Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10811
Peer-review started: July 12, 2022
First decision: August 1, 2022
Revised: August 10, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 78 Days and 17.8 Hours
Serum procalcitonin (PCT) is widely used to diagnose bacterial infection and sepsis. However, PCT may be elevated in some neoplasms. It is important to distinguish infection from no infection in such neoplasms. The relationship between hepatocellular carcinoma (HCC) and PCT is unknown.
A 62-year-old male was admitted due to a hepatic lesion of unknown origin. The patient had an elevated PCT level. Infectious diseases were excluded after app
HCC cells can secrete PCT in the absence of infection and PCT may be used as a marker to monitor the efficacy of tumor therapy.
Core Tip: Serum procalcitonin (PCT) is widely used to diagnose bacterial infection and sepsis. However, PCT may be elevated in some neoplasms. It is important to distinguish infection from no infection in such neoplasms. Here, we report a patient with hepatocellular carcinoma (HCC) with elevated PCT and no evidence of infection. There are no reports on the association between serum PCT levels and HCC. Furthermore, tumor markers were normal in this patient and PCT levels decreased after surgery. Thus, PCT may be used as a marker to monitor the efficacy of tumor therapy.
- Citation: Zeng JT, Wang Y, Wang Y, Luo ZH, Qing Z, Zhang Y, Zhang YL, Zhang JF, Li DW, Luo XZ. Elevated procalcitonin levels in the absence of infection in procalcitonin-secretin hepatocellular carcinoma: A case report. World J Clin Cases 2022; 10(29): 10811-10816
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10811.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10811
Procalcitonin (PCT) is widely used to identify infection in the clinic[1,2]. However, serum PCT levels may be elevated in the absence of infection, such as trauma, mechanical injury, burn, surgery and neoplasms[3,4]. It is important to distinguish infection from no infection when PCT is elevated, especially in cancer patients. Here, we report a patient with hepatocellular carcinoma (HCC) and elevated PCT with no evidence of infection.
The 62-year-old male patient was admitted to Chongqing University Cancer Hospital in December 2017 with a liver tumor of unknown origin. Five days later, the patient suddenly experienced a fever (38.3 ℃) without chills, cough, or diarrhea.
The patient's symptoms began five days before visiting the hospital.
There was no history of jaundice, weight loss or abdominal pain. His medical history included left rib rupture caused by trauma from a traffic accident in July 2007. No history of alcohol misuse or smoking was reported.
The physical examination showed no positive signs.
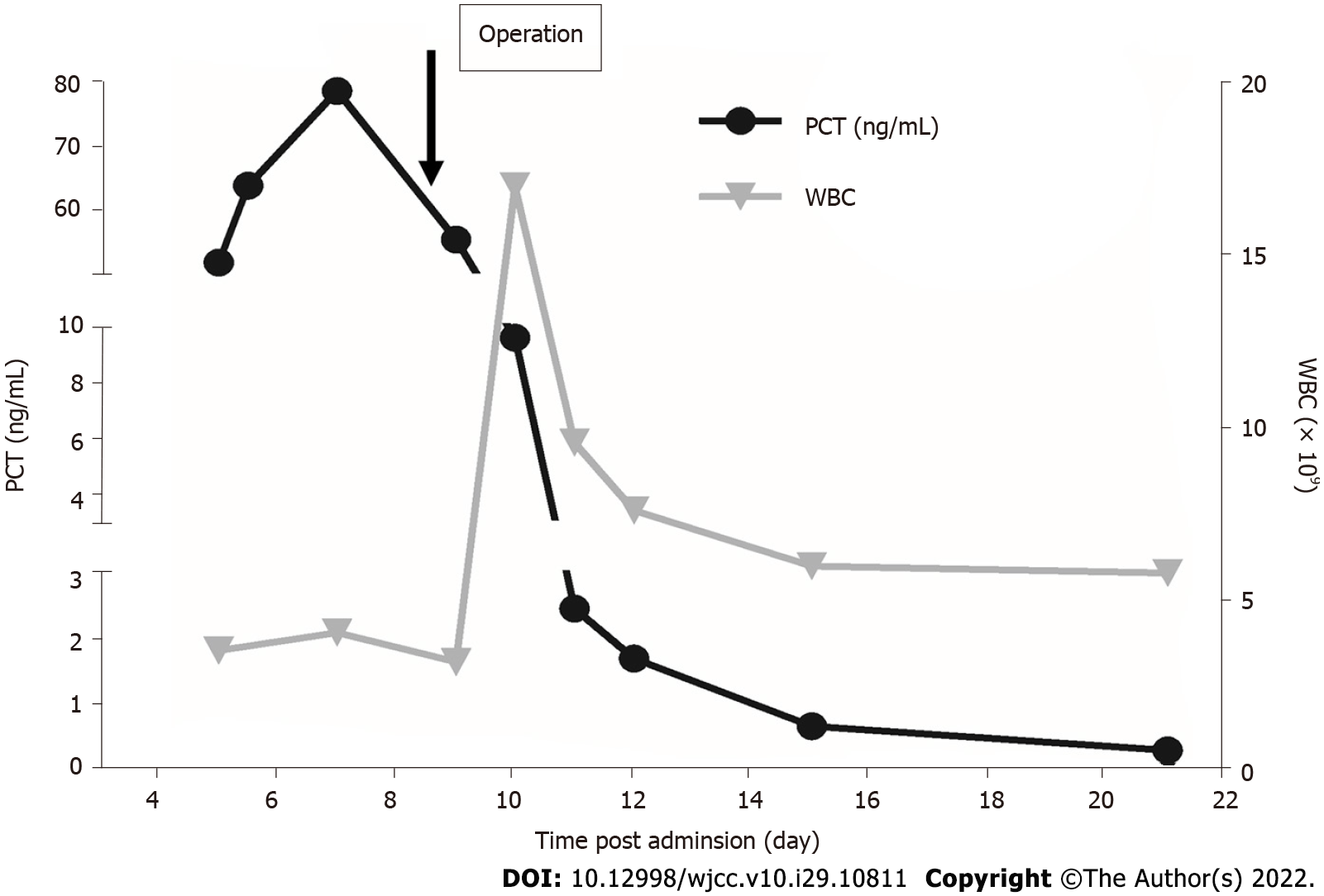
Routine blood analysis was normal. Chronic hepatitis was negative, and liver function was normal and graded as A (score 5) according to the Child-Turcotte-Pugh classification. His alpha-fetoprotein level was 2.56 ng/mL (normal range, 0-8.1 ng/mL), carbohydrate antigen 19-9 was 16.84 U/mL (normal range, 0-30.9 U/mL) and carcinoembryonic antigen was 3.69 ng/mL (normal range, 1-5 ng/mL). Five days later, the patient suddenly experienced a fever (38.3 ℃) without chills, cough, or diarrhea. Blood levels of PCT were 51.62 ng/mL (normal range: 0-0.5 ng/mL) (Figure 1). However, blood analysis showed that C-reactive protein and leukocyte count were normal. In addition, blood culture was negative.
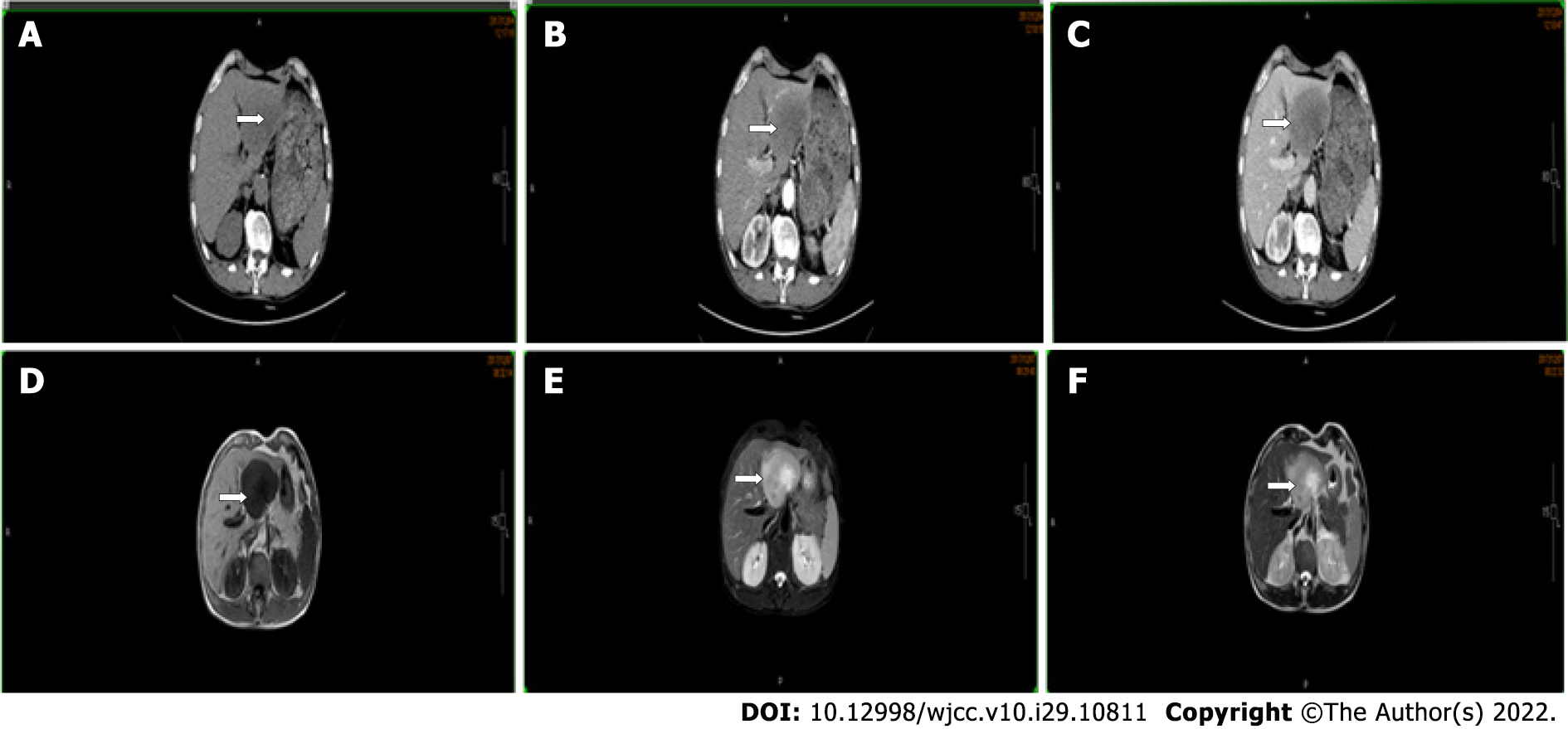
Chest radiography was normal. No tumors were found on esophagogastroduodenoscopy and colonoscopy. Contrast-enhanced computed tomography showed an 80-mm hypo-vascular tumor in the left lateral liver lobe and magnetic resonance imaging revealed a hyperintense tumor on T2-weighted images and diffusion-weighted imaging in the left lateral liver lobe (Figure 2).
The liver lesion was of unknown origin, and a biopsy was recommended. The patient refused to undergo percutaneous biopsy. Therefore, exploratory laparotomy was performed after multidisciplinary consultation.
The patient experienced fever and had an elevated PCT level. However, blood analysis showed normal C-reactive protein and leukocyte count. Blood culture was also negative. After excluding elevated PCT caused by infection, dynamic follow-up was recommended.
As the liver lesion was of unknown origin, there was no evidence of chronic hepatitis and negative findings on esophagogastroduodenoscopy and colonoscopy, a biopsy was recommended. The patient refused to undergo a percutaneous biopsy, and as there were no extrahepatic lesions, an exploratory laparotomy was indicated following a multidisciplinary consultation.
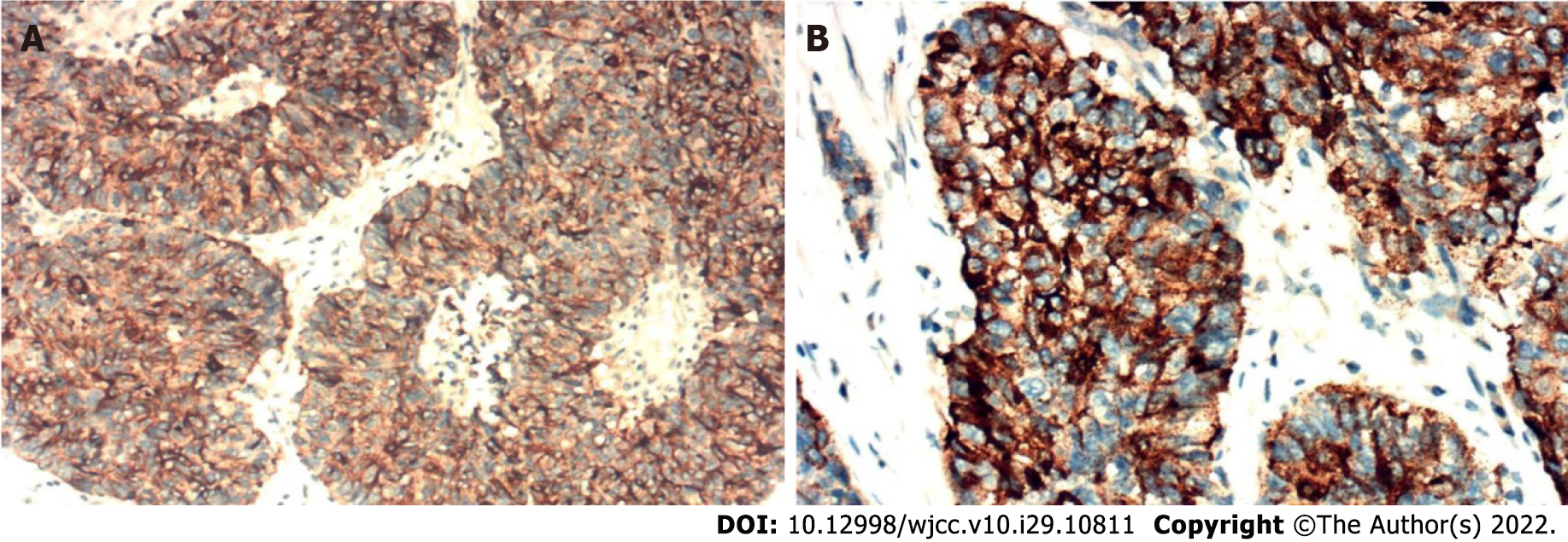
HCC was diagnosed by histopathology and no evidence of infection was observed. Furthermore, immunohistochemical analyses revealed that the tumor cells secreted PCT (clone 44D9, NB120-14817, Novus Biologicals, Littleton, CO, United States) (Figure 3). Therefore, the patient was diagnosed with a PCT-secreting HCC.
The patient underwent exploratory laparotomy. No tumors were found at extrahepatic sites and a left lateral hepatectomy was performed.
A marked decrease in PCT level was observed after surgery and gradually returned to normal on day 16 (Figure 1). The patient recovered with an uneventful postoperative course. There was no recurrence in the liver and PCT level was normal during the 3-year follow-up period.
HCC is the third leading cause of cancer mortality, and is a major cause of death in patients with cirrhosis[5]. Most patients are diagnosed by invasive imaging which has the characteristics of intense contrast uptake in the arterial phase followed by extracellular contrast wash-out in the venous and/or delayed phases. Our patient had no risk factors for HCC, no typical imaging features of HCC and normal tumor marker levels; therefore, a secondary lesion was suspected. Due to negative findings on esophagogastroduodenoscopy and colonoscopy, a biopsy was recommended. The patient chose to undergo surgery, and exploratory laparotomy was indicated following a multidisciplinary consultation. No tumors were identified at extrahepatic sites and left lateral hepatectomy was performed. HCC was diagnosed by histopathology.
PCT, the prototype of a hormokine mediator, is released from all cell types throughout the body during microbial infections and is regarded as a reliable marker of sepsis[2]. A systematic review[1] of the diagnostic accuracy of PCT in bacteremia showed that the optimal and most widely used cut-off value was 0.5 ng/mL, offering a sensitivity of 76% and specificity of 69%. Serum PCT levels may be elevated in the presence of neoplasms, especially in medullary thyroid carcinoma and small-cell lung carcinoma[6]. Nevertheless, the relationship between PCT levels and HCC is unclear. In the present report, the patient had elevated PCT levels with no signs of infection. There are reports that some tumors may secrete PCT. Thus, we suspected that the elevation in PCT may be associated with the liver lesion rather than infection following a multidisciplinary consultation. Exploratory laparotomy was indicated, and the patient subsequently underwent left lateral hepatectomy. The PCT level decreased dramatically and was normal on day 16. There were no features of inflammation on histopathological and immunohistochemical analyses which showed that tumor cells were positive for PCT. Thus, the elevated PCT level was associated with the tumor rather than infection. It was later confirmed that the HCC secreted PCT. To our knowledge, there are no reports on the association between serum PCT levels and HCC. Furthermore, the tumor markers were normal in this patient. The change in PCT may reflect treatment efficacy. Further research is needed to determine the specificity of PCT in patients with HCC and in particular to explore the potential usefulness of PCT in monitoring the response to treatment in this type of HCC.
The unique characteristics of this case should also be noted. First, we report, for the first time, that HCC can lead to an elevation in PCT. Second, PCT may be used as a marker in patients whose tumor markers are normal and the dynamic change in PCT may reflect treatment efficacy.
The limitations in this study are that only one patient was included and measurement of calcitonin level was not performed.
HCC can secrete PCT in the absence of infection and may be used as a marker to monitor the efficacy of tumor therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Shah SIA, Saudi Arabia S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1124] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 3. | Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 383] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 4. | Takahashi K, Ozawa E, Nakao K, Aoki S, Takase Y. Hepatobiliary and Pancreatic: A procalcitonin-secreting and calcitonin-secreting pancreatic neuroendocrine carcinoma. J Gastroenterol Hepatol. 2019;34:964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20369] [Article Influence: 2036.9] [Reference Citation Analysis (19)] |
| 6. | Avrillon V, Locatelli-Sanchez M, Folliet L, Carbonnaux M, Perino E, Fossard G, Desseigne M, Freymond N, Geriniere L, Perrot E, Souquet PJ, Couraud S. Lung cancer may increase serum procalcitonin level. Infect Disord Drug Targets. 2015;15:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |