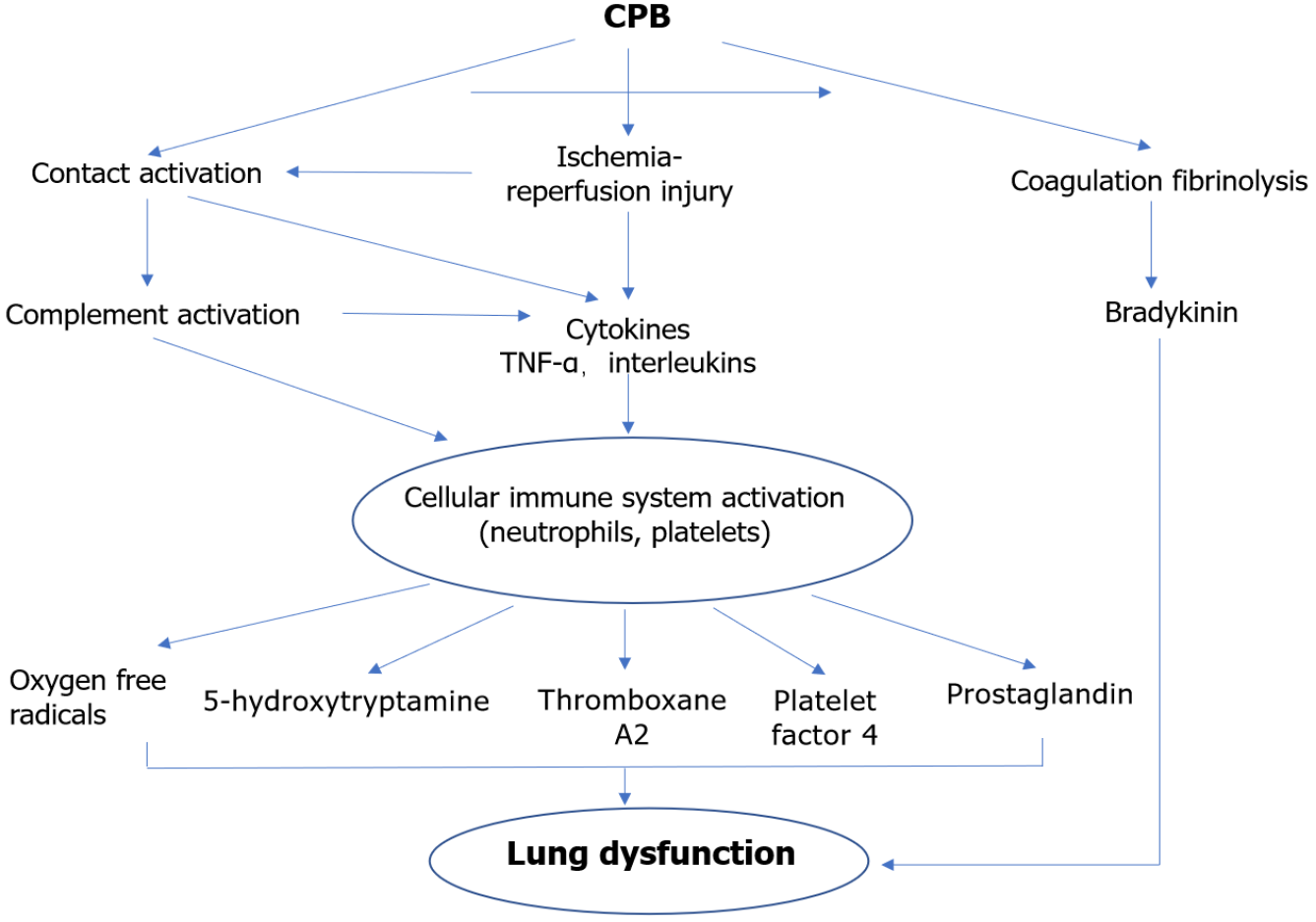
Our understanding of the SIRS to CPB began with the study of Kirklin et al[16] in the 1980s. SIRS is characterized by activation of platelets, neutrophils and macrophages, and cascades (coagulation, fibrinolytic, and kallikrein), which result in increased endothelial permeability and vascular and parenchymal damage[17,18]. These inflammatory responses are associated with the development of lung injury after CPB (Figure 1).
The contact of blood elements with the artificial surfaces of the CPB machine primes, heparin administration, and damage to the hematologic system from incisional, anesthesia and surgery can activate the complement system. C3a expression is activated mainly by CPB via the alternative pathway, and C4a expression is activated by the heparin protamine complex via the classical pathway. Complement (especially C3, C4a and C5a) promotes the release of mast cells and basophils in response to inflammatory mediators such as histamine, resulting in increased permeability of pulmonary epithelial cells and vasodilation[19].
There is increased expression of CD18 and CD11b adhesion molecules on the surface of neutrophils after chemotaxis of interleukin (IL)-8 and induction of C5a[20]. Activated neutrophils release proteolytic enzymes and oxygen-free radicals (O2- and HO), which directly or indirectly damage pulmonary vascular endothelial cells and promote their apoptosis, leading to increased intrapulmonary shunt fraction and pulmonary vascular resistance, and increased lung permeability with interstitial edema[21].
C3a is a powerful platelet aggregation agent. Heparin, hypothermia and duct disruption in CPB release platelets, and platelet activation leads to platelet aggregation, adhesion, consumption and thrombi formation by adsorption with fibrin[22]. Moreover, in late CPB, neutrophils adhere and transmigrate into the lung parenchyma and platelets are retained to block the pulmonary microcirculation. Activated platelets release 5-hydroxytryptamine, prostaglandin, thromboxane A2 and platelet factor 4, which damage pulmonary vascular endothelial cells and mediate lung parenchymal damage locally through cellular and tissue injury. Xiao et al[23] found that peripheral circulating platelets in CPB lung injury were significantly decreased. In addition, Kunitomo et al[24] reported that isolating 20% of platelets from blood before CPB and returning them after surgery significantly improved postoperative cardiopulmonary function, which may be related to the reduced effect of CPB on platelet number and function.
Complement activation, ischemia reperfusion and cytokine interactions can lead to release of cytokines, with tumor necrosis factor (TNF)-α[25] and IL-1, IL-6, IL-8 and IL-10 being the main ones clearly associated with lung injury[26]. TNF-α and IL-1 synergistically activate nuclear factor-κB to promote generation of cytokines and polymorphonuclear cells (PMNs), exacerbating the cascade of cell death signals. These, in turn, lead to endothelial cell swelling, plasma and protein extravasation into the interstitial tissue, aggregation of PMNs and macrophages at the injury site, and, finally, impedance of intra-alveolar cellular perfusion and oxygen exchange, causing lung injury.
Factor XII is activated by blood contact with CPB ducts and by vascular endothelial cell injury resulting in subendothelial collagen exposure, then factor XIIa activates the endogenous coagulation system and kallikrein, bradykinin production, vasodilation and increased permeability. Meanwhile, enhanced fibrinolysis and fibrin degradation products during CPB can lead to lung injury.
The lungs have a dual blood supply from the bronchial artery and pulmonary artery. It has been shown that, under normal physiological conditions, the bronchial artery blood flow is 3%–5% of the total blood flow to the lungs[27]. The vena cava is cut off during CPB, and the metabolic demands of the lungs are totally dependent on oxygen supply from the bronchial arteries. Therefore, the lungs are excluded from the systemic circulation ischemia and hypoxia. Subsequently, ATP and lung surfactant are affected after CPB. Finally, lung permeability increases along with protein exudation. At this time, the lungs are in a hypoxic and relatively hypermetabolic state and are susceptible to endothelial cell injury. Vascular endothelial cells produce a large number of cytotoxic enzymes such as myeloperoxidase (MPO), leading to I/R injury. It has been shown that I/R injury leading to Na+ pump inactivation and Ca2+ overload is an important factor triggering lung tissue injury[28,29]. In addition, mitochondria are important targets of intracellular Ca2+ overload attack, and intracellular Ca2+-dependent proteases are activated, causing impaired energy metabolism and release of cytochrome C (CytC) and apoptosis-inducing factor from the inner and outer mitochondrial membrane gap. Meanwhile, reduced synthesis of endothelium-derived relaxing factor (NO) due to I/R injury can also mediate lung parenchymal injury[30].
Systemic inflammation, surgical trauma, and reperfusion after ischemia play a pivotal role in oxidative stress by initiating a series of biochemical events that result in the generation of excessive amount of ROS[31,32]. Lipid peroxidation is closely related to apoptosis[33]. During CPB, ischemic injury occurs when the blood supply to tissue is suboptimal and accompanied by cellular ATP depletion due to its degradation by hypoxanthine. During periods of stress, cell membrane surface NADH/NADPH oxidase is activated. Meanwhile, ROS levels can increase drastically, leading to substantial damage to many cellular molecules such as lipids, proteins and DNA[34]. Furthermore, ROS results in the production of CytC and damaged mitochondrial membranes with disruption of the alveolar barrier and apoptosis of alveolar epithelial cells[35].
Intralipid, is a safe lipid emulsion for intravenous application, which has been widely utilized as a vehicle for different drugs like propofol and etomidate[14]. It is also used for parenteral nutrition to supplement the body with energy and essential fatty acids. In addition, intralipid has been used in the treatment of cardiotoxicity caused by overdose of local anesthetics such as bupivacaine[36]. Recent animal studies have shown that postischemic administration of lipid emulsion protects the heart against I/R injury[14,37,38]. Meanwhile, clinical studies have also demonstrated that intralipid postconditioning reduces the release of markers of myocardial injury after heart valve replacement and has a cardioprotective effect[39]. In addition, animal studies also suggest that intralipid mitigates impaired pulmonary function induced by I/R through attenuation of local cellular injury and the subsequent SIRS[40]. Therefore, we hypothesize that there is a pulmonary protective function of intralipid, and it is necessary to explore its potential mechanism of action.
The lungs are especially susceptible to the inflammatory attack and I/R injury ascribed to the use of CPB[4]. Oxidative stress and massive release of ROS caused by I/R-induced lung injury, which can lead to functional changes and apoptosis of pulmonary microvascular endothelial cells, result in increased capillary permeability, impaired pulmonary diffusion function, and accumulation of fluid in the interstitial space[41,42]. In solid organs, electron conduction defects occur in ischemic/hypoxic cells, leading to irreparable mitochondrial damage, which is a key mechanism of I/R-induced lung injury[43]. Therefore, maintaining mitochondrial functional homeostasis makes it possible to mitigate I/R damage. Mitochondria store the energy generated as electrochemical potential energy in the inner membrane, resulting in an asymmetric distribution of H+ and other ion concentrations on both sides of the inner membrane to form the ΔΨm[44], which is reflective of metabolic function[45]. ΔΨm is essential for maintaining mitochondria for oxidative phosphorylation and production of ATP, and stability of ΔΨm depends on normal respiratory chain complex activity, proton flow and ATP synthesis[46]. Alteration in the activity of mitochondrial respiratory enzyme complexes during reperfusion of various tissues, which results in an excess of free radicals derived from oxygen and cellular ATP imbalance, has been reported in organs such as the heart, liver and brain[47,48]. Moreover, Sommer et al[49] found that the lungs suffer from the same mitochondrial damage as other solid organs in the pathological situation of I/R, and that changes in the degree of ΔΨm polarization are critical for the development of lung mitochondrial dysfunction[49]. In the postischemic reperfusion phase in the lungs, respiratory chain complex dysfunction, lipid membrane oxidation, and ATP reduction impair the stability of ΔΨm. The above studies suggest that maintaining the stability of ΔΨm is important to reduce lung I/R injury during the early postischemic reperfusion phase.
There is abundant evidence that intralipid can exert myocardial protective effects by inhibiting the opening of mPTP[14]. I/R injury leads to mitochondrial respiratory chain damage and impaired oxidative phosphorylation[50,51]. Mitochondrial damage generates reactive oxygen clusters through complexes I and III and ROS promote oxidative stress. Meanwhile, ROS act as signaling molecules for apoptosis by decreasing mitochondrial ΔΨm and increasing mPTP opening. In the early phase of reperfusion, Ca2+ overload and oxidative stress due to ischemia cause the opening of mPTP[52]. mPTP opening is a key event in cell death after I/R because it causes an abrupt increase in the permeability of the inner mitochondrial membrane to solutes with molecular weights up to 1500 Da, which further causes a decrease in mitochondrial membrane polarization, leading to a decrease in CytC release and matrix swelling, activating caspase 3- and 9-dependent apoptotic cascade responses[52,53]. As mentioned earlier, numerous studies have suggested that intralipid can inhibit the opening of mPTP in cardiomyocytes, therefore, the three aspects of mitochondrial respiratory chain, mPTP and ΔΨm make it possible for intralipid to improve mitochondrial function in pulmonary vascular endothelial cells for lung protection.
Despite the advances in extracorporeal circulation and surgical techniques of recent years, a significant proportion of patients still have a poor outcome. Ischemic and pharmacological preconditioning and postconditioning has been reported to ease I/R injury[38,54]. However, the lack conditions in the clinical setting are not as precisely defined as in the laboratory; therefore, ischemic modulation has limited clinical application[55]. Besides, it is reported that remote ischemic preconditioning does not reduce morbidity or mortality in patients undergoing cardiac surgery with CPB[56]. Therefore, from the view of clinical practice, pharmacological preconditioning or postconditioning is especially promising. Intralipid is a necessary fatty acid carrier and may be a promising approach to improve outcomes after CPB.
Intralipid can be used to treat the cardiotoxicity caused by local overdose of anesthetics and has been clinically proven[57]. The most supported dosing regimen is an intravenous bolus of 1–1.5 mL/kg 20% intralipid. Approximately 12 mL/kg intralipid is the upper limit for initial dosing in adults and 15 mL/kg in children[57-59]. Previous research has provided evidence that intralipid reduces myocardial infarct size and improves cardiac function by pre-reperfusion infusion[38,60,61]. Yuan et al[62] recently conducted a randomized controlled trial using intralipid to assess prognosis and cardiac function in adult cardiac surgery patients after extracorporeal circulation. A single intravenous bolus of intralipid (2 mL/kg, 20%) did not cause abnormal lipid metabolism, with no perioperative hepatic or renal dysfunction, or other related complications[39,62,63]. The duration of intravenous infusion of intralipid should be > 10 min as rapid administration of large amounts increases the risk of fat embolism. The dose of intralipid is selected on the basis of the pill dose when it is used in rescuing the cardiac arrest caused by local anesthetic toxicity, so it leads to a difference in the drug dose. Notably, some studies have found that short infusions of intralipid can cause elevated free fatty acids, induce insulin resistance and increase risk of hyperglycemic events[64], while hyperglycemia is an independent risk factor for mortality and postoperative complications in coronary heart disease surgery[65]. These may limit the use of intralipid on-pump CABG. However, Javaherforoosh Zadeh et al[66] showed that using adjusted tight glycemic control to a level that is near to normal during cardiac surgery may reduce hyperglycemic complications. Due to the lack of research on proper intravenous dosage of intralipid in lung injury after CPB, more data are needed to confirm the specific dose to be used.
The prevention and treatment of lung injury after CPB face many challenges. First, with the advent of extracorporeal circulation and improvement in surgical techniques, the incidence of postoperative complications of cardiac surgery should be minimal. However, the incidence of pulmonary complications and mortality rates are high. Second, the mechanism of lung injury caused by CPB is not clear and needs to be studied in depth. Third, there is a lack of effective treatment methods. The development and use of therapeutic agents against the lung injury after CPB are important. If intralipid demonstrates benefit, it would have rapid uptake globally and have tremendous impact.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Glumac S, Karim HMR S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Costa MA, Trentini CA, Schafranski MD, Pipino O, Gomes RZ, Reis ES. Factors Associated With the Development of Chronic Post-Sternotomy Pain: a Case-Control Study. Braz J Cardiovasc Surg. 2015;30:552-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Ball L, Costantino F, Pelosi P. Postoperative complications of patients undergoing cardiac surgery. Curr Opin Crit Care. 2016;22:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 421] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 4. | Huffmyer JL, Groves DS. Pulmonary complications of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015;29:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 998] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 6. | Suehiro S, Shimizu K, Imai K, Niii A, Akeho K, Nakata H, Yamaguchi A, Matsumoto KI, Oda T. Polymer-coated cardiopulmonary bypass circuit attenuates upregulation of both proteases/protease inhibitors and platelet degranulation in pigs. Perfusion. 2017;32:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Luc JGY, Aboelnazar NS, Himmat S, Hatami S, Haromy A, Matsumura N, Vasanthan V, White CW, Mengel M, Freed DH, Nagendran J. A Leukocyte Filter Does Not Provide Further Benefit During Ex Vivo Lung Perfusion. ASAIO J. 2017;63:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Ziyaeifard M, Alizadehasl A, Massoumi G. Modified ultrafiltration during cardiopulmonary bypass and postoperative course of pediatric cardiac surgery. Res Cardiovasc Med. 2014;3:e17830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hofmann B, Kaufmann C, Stiller M, Neitzel T, Wienke A, Silber RE, Treede H. Positive impact of retrograde autologous priming in adult patients undergoing cardiac surgery: a randomized clinical trial. J Cardiothorac Surg. 2018;13:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Whitlock R, Teoh K, Vincent J, Devereaux PJ, Lamy A, Paparella D, Zuo Y, Sessler DI, Shah P, Villar JC, Karthikeyan G, Urrútia G, Alvezum A, Zhang X, Abbasi SH, Zheng H, Quantz M, Yared JP, Yu H, Noiseux N, Yusuf S. Rationale and design of the steroids in cardiac surgery trial. Am Heart J. 2014;167:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Whitlock RP, Devereaux PJ, Teoh KH, Lamy A, Vincent J, Pogue J, Paparella D, Sessler DI, Karthikeyan G, Villar JC, Zuo Y, Avezum Á, Quantz M, Tagarakis GI, Shah PJ, Abbasi SH, Zheng H, Pettit S, Chrolavicius S, Yusuf S; SIRS Investigators. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:1243-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 12. | Glumac S, Kardum G, Sodic L, Bulat C, Covic I, Carev M, Karanovic N. Longitudinal assessment of preoperative dexamethasone administration on cognitive function after cardiac surgery: a 4-year follow-up of a randomized controlled trial. BMC Anesthesiol. 2021;21:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: A randomised controlled trial. Eur J Anaesthesiol. 2017;34:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, Eghbali M. Phosphorylation of GSK-3β mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology. 2011;115:242-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Byrne J, McGuinness J, Chen G, Hill AD, Redmond MJ. Intravenous omega-3, a technique to prevent an excessive innate immune response to cardiac surgery in a rodent gut ischemia model. J Thorac Cardiovasc Surg. 2011;141:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845-857. [PubMed] |
| 17. | Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 622] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 19. | Eichler W, Bechtel JF, Schumacher J, Wermelt JA, Klotz KF, Bartels C. A rise of MMP-2 and MMP-9 in bronchoalveolar lavage fluid is associated with acute lung injury after cardiopulmonary bypass in a swine model. Perfusion. 2003;18:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Rinder CS, Smith MJ, Rinder HM, Cortright DN, Brodbeck RM, Krause JE, Smith BR. Leukocyte effects of C5a-receptor blockade during simulated extracorporeal circulation. Ann Thorac Surg. 2007;83:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Xu XW, Yang XM, Jin ZX, Zhu SJ. [Effect of Ginkgo biloba extract on the function of alveolar polymorphonuclear neutrophils in severe acute pancreatitis rats complicated with lung injury]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:460-465. [PubMed] |
| 22. | Rinder CS, Rinder HM, Smith MJ, Fitch JC, Tracey JB, Chandler WL, Rollins SA, Smith BR. Antithrombin reduces monocyte and neutrophil CD11b up regulation in addition to blocking platelet activation during extracorporeal circulation. Transfusion. 2006;46:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Xiao da W, Yang M, Yang J, Hon KL, Fok FT. Lung damage may induce thrombocytopenia. Platelets. 2006;17:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Kunitomo R, Kitamura N, Utoh J, Nishimura K, Sakaguchi H, Uemura S, Hagiwara S. Concentrated platelets harvesting before cardiopulmonary bypass improved cardiac and pulmonary function. J Cardiovasc Surg (Torino). 2002;43:161-165. [PubMed] |
| 25. | Lai WY, Wang JW, Huang BT, Lin EP, Yang PC. A Novel TNF-α-Targeting Aptamer for TNF-α-Mediated Acute Lung Injury and Acute Liver Failure. Theranostics. 2019;9:1741-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 26. | Butt Y, Kurdowska A, Allen TC. Acute Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med. 2016;140:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 666] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 27. | Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 273] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Zhang C, Guo Z, Liu H, Shi Y, Ge S. Influence of levosimendan postconditioning on apoptosis of rat lung cells in a model of ischemia-reperfusion injury. PLoS One. 2015;10:e0114963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Consolini AE, Ragone MI, Bonazzola P, Colareda GA. Mitochondrial Bioenergetics During Ischemia and Reperfusion. Adv Exp Med Biol. 2017;982:141-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Karu I, Taal G, Zilmer K, Pruunsild C, Starkopf J, Zilmer M. Inflammatory/oxidative stress during the first week after different types of cardiac surgery. Scand Cardiovasc J. 2010;44:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Ulus AT, Aksoyek A, Ozkan M, Katircioglu SF, Basu S. Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free Radic Biol Med. 2003;34:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Jamal M, Masood A, Belcastro R, Lopez L, Li J, Belik J, Jankov RP, Keith Tanswell A. Lipid hydroperoxide formation regulates postnatal rat lung cell apoptosis and alveologenesis. Free Radic Biol Med. 2013;55:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 34. | Hasunuma H, Shimizu N, Yokota H, Tatsuno I. Azacitidine decreases reactive oxygen species production in peripheral white blood cells: A case report. World J Clin Cases. 2020;8:5657-5662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Zakkar M, Guida G, Suleiman MS, Angelini GD. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev. 2015;2015:189863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 36. | Foxall G, McCahon R, Lamb J, Hardman JG, Bedforth NM. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007;62:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Liu SL, Wang Y, Wang RR, Chai YF, Wu W, Huang H, Liu J. [Protective effect of intralipid on myocardial ischemia/reperfusion injury in isolated rat heart]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20:227-230. [PubMed] |
| 38. | Li J, Iorga A, Sharma S, Youn JY, Partow-Navid R, Umar S, Cai H, Rahman S, Eghbali M. Intralipid, a clinically safe compound, protects the heart against ischemia-reperfusion injury more efficiently than cyclosporine-A. Anesthesiology. 2012;117:836-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Zhou RH, Yu H, Yin XR, Li Q, Chen C, Xiong JY, Qin Z, Luo M, Tan ZX, Liu T. Effect of intralipid postconditioning on myocardial injury in patients undergoing valve replacement surgery: a randomised controlled trial. Heart. 2017;103:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Xia F, Xia Y, Chen S, Chen L, Zhu W, Chen Y, Papadimos TJ, Xu X, Liu L. Lipid emulsion mitigates impaired pulmonary function induced by limb I/R in rats through attenuation of local cellular injury and the subsequent systemic inflammatory response/inflammation. BMC Anesthesiol. 2017;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 591] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 42. | Apostolakis E, Filos KS, Koletsis E, Dougenis D. Lung dysfunction following cardiopulmonary bypass. J Card Surg. 2010;25:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17:3341-3349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Teodoro JS, Palmeira CM, Rolo AP. Mitochondrial Membrane Potential (ΔΨ) Fluctuations Associated with the Metabolic States of Mitochondria. Methods Mol Biol. 2018;1782:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Sakamuru S, Attene-Ramos MS, Xia M. Mitochondrial Membrane Potential Assay. Methods Mol Biol. 2016;1473:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 46. | Lecoeur H, Langonné A, Baux L, Rebouillat D, Rustin P, Prévost MC, Brenner C, Edelman L, Jacotot E. Real-time flow cytometry analysis of permeability transition in isolated mitochondria. Exp Cell Res. 2004;294:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Schild L, Reinheckel T, Wiswedel I, Augustin W. Short-term impairment of energy production in isolated rat liver mitochondria by hypoxia/reoxygenation: involvement of oxidative protein modification. Biochem J. 1997;328 ( Pt 1):205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol. 2009;46:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Sommer SP, Sommer S, Sinha B, Wiedemann J, Otto C, Aleksic I, Schimmer C, Leyh RG. Ischemia-reperfusion injury-induced pulmonary mitochondrial damage. J Heart Lung Transplant. 2011;30:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H2770-H2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol. 1983;244:H743-H748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5324] [Cited by in RCA: 5482] [Article Influence: 195.8] [Reference Citation Analysis (0)] |
| 53. | Palmer JW, Tandler B, Hoppel CL. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am J Physiol. 1986;250:H741-H748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 55. | Iliodromitis EK, Andreadou I, Iliodromitis K, Dagres N. Ischemic and postischemic conditioning of the myocardium in clinical practice: challenges, expectations and obstacles. Cardiology. 2014;129:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Pierce B, Bole I, Patel V, Brown DL. Clinical Outcomes of Remote Ischemic Preconditioning Prior to Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Karcioglu O. Use of lipid emulsion therapy in local anesthetic overdose. Saudi Med J. 2017;38:985-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Neal JM, Barrington MJ, Fettiplace MR, Gitman M, Memtsoudis SG, Mörwald EE, Rubin DS, Weinberg G. The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on Local Anesthetic Systemic Toxicity: Executive Summary 2017. Reg Anesth Pain Med. 2018;43:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 59. | Neal JM, Woodward CM, Harrison TK. The American Society of Regional Anesthesia and Pain Medicine Checklist for Managing Local Anesthetic Systemic Toxicity: 2017 Version. Reg Anesth Pain Med. 2018;43:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 60. | Lou PH, Lucchinetti E, Zhang L, Affolter A, Schaub MC, Gandhi M, Hersberger M, Warren BE, Lemieux H, Sobhi HF, Clanachan AS, Zaugg M. The mechanism of Intralipid®-mediated cardioprotection complex IV inhibition by the active metabolite, palmitoylcarnitine, generates reactive oxygen species and activates reperfusion injury salvage kinases. PLoS One. 2014;9:e87205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Umar S, Li J, Hannabass K, Vaillancourt M, Cunningham CM, Moazeni S, Mahajan A, Eghbali M. Free Fatty Acid Receptor G-protein-coupled Receptor 40 Mediates Lipid Emulsion-induced Cardioprotection. Anesthesiology. 2018;129:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Yuan Y, Xiong H, Zhang Y, Yu H, Zhou RH. Intralipid postconditioning in patients of cardiac surgery undergoing cardiopulmonary bypass (iCPB): study protocol for a randomized controlled trial. Trials. 2020;21:953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Yu H, Li Q, Chen C, Li T, Xiong JY, Qin Z, Luo M, Tan ZX, Liu T, Yu H, Yin XR, Zhou RH. Effect of intralipid on myocardial injury during valve replacement surgery with concomitant radiofrequency ablation: A randomized controlled trial. Medicine (Baltimore). 2018;97:e9603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Park E, Wong V, Guan X, Oprescu AI, Giacca A. Salicylate prevents hepatic insulin resistance caused by short-term elevation of free fatty acids in vivo. J Endocrinol. 2007;195:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Magee MJ, Dewey TM, Acuff T, Edgerton JR, Hebeler JF, Prince SL, Mack MJ. Influence of diabetes on mortality and morbidity: off-pump coronary artery bypass grafting versus coronary artery bypass grafting with cardiopulmonary bypass. Ann Thorac Surg. 2001;72:776-80; discussion 780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Javaherforoosh Zadeh F, Azemati S. Adjusted tight control blood glucose management in diabetic patients undergoing on pump coronary artery bypass graft. A randomized clinical trial. J Diabetes Metab Disord. 2020;19:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |