Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1122
Peer-review started: July 14, 2021
First decision: October 22, 2021
Revised: October 26, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 21, 2022
Processing time: 184 Days and 23.3 Hours
Distant metastasis of colorectal cancer to the anus is very rare, with only 30 related cases published in PubMed thus far. Therefore, recurrence of colorectal cancer derived anus metastases is rarely seen and less presented.
Here we report an 80-year-old male patient who underwent radical resection for sigmoid colon cancer in January 2010 and another surgery for anal fistula resection in December 2010. Postoperative pathology of the anal fistula revealed a metastatic moderately differentiated adenocarcinoma. The patient subsequently received chemotherapy and radiotherapy. In May 2020, after the patient reported symptoms of anal swelling and pain, computed tomography and magnetic resonance imaging revealed a perianal abscess. Perianal mass biopsy was performed, and the postoperative pathological diagnosis was metastatic moderately differentiated adenocarcinoma.
This case highlights that there is a risk of recurrence of anal metastasis of colorectal cancer even after 10 years of follow-up. We also reviewed the literature and discuss potential mechanisms for anal metastasis of colorectal cancer, thus providing some suggestions for treatment of these cases.
Core Tip: Metastasis of colorectal cancer to the anus is very rare. We describe a patient who had a local anal metastatic recurrence after chemotherapy and local anal radiotherapy. This case highlights that there is a risk of recurrence of anal metastasis of colorectal cancer even after 10 years of follow-up.
- Citation: Meng LK, Zhu D, Zhang Y, Fang Y, Liu WZ, Zhang XQ, Zhu Y. Recurrence of sigmoid colon cancer–derived anal metastasis: A case report and review of literature. World J Clin Cases 2022; 10(3): 1122-1130
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1122
The incidence of colorectal cancer is 38.7 per 100000 and 50%-60% of patients develop distant metastases with the liver being the most common site of involvement[1]. The most common seeding metastatic site of colorectal cancer is the anastomosis[2]. In contrast, metastasis at the anus is rare, with only 30 cases published in PubMed thus far. Due to the limited number of cases and insufficient information for anal metastasis of colorectal cancer, the diagnosis of such patients is difficult. In addition, there is currently no standard treatment and postoperative management strategy for anus metastasis of colorectal cancer. In most cases, patients receive surgical treatment and some are also treated with radiotherapy or chemotherapy. Patients who receive surgical treatment typically exhibit a good prognosis with a low recurrence rate. We reviewed and analyzed the relevant literature to provide more information to help clinicians better recognize and treat similar cases in the future.
In May 2020, an 80-year-old man presented with symptoms including anal swelling and pain.
Patient’s symptoms started a month ago with recurrent episodes of anal swelling and pain, as well as blood in the stool and diarrhea.
The patient went to hospital for colonoscopy due to repeated blood in stool in January 2010. A mass in sigmoid was found and pathology showed moderately differentiated adenocarcinoma. Subsequently, he underwent an open radical resection of the sigmoid colon in January 2010. Postoperative pathological examination showed moderately differentiated adenocarcinoma, pT3N1M0, with invasion to the serosal layer; the margin was free and 1 of 29 Lymph nodes was positive. In December 2010, he complained of anal swelling and pain and subsequently underwent anal fistula resection. Postoperative pathology revealed moderately differentiated adenocarcinoma and the margin was free. In December 2010, the patient began 6 cycles of chemotherapy with the FOLFIRI regimen and one course of local anal radiotherapy (45 Gy in 25 fractions). In September 2019, he was admitted to the Department of Hematology for four rounds of Azacytidine chemotherapy for myelodysplastic syndrome (MDS).
The patient did not have any history of anal disease. His family history was unremarkable.
Our physical examination found an approximate 3 cm × 3 cm perianal ring-shaped mass with obvious tenderness.
Blood count shows lymphocyte count 1.03 × 109/L, red blood cell count 3.52 × 1012/L, hemoglobin 114 g/L and albumin 33.2 g/L. Blood tests for cancer-associated markers revealed the carcinoembryonic antigen (CEA) of 5.95 ng/mL and carbohydrate antigen 199 (CA199) of 20.59 U/mL. Fecal occult blood test was positive.
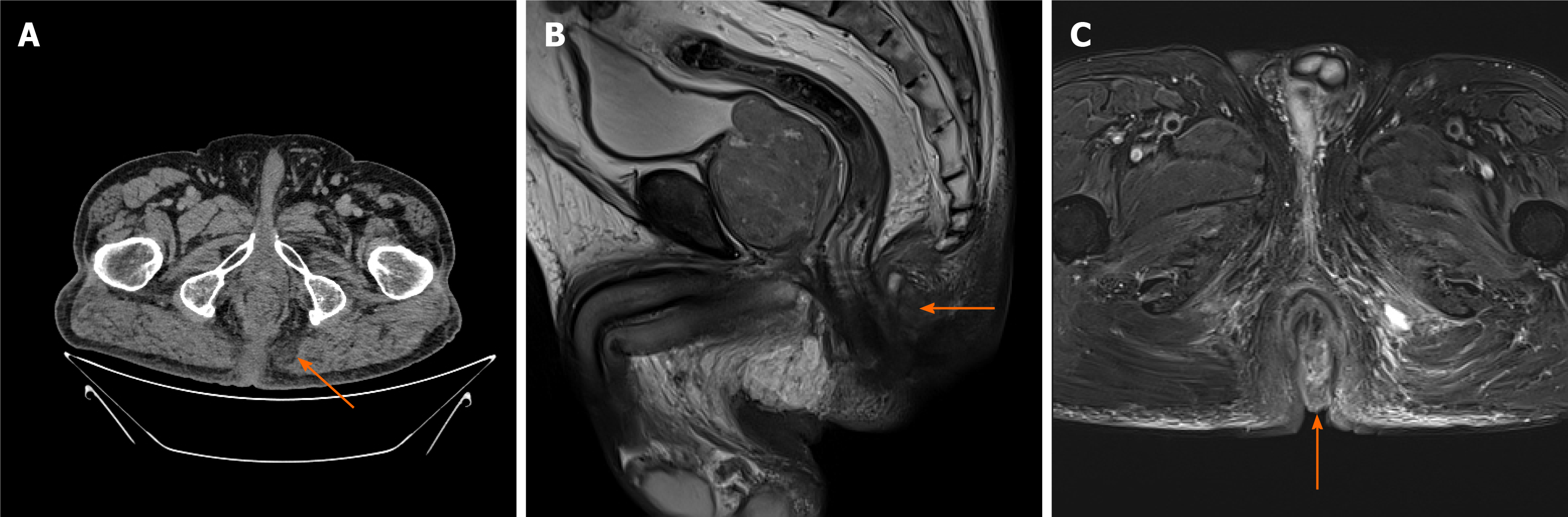
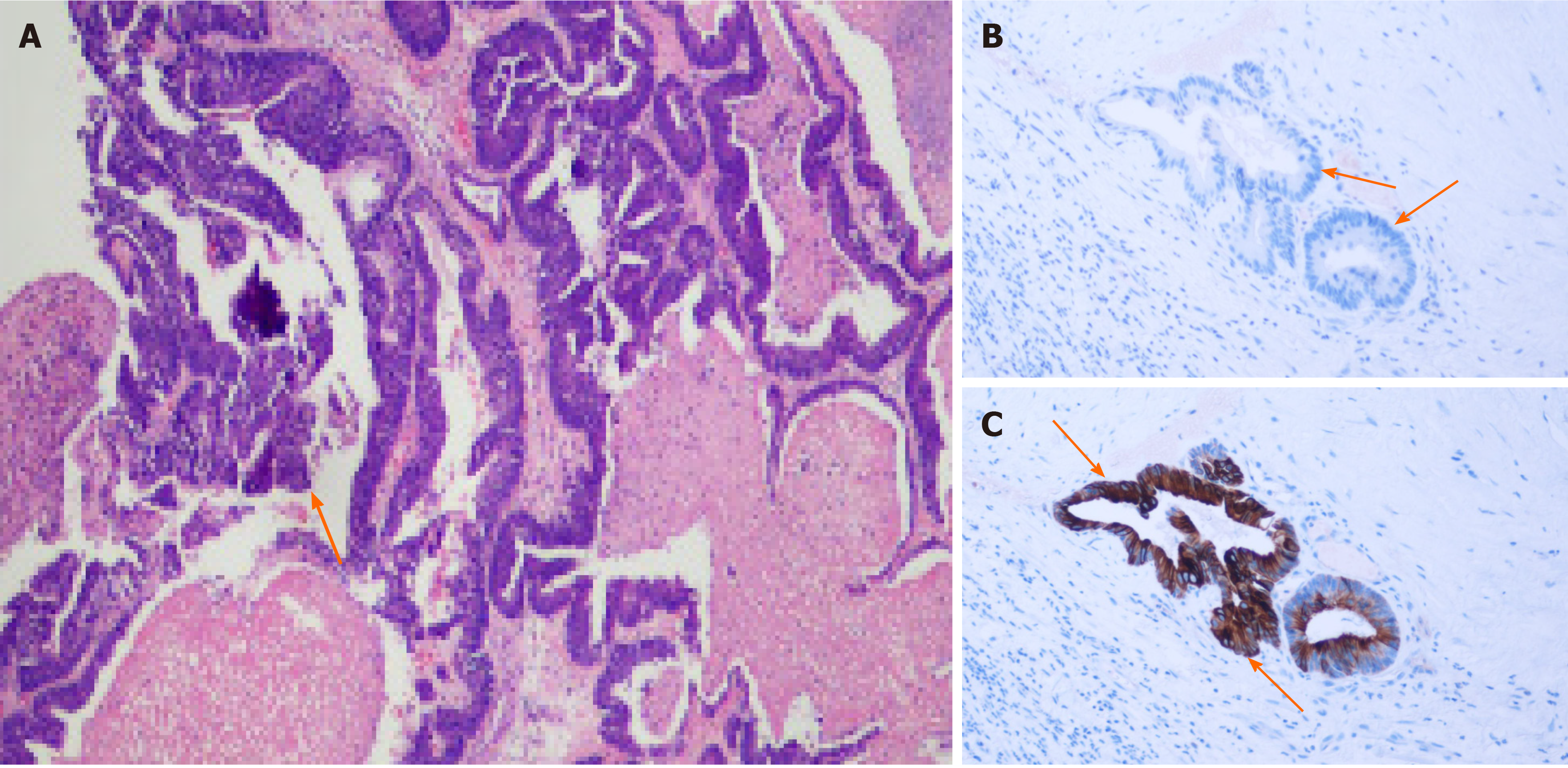
Colonoscopy did not detect any mass or abnormality. Computed tomography found low-density shadows on the posterior edge of the anal canal. Magnetic resonance imaging further confirmed that the 22.8 mm × 24.2 mm lesion went through the external sphincter. The internal fistula was located at 6 o’clock on the posterior edge of the anal canal; the external fistula was at the left side of the buttocks; and the subcutaneous soft tissue signal of the buttocks was increased (Figure 1). Biopsy test of the anal mass was performed by resecting the most obvious swollen part at the lithotomy position. Postoperative pathology of this soft and poorly structured tissue showed moderately differentiated adenocarcinoma with large amounts of necrotic tissue that was positive for cytokeratin 20 (CK20) and negative for cytokeratin 7 (CK7) (Figure 2).
Based on pathology as well as the patient’s history, the final diagnosis was metastatic anal cancer derived from sigmoid colon cancer.
The patient underwent a biopsy test of the anal mass.
We planned to perform abdominoperineal resection (APR) after chemotherapy for MDS in another hospital. However, the patient died due to MDS in November 2020.
The most common distant metastasis site of colorectal cancer is the liver[3]. Regarding implantation metastases, the most common ones are observed at anastomoses and biopsy sites, and some studies have reported metastases at fistulas and hemorrhoids[2,4-9]. Metastasis of sigmoid colon cancer to the anus is very rare[10], and so far, the underlying mechanism remains unknown. One possible explanation for these metastases is that improper operation during surgery may cause tumor cells to fall off and relocate, but in general, tumor cells do not easily implant to intact mucosa. However, the intestinal mucosa could possibly be damaged when surgical instruments or fingers are used to expand the anus during surgery. In this case, damaged intestinal mucosa might become an adhesion target for tumor cells, which would then colonize and begin to proliferate[5,6,8,11]. This phenomenon has been observed in mouse models. For example, Hubens et al[12] observed that mice with damaged intestinal mucosal develop gut tumors after colorectal cancer cells perfuse into the colon, while no mice with intact intestinal mucosa showed tumor growth. Another possible explanation is that tumor cells were already implanted into the existing fistula before resection of the primary tumor. Occasionally, clinical symptoms appear when the tumor grows to a sufficient size[13]. In addition, one study reported the same DNA aneuploid cell line in sigmoid colon tumors and perianal tumors[14]. These findings supported a potential metastasis mechanism of tumor cells migrating from the colon to anus. More cases and studies are warranted to better elucidate the underlying mechanisms.
Diagnostic criteria have not been established in metastatic anal cancer. First, colorectal cancer cases with first symptoms as anal fistula and perianal abscess should be excluded[4]. Additionally, diagnosis of metastatic anal cancer should include primary tumors in the colon with five exclusion criteria for primary anal fistula cancer: (1) More than 10 years of history of anal fistula; (2) Induration and severe pain at the anal fistula; (3) Mucus secretion; (4) Internal opening in the anus and anal recess; and (5) No tumor on the cranial side of the anal fistula[15]. More importantly, immunohistochemical staining of CK7 and CK20 biomarkers is usually used to confirm the presence of a metastatic tumor. Anal tissue shows strong positive expression only for CK7, while colorectal tumor tissue shows positive CK20 expression[16,17]. Immunohistochemical analyses of the tumor in the current case were CK20 positive and CK7 negative, consistent with our diagnosis as metastatic anal cancer.
We further reviewed previous publications of these cases. Guiss[18] published the first case report of sigmoid colon cancer implanted anal fistula in 1954. We retrieved 25 papers from PubMed describing a total of 30 cases of colorectal cancer metastasis to the anus (Table 1). Among the 30 patients, there was only one female, and the mean patient age was 60.2 yr. Seventeen patients (56.7%) had a history of anal disease. Most patients complained of anal abscess and induration as first symptoms. All primary tumors were located in or below the descending colon; 13 tumors (43.3%) were located in the colon, 12 tumors (40%) were at the junction of the rectum and sigmoid colon, and the remaining 5 tumors (16.7%) were in the rectum. This location information may support the idea that seeding metastasis, instead of hematogenous or lymphatic was more likely the cause of anal metastasis formation. All 30 patients underwent radical primary tumor resection; 16 cases (53.3%) had synchronous metastases and the rest 14 cases (46.7%) had metachronous metastases at approximately 8.5 mo post-surgery. Due to the limited number of cases, there is currently no standard treatment method for colorectal cancer–derived anus metastasis. Surgery is still the most common treatment method. Among the 30 cases, 13 patients (43.3%) received APR surgery, 15 patients (50%) underwent additional local lesion resection after radical colorectal surgery, and 3 patients (10%) did not undergo surgery because of extensive tumor metastasis or disapproval of the surgical plan. Overall, postoperative pathology was mostly moderately or well-differentiated adenocarcinoma. Notably, only 11 patients received radiotherapy and chemotherapy during the perioperative period. However, the prognosis of most patients was good. The average follow-up time for patients was 29.9 mo. Only one patient died 10 mo after surgery from extensive peritoneal metastasis[19].
| Ref. | Sex | Age | Anal disease history | Primary tumor | BVI | Lymph nodes | Anal metastasis | Follow-up time (mo) and recurrence | |||||||
| Location | Surgery | Stage | Pathology | Symptoms | Time after primary surgery | Surgery | Pathology | Chemo or radiotherapy | |||||||
| Guiss[18], 1954 | M | 63 | NS | SC | APR | A | NS | NS | NS | Pain, blood in stool | Synchronous | APR | NS | NS | 14/NA |
| Killingbacket al[24], 1965 | M | 63 | 8 yr of AF | SC | APR | A | WDA | - | - | Perianal abscess | Synchronous | APR | WDA | NS | NA |
| Parnes[25], 1976 | M | 56 | Yes | RS | APR | B | NS | - | - | Perianal abscess | 3 mo | LR | NS | No | 18/NA |
| Rollinson et al[26], 1984 | M | 65 | 20 yr of AF | RS | APR | NS | MDA | - | - | Perianal abscess | Synchronous | APR | MDA | No | 10/NA |
| Norgren et al[11], 1985 | M | 60 | No | R | AR | B | MDA | - | - | Perianal abscess, blood in stool | 4 mo | LR | MDA | NS | NS |
| Scott et al[14], 1988 | F | 70 | No | SC | SCR | B | NS | - | - | Pain, blood in stool | 3 mo | LR | NS | No | NS |
| Thomas et al[9], 1992 | M | 68 | 17 yr of AF | SC | APR | B | MDA | NS | NS | Perianal abscess with mass | Synchronous | APR | MDA | NS | NS |
| Wind et al[27], 1999 | M | 70 | No | R | LAR | A | MDA | - | - | No | 6 mo | LR | MDA | No | 30/NA |
| Isbister[13], 2000 | M | 39 | 1 yr of AF | SC | Hartmann | C | MDA | - | + | Pain | 12 mo | LR | MDA | No | NS |
| M | 47 | 20 yr of AF | RS | AR | C | MDA | - | + | Perianal abscess | 12 mo | No | MDA | No | NS | |
| M | 69 | 20 yr of AF | SC | SCR | C | NS | + | + | Perianal mass | synchronous | No | NS | No | NS | |
| Shinohara et al[28], 2001 | M | 36 | 16 yr of AF | R | AR | C | MDA | + | + | No | 21 d | LR | MDA | NS | 6/liver metastasis |
| Kouraklis et al[29], 2002 | M | 75 | 1 yr of AF | RS | APR | B | MDA | - | - | Perianal abscess | Synchronous | APR | MDA | NS | NS |
| Hyman et al[30], 2003 | M | 66 | 15 yr of AF | SC | APR | B | MDA | - | - | Perianal abscess | Synchronous | APR | MDA | NS | 12/NA |
| Gupta et al[8], 2005 | M | 44 | NS | DC | DCR | C | MDA | - | - | Perianal abscess | Synchronous | LR | MDA | NS | 36/NA |
| Hamada et al[17], 2005 | M | 53 | 7 yr of AF | RS | AR | B | W | - | - | Blood in stool | 20 d | LR | WDA | After surgery | 12/NA |
| Ishiyama et al[19], 2006 | M | 53 | 20 yr of AF | R | LAR | C | MDA | - | + | Pain | Synchronous | LR | MDA | NS | 10/death due to peritoneal metastasis |
| Sandiford et al[31], 2006 | M | 72 | 7 yr of AF | RS | SCR + LR | C | MDA | - | - | Blood in stool, diarrhea | Synchronous | SCR + LR | NS | After surgery | 14/NA |
| Gravante et al[15], 2008 | M | 64 | No | DC | DCR | A | MDA | - | - | No | 1 mo | APR | MDA | After surgery | 14/NA |
| Wakatsuki et al[32], 2008 | M | 57 | 7 yr of AF | RS | AR | C | MDA | - | + | Anal mass | 27 mo | LR | MDA | NS | 43/NA |
| Yokoyama et al[33], 2010 | M | 72 | NS | SC | SCR | C | MDA | - | + | Blood in stool | 26 mo | APR | MDA | NS | 132/Lung metastasis |
| Takahashi et al[34], 2011 | M | 61 | 5 yr of AF | RS | APR | C | MDA | + | + | Anal mass | Synchronous | APR | MDA | Before surgery | 36/NA |
| M | 47 | No | SC | Pelvic lymphadenectomy | C | MDA | + | + | Perianal abscess | Synchronous | APR | MDA | Before surgery | 87/NA | |
| M | 59 | No | R | AR | C | MDA | - | + | Anal mass | 6 mo | APR | MDA | After surgery | 3/NA | |
| Benjelloun et al[35], 2012 | M | 55 | 10 yr of AF | RS | AR + LR | B | WDA | - | - | Anal mass | Synchronous | AR + LR | WDA | Before surgery | 36/NA |
| M | 68 | No | RS | AR + LR | B | WDA | - | - | Perianal abscess | Synchronous | AR + LR | WDA | Before surgery | 36/NA | |
| Hakoda et al[36], 2017 | M | 65 | NS | R | AR | C | MDA | + | + | Pain | 18 mo | APR | MDA | NS | 18/NA |
| Fathallah et al[6], 2018 | M | 49 | No | SC | SCR | C | MDA | - | + | Perianal abscess, diarrhea | Synchronous | No | MDA | Before surgery | NS |
| Ikeda et al[5], 2019 | M | 69 | Yes | RS | Hartmann | B | WDA | - | - | Perianal abscess | 1 mo | LR | WDA | Before surgery | 31/NA |
| Badiani et al[37], 2020 | M | 70 | No | RS | APR | B | MDA | - | - | Blood in anus | Synchronous | APR | MDA | Before surgery | NS |
| Our case | M | 80 | No | SC | SCR | C | MDA | - | + | Perianal abscess | 12 mo | LR | MDA | After surgery | 10 yr/recurrence |
| Recurrence | Perianal abscess | 10 yr | LR | MDA | No | 5/death due to MLD | |||||||||
Compared with the previously reported cases, our cases show some unique characteristics and findings. The patient received radiotherapy and chemotherapy after resection of local anal metastatic lesions in 2010, but recurrence of local anal tumor still occurred 10 years later. However, this patient needed chemotherapy for MDS with ring sideroblasts and with multilineage dysplasia (RS-MLD), so only a perianal mass biopsy was performed to confirm the diagnosis. Although APR surgery was planned after the chemotherapy, the patient still required management for RS-MLD and died 5 mo later.
The main surgical treatment options are APR and local resection. Although APR is more effective in reducing the risk of residual tumor cells, the life quality of patients is relatively poor. Therefore, we suggest that local resection should be considered first to ensure that patients have a better quality of life after surgery when the anus tumor does not aggressively grow. In addition, Ikeda et al[5] indicated that tumors should be treated first when the patient exhibits other anal diseases. Otherwise, it is possible that the tumor cells may easily implant on the anal wound and cause anal recurrence. Regarding perioperative radiotherapy and chemotherapy, a retrospective study of metastatic anal cancer patients from 1950 to 2011 found that the combination of preoperative or postoperative radiotherapy, chemotherapy and radical surgical resection provided patients with better survival compared with patients receiving surgeries only[20]. At present, there is no standard postoperative follow-up management guideline, so we should extend the postoperative follow-up time for such patients to detect the disease and provide treatment in a timely manner.
The current patient reported no anal disease before the first radical surgery. Although without immune-histological result, histological features of this anal mass were moderately differentiated gland cancer, similar to primary tumor in sigmoid colon. Considering the anatomical structure of the colon and anus and combined with the patient’s medical history, we therefore believe that the lesion was derived from sigmoid colon tumor cells. Since any shed tumor cells would not be implanted on the intact intestinal mucosa, as discussed above, we assume that this may be from stapler use that damaged the anal mucosa during the operation. The patient showed a relapse at the anus, and the colonoscopy showed no tumor in the colon. Moreover, immunohistological results showed the tumor was derived from colon, so it was possible that a small amount of tumor cells had remained in the anus. Moreover, chemotherapy for MDS for 4 mo potentially impaired the patient’s immune system, causing any remaining tumor cells to proliferate.
Norgren et al[11] and Tranchart et al[21] also reported cases of recurrence of local scars in the anus caused by the use of staplers and retractors during operation. Therefore, surgeons should be aware of the importance of protecting the mucosa during surgical procedures, for example during staple use and retraction. Another study reported the presence of tumor cells in washing solution after rectal washing during surgery. Therefore, sterile water or 5% povidone-iodine and other cytotoxic solutions may be useful to wash the surgical area to reduce the numbers of any remaining tumor cells and prevent local recurrence[22,23]. A close follow-up around the anus after surgery is also recommended.
Metastasis of colorectal cancer to the anus is very rare. The clinical symptoms are similar to benign anal diseases like perianal abscesses and anal fistula, which makes the diagnosis of metastasis of colorectal cancer to the anus more difficult. Currently, pathological examination and staining of CK7 and CK20 markers can contribute to diagnosis of anal metastases. In addition, surgeons should pay attention to protecting the normal mucosa during operation to reduce the possibility of implant metastasis caused by iatrogenic injury. During surgery, surgical area irrigation with cytotoxic solution is also recommended to reduce the number of remaining tumor cells. For patients with anal metastasis, the follow-up time after surgery should be extended. Accumulating more clinical data is necessary to establish treatment and postoperative management standards for colorectal cancer–derived anal metastasis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kanat O S-Editor: Li JH L-Editor: A P-Editor: Li JH
| 1. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 959] [Article Influence: 239.8] [Reference Citation Analysis (16)] |
| 2. | Liasis L, Papaconstantinou HT. Colorectal cancer implant in an external hemorrhoidal skin tag. Proc (Bayl Univ Med Cent). 2016;29:194-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Lan Q, Lai W, Zeng Y, Liu L, Li S, Jin S, Zhang Y, Luo X, Xu H, Lin X, Chu Z. CCL26 Participates in the PRL-3-Induced Promotion of Colorectal Cancer Invasion by Stimulating Tumor-Associated Macrophage Infiltration. Mol Cancer Ther. 2018;17:276-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Gomes RM, Kumar RK, Desouza A, Saklani A. Implantation metastasis from adenocarcinoma of the sigmoid colon into a perianal fistula: a case report. Ann Gastroenterol. 2014;27:276-279. [PubMed] |
| 5. | Ikeda T, Nanashima A, Ichihara A, Kitamura E, Nagatomo K, Tanaka H. A rare case of rectal cancer with perianal metastasis: a case report. World J Surg Oncol. 2019;17:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Fathallah N, Somsouk C, Morcelet MC, Prévot S, de Parades V. [Anal metastasis of sigmoid adenocarcinoma, you had to think about it! Presse Med. 2018;47:829-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Futami R, Shimanuki K, Sugiura A, Tsuchiya Y, Kaneko M, Okawa K, Mineta S, Sugiyama Y, Akimaru K, Tajiri T. Recurrence of colonic cancer twice at the site of stapled colorectal anastomosis. J Nippon Med Sch. 2007;74:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Gupta R, Kay M, Birch DW. Implantation metastasis from adenocarcinoma of the colon into a fistula-in-ano: a case report. Can J Surg. 2005;48:162-163. [PubMed] |
| 9. | Thomas DJ, Thompson MR. Implantation metastasis from adenocarcinoma of sigmoid colon into fistula in ano. J R Soc Med. 1992;85:361. [PubMed] |
| 10. | Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J Gastroenterol. 2015;21:11767-11776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (2)] |
| 11. | Norgren J, Svensson JO. Anal implantation metastasis from carcinoma of the sigmoid colon and rectum--a risk when performing anterior resection with the EEA stapler? Br J Surg. 1985;72:602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Hubens G, Lafullarde T, Van Marck E, Vermeulen P, Hubens A. Implantation of colon cancer cells on intact and damaged colon mucosa and serosa: an experimental study in the rat. Acta Chir Belg. 1994;94:258-262. [PubMed] |
| 13. | Isbister WH. Unusual 'recurrence' sites for colorectal cancer. Dig Surg. 2000;17:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Scott NA, Taylor BA, Wolff BG, Lieber MM. Perianal metastasis from a sigmoid carcinoma--objective evidence of a clonal origin. Report of a case. Dis Colon Rectum. 1988;31:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Gravante G, Delogu D, Venditti D. Colosigmoid adenocarcinoma anastomotic recurrence seeding into a transsphincteric fistula-in-ano: a clinical report and literature review. Surg Laparosc Endosc Percutan Tech. 2008;18:407-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Ramalingam P, Hart WR, Goldblum JR. Cytokeratin subset immunostaining in rectal adenocarcinoma and normal anal glands. Arch Pathol Lab Med. 2001;125:1074-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Hamada M, Ozaki K, Iwata J, Nishioka Y, Horimi T. A case of rectosigmoid cancer metastasizing to a fistula in ano. Jpn J Clin Oncol. 2005;35:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Guiss RL. The implantation of cancer cells within a fistula in ano: case report. Surgery. 1954;36:136-139. [PubMed] |
| 19. | Ishiyama S, Inoue S, Kobayashi K, Sano Y, Kushida N, Yamazaki Y, Yanaga K. Implantation of rectal cancer in an anal fistula: report of a case. Surg Today. 2006;36:747-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Anwar S, Welbourn H, Hill J, Sebag-Montefiore D. Adenocarcinoma of the anal canal - a systematic review. Colorectal Dis. 2013;15:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Tranchart H, Benoist S, Penna C, Julie C, Rougier P, Nordlinger B. Cutaneous perianal recurrence on the site of Lone Star Retractor after J-pouch coloanal anastomosis for rectal cancer: report of two cases. Dis Colon Rectum. 2008;51:1850-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Terzi C, Unek T, Sağol O, Yilmaz T, Füzün M, Sökmen S, Ergör G, Küpelioğlu A. Is rectal washout necessary in anterior resection for rectal cancer? World J Surg. 2006;30:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Okoshi K, Kono E, Tomizawa Y, Kinoshita K. Can rectal washout reduce anastomotic recurrence after anterior resection for rectal cancer? Surg Today. 2020;50:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Killingback M, Wilson E, Hughes ES. Anal metastases from carcinoma of the rectum and colon. Aust N Z J Surg. 1965;34:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Parnes IH. An interesting case of cancer of the sigmoid with concomitant cancer in an anal fistula. Mt Sinai J Med. 1976;43:476-479. [PubMed] |
| 26. | Rollinson PD, Dundas SA. Adenocarcinoma of sigmoid colon seeding into pre-existing fistula in ano. Br J Surg. 1984;71:664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Wind P, Douard R, Poupardin E, Cugnenc PH. Anal implantation of exfoliated tumour cells from a rectal adenocarcinoma after colorectal stapled anastomosis. Eur J Surg. 1999;165:905-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Shinohara T, Hara H, Kato Y, Asano M, Nakazawa Y, Kato T, Nogaki T, Yamashita Y. Implantation of rectal cancer cells in a fistula in ano: report of a case. Surg Today. 2001;31:1094-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Kouraklis G, Glinavou A, Kouvaraki M, Raftopoulos J, Karatzas G. Anal lesion resulting from implantation of viable tumour cells in a pre-existing anal fistula. A case report. Acta Chir Belg. 2002;102:212-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Hyman N, Kida M. Adenocarcinoma of the sigmoid colon seeding a chronic anal fistula: report of a case. Dis Colon Rectum. 2003;46:835-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Sandiford N, Prussia PR, Chiappa A, Zbar AP. Synchronous mucinous adenocarcinoma of the rectosigmoid seeding onto a pre-existing anal fistula. Int Semin Surg Oncol. 2006;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Wakatsuki K, Oeda Y, Isono T, Yoshioka S, Nukui Y, Yamazaki K, Nabeshima S, Miyazaki M. Adenocarcinoma of the rectosigmoid colon seeding into pre-existing anal fistula. Hepatogastroenterology. 2008;55:952-955. [PubMed] |
| 33. | Yokoyama Y, Nishimura Y, Yatsuoka T, Sakamoto H, Tanaka Y, Kurosumi M. [A case of anal metastasis from sigmoid colon cancer in a long-term survivor who had repeated local excisions]. Gan To Kagaku Ryoho. 2010;37:2585-2587. [PubMed] |
| 34. | Takahashi H, Ikeda M, Takemasa I, Mizushima T, Yamamoto H, Sekimoto M, Doki Y, Mori M. Anal metastasis of colorectal carcinoma origin: implications for diagnosis and treatment strategy. Dis Colon Rectum. 2011;54:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Benjelloun el B, Aitalalim S, Chbani L, Mellouki I, Mazaz K, Aittaleb K. Rectosigmoid adenocarcinoma revealed by metastatic anal fistula. The visible part of the iceberg: a report of two cases with literature review. World J Surg Oncol. 2012;10:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Hakoda K, Yoshimitsu M, Emi M, Hirai Y, Kamigaichi A, Osawa M, Kuraoka N, Komo T, Tsubokawa N, Yamakita I, Miguchi M, Aoki Y, Nakashima A, Kano M, Oishi K, Kohashi T, Kaneko M, Funakoshi M, Hihara J, Mukaida H, Hirabayashi N. [Abdominoperineal Resection for Anal Metastasis of Rectal Cancer]. Gan To Kagaku Ryoho. 2017;44:1364-1366. [PubMed] |
| 37. | Badiani S, Cooper E, Berney CR. A Fall Worth It: Cutaneous Metastatic Deposit of a Distant Colorectal Cancer With Fistula-in-Ano. Cureus. 2020;12:e9979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |