Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10794
Peer-review started: July 7, 2022
First decision: August 1, 2022
Revised: August 10, 2022
Accepted: September 5, 2022
Article in press: September 5, 2022
Published online: October 16, 2022
Processing time: 84 Days and 4.7 Hours
Castleman's disease (CD), also known as vascular follicular lymphadenopathy is a rare proliferative disease of lymphoid tissue of unknown etiology that is clinically classified as unicentric CD (UCD) or multicentric CD (MCD) depending on lymph node involvement. At present, idiopathic MCD (iMCD) is treated with inter
This paper reports on a case of MCD in a 49-year-old female with persistent peri
The combination of glucocorticoids with tofacitinib is an effective regimen for the treatment of CD.
Core Tip: Castleman's disease (CD) is a rare proliferative disease of lymphoid tissue. The exact pathogenesis of CD remains unclear and is thought to be related to autoimmune diseases, viral infections and malignancies. The current preferred treatment for idiopathic multicentric CD (iMCD) is interleukin-6 inhibitors but there are still some patients who do not respond well to IL-6 inhibitors. In this paper, we report on a patient with iMCD having multiregional lymph node enlargement combined with malignant ascites who improved after treatment with janus kinase inhibitors.
- Citation: Liu XR, Tian M. Glucocorticoids combined with tofacitinib in the treatment of Castleman's disease: A case report. World J Clin Cases 2022; 10(29): 10794-10802
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10794.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10794
Castleman's disease (CD), also known as vascular follicular lymphadenopathy or giant lymph node hyperplasia is a rare proliferative disease of lymphoid tissue of which the exact pathogenesis is still unclear and thought to be related to autoimmune diseases, viral infections and malignancies[1]. The main pathological features are hyperplasia of lymphoid follicles, blood vessels and plasma cells. The disease tends to occur in any age group and is not related to sex. It can occur in all areas where lymph nodes are present but most often occurs in the mediastinum, followed by the head and neck.
CD was first described by Castleman et al[2] in the 1950s and its etiology and pathogenesis are still unclear. In terms of pathology, CD is classified mainly as a hyaline vascular subtype of CD (HV-CD), the plasma cell subtype of CD (PC-CD) or the mixed type of CD (MT-CD). Clinically, CD can be divided into unicentric CD (UCD) and multicentric CD (MCD) according to the number of lymph node-affected areas. MCD can be further divided into human herpesvirus type (HHV)-8-positive MCD and HHV-8-negative MCD according to whether they are infected with HHV8, and the latter can be further divided into idiopathic MCD (iMCD)[3]. The current preferred treatment for iMCD is interleukin (IL)-6 inhibitors, however, some patients do not respond well to IL-6 inhibitors. In this paper, we report on a patient with iMCD with multiregional lymph node enlargement combined with malignant ascites who improved after treatment with a janus kinase (JAK) inhibitor.
A 49-year-old female was admitted to our hospital with persistent thoracoabdominal effusion for 1 year.
One year ago, there was no obvious inducement of abdominal distension, abdominal pain, nausea, vomiting, hematemesis, black stool or edema of either lower limb. She had been to other hospitals many times, where the examination showed that there was peritoneal effusion but the cause of ascites was not clear. Abdominal distension worsened before May and the other hospitals considered "tuberculous peritonitis". The symptoms did not improve significantly after regular anti-tuberculosis treatment. Weight loss of 5 kg occurred over the previous 6 mo.
A history of cesarean section performed in Chishui People's Hospital 19 years ago and cervical polypectomy performed in Chishui People's Hospital 2 years ago. Hypothyroidism was diagnosed in Chishui People's Hospital in the same year and levothyroxine sodium tablets were taken regularly.
She denied any family history of malignant tumors.
The physical examination showed enlarged lymph nodes that could be reached in the right armpit, bilateral neck and groin; abdominal bulge; turbid sounds on percussion; positive mobile turbid sounds; and no significant abnormalities in the rest of the examination.
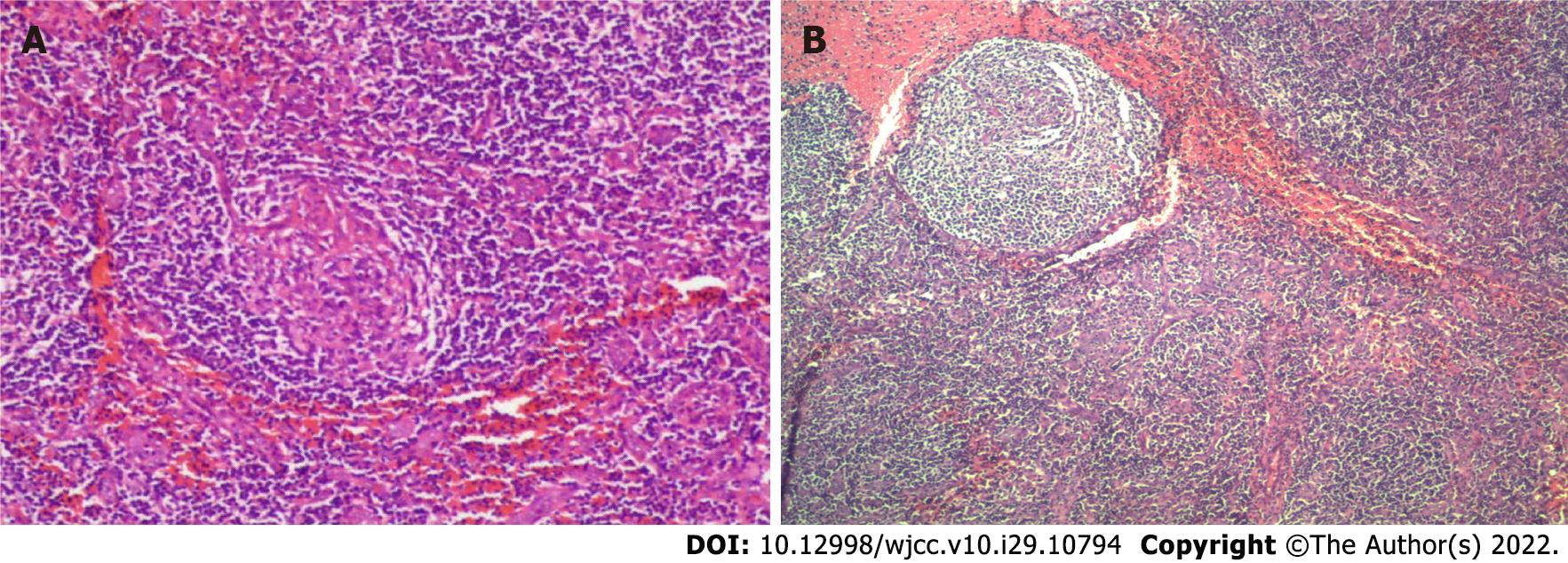
Other hospital biochemical examinations revealed total protein at 29.9-39.7 g/L, albumin at 17.4-25.7 g/L, lactate dehydrogenase at 62-73.1 U/L, and adenosine deaminase at 3.4 U/L, with no acid-fast bacilli. The routine test results of three abdominal punctures and drainage showed that serous mucin was qualitatively positive (+), the li fanka test was weakly positive, and more lymphocytes and proliferative mesothelial cells were observed. Ascites exfoliative cytology revealed no malignant cells. The patient was both negative for acid-fast bacilli in the ascites and free of mycobacterium tuberculosis growth by mycobacterium culture of the ascites. A biopsy of the right lobe of the liver was performed. The results of the pathological biopsy showed that in the liver tissue, a small amount of lymph node infiltration was observed in the hepatic sinus and portal area. A resection biopsy of the left inguinal lymph nodes showed that the 3 left inguinal lymph nodes had reactive hyperplasia. Because the diagnosis was not clear, the right axillary lymph nodes were collected after hospitalization in our hospital and sent to Beijing Boren Hospital for examination. Immunohistochemistry showed the following: Ki-67 [granulosa cell (GC) +, the remaining 5% +], B cell lymphoma (GC -), CD3 (T-region +), CD20 (focal +), CD21 (follicle dendritic cell +), CD10 (granulocyte +, GC weak +), CD138 (small amount +), and HHV8 (-). The in-situ hybridization results showed ebv-eber (-). By pathology, in the right armpit, the following were observed: Benign hyperplasia of the lymph nodes, shrinkage of lymph follicles in the nodes, obvious hyperplasia of the mantle area showing an "onion skin"-like structure and proliferation of blood vessels between and within follicles showing Castleman’s disease-like changes (Figure 1A). This was accompanied by reactive hyperplasia of the lymph nodes, and no tumor or tuberculosis was found (in this case, small lymph nodes were sent for examination, lymph follicles were reduced, the mantle area was significantly proliferated, showing an "onion skin"-like structure, and follicles and intrafollicular vascular proliferation were seen) (Figure 1B). In the bone marrow examination, 36 megakaryocytes and scattered or small piles of platelets were found in the whole film. The detection of T cells in tuberculosis infection results were positive, and the tuberculin test results were (++). The interleukin 6 level was determined by chemiluminescence to be 5.5 pg/mL. No obvious abnormalities in immunoglobulin quantification, antinuclear antibody, antinuclear antibody spectrum, female tumor-associated antigen, delivered IgG4, or anticardiolipin antibody were found. Analysis of the blood showed a hemoglobin level of 113 g/L, an erythrocyte sedimentation rate (ESR) level of 29 mm/h, a hypersensitive C-reactive protein (CRP) level of 28.922 mg/L and no obvious abnormalities in liver function or renal function (Table 1).
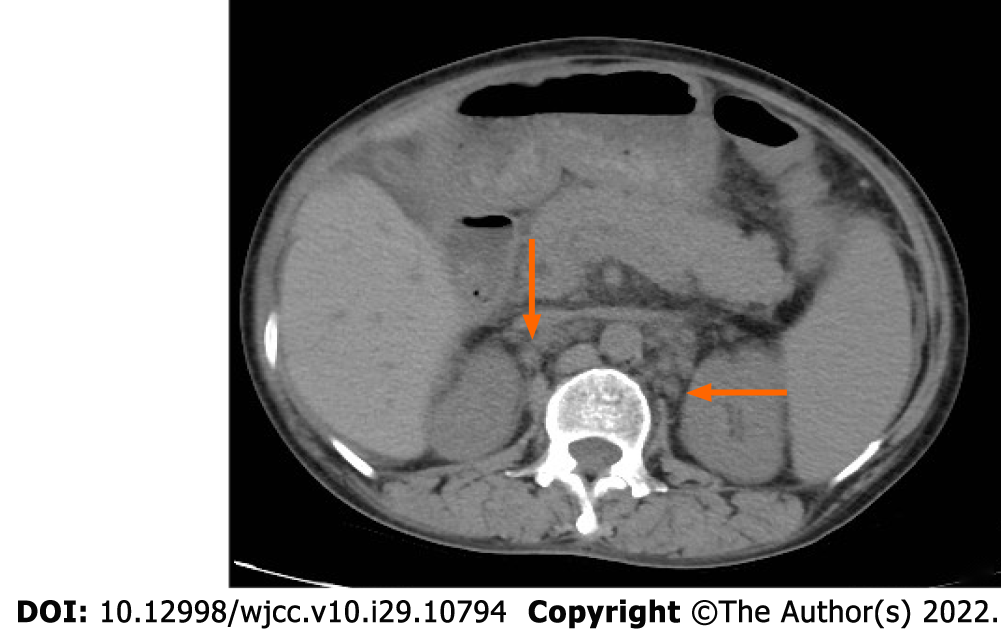
In other hospitals, multiple chest and abdominal computed tomography (CT) examinations showed a small amount of pleural and pericardial effusion and a large amount of abdominal and pelvic effusion. Multiple lymph nodes in the mediastinum, bilateral hilus, bilateral armpits, right septal angle, abdominal pelvic cavity, and bilateral inguinal area were observed to be increased. The lymph nodes in the hilar region and portal space were enlarged and the spleen was enlarged. The CT of the chest and abdomen again in our hospital still showed a large amount of pleural and peritoneal effusion, hepatosplenomegaly and enlargement of multiple lymph nodes in the chest. Later, to exclude tumor diseases, positron emission tomography (PET)-CT examination was performed. PET-CT examination was added to exclude tumor diseases. The results showed that the lymph nodes with low metabolism on both sides of the neck were 0.3-1.0 cm in size (suvmax2.5); there were multiple mild metabolic lymph nodes in the mediastinum and bilateral armpits, with a size of 0.5-1.5 cm (suvmax3.1); retroperitoneal, superior mesenteric, paraaortic, pelvic and bilateral inguinal multiple mild metabolic lymph nodes, with a size of 0.4-1.6 cm (suvmax1.6); slightly enlarged spleen with slightly increased metabolism (suvmax2.3); and abdominal and pelvic effusion. The results of the color Doppler ultrasound of superficial organs demonstrated that lymph nodes were detected in the bilateral neck, armpit, groin area, and right supraclavicular area that showed some morphological abnormalities. The chest CT results demonstrated a small amount of pleural effusion on both sides and a small amount of pericardial effusion, and the mediastinum, bilateral axillary and clavicles lymph nodes were observed to be increased and partially enlarged. The results of the abdominal CT demonstrated that the number of lymph nodes in the abdominal cavity, retroperitoneum, and bilateral inguinal areas increased and there was abdominal and pelvic effusion (Figure 2). The fiberoptic bronchoscopy tuberculosis gene test was negative.
The patient had a large amount of ascites for a long time and the laboratory results showed that ESR and CRP increased and that albumin decreased. The results of several abdominal CT and PET-CT scans showed that there were multiple lymphadenopathies and splenomegaly in the whole body, and the immunohistochemistry analysis of the right axillary lymph tissue showed HHV8 (-). According to the diagnostic criteria of iMCD (Table 2), this patient was considered to have iMCD. The pathological biopsy results of the right axillary lymph tissue showed that the lymph follicles were reduced, the sheath area was proliferated, some of them showed an "onion skin"-like structure, the T area was proliferated, the small lymphocytes had proliferated, the blood vessels had increased significantly, the endothelium was swollen with some of it showing a glassy change, and the blood vessels between and within the follicles had proliferated showing Castleman disease-like changes. The pathological results of the patient were classified as hypervascular type (HyperV).
| Items | Before treatment | After treatment | Reference range |
| ESR in mm/h | 29 | 6 | < 38 |
| HB in g/L | 128 | 119 | 115-150 |
| PLT as × 109/L | 229 | 213 | 100-300 |
| CRP (mg/L) | 28.922 | 2.630 | 0.068-8.200 |
| ALT in U/L | 17 | 14 | 7-40 |
| AST in U/L | 15 | 14 | 13-35 |
| ANA spectrum | Negative | Not reviewed | Negative |
| Acid fast bacillus smear | Acid fast bacteria not found | Not reviewed | Acid fast bacteria not found |
| Female tumor-associated antigen | Negative | Not reviewed | Negative |
| ACA | Negative | Not reviewed | Negative |
| IL-6 in pg/mL | 5.5 | Not reviewed | < 7.0 |
| T-SPOT | Positive | Not reviewed | Negative |
Intravenous infusion of methylprednisolone (100 mL of 0.9% sodium chloride + 40 mg methylprednisolone) was administered once a day and oral administration of tofacitinib citrate 5 mg was administered twice a day. After 1 wk in the hospital, the patient was discharged and continued to take tofacitinib 5 mg twice a day. The hormone was changed to oral 40 mg once a day and gradually reduced to discontinuation. Up to now, the patient has been reviewed regularly and her condition is stable without recurrence.
After 1 wk of treatment, the patient's condition was significantly improved and the erythrocyte sedimentation rate and blood CRP were reduced to normal levels. CT showed that ascites and pericardium in the abdominal cavity and thoracic cavity were obviously reduced. After discharge, the patient continued to take tofacitinib orally until now with stable condition and no recurrence. It indicates that tofacitinib plus glucocorticoid is effective in the treatment of iMCD. Therefore, this treatment is effective.
The exact pathogenesis of CD is not clear but it is generally believed to be related to IL-6 and HHV-8. CD is usually diagnosed by lymph node pathological biopsy. According to the pathological morphology, it can be classified as HV-CD, PC-CD or MT-CD. HV-CD is the most common pathological type of CD and is easily confused with follicular lymphoma. Under the microscope, there is an enlarged lymphoid follicular structure, obvious thickening in the blood vessels, and glass-like changes in later stages. The follicle is surrounded by multilayer circular lymphocytes to form a special onion skin structure. The pathological feature of PC-CD is the proliferation of plasma cells at all levels between follicles. Russell bodies can be observed. The reticular lymph nodes are large and there are few transparent vessels. Generally, there is no typical onion skin structure, which is prone to anemia, hypergammaglobulinemia and hypoproteinemia. MT-CD has the characteristics of both HV-CD and PC-CD. Clinically, according to the different involvement of lymph nodes, CD is classified as UCD or MCD. The former usually involves single regional lymph nodes, while the latter usually involves multiregional lymph nodes. Patients with HV-CD are more likely to have UCD, while patients with PC-CD are more likely to have MCD[4]. MCD is divided into HHV-8-positive MCD and HHV-8-negative MCD according to whether they are infected with human herpesvirus type 8 (HHV-8). The latter is further divided into asymptomatic MCD (aMCD) and idiopathic MCD (iMCD)[3]. At present, many scholars believe that HV occurs only in UCD, but HV characteristics have recently been found in iMCD patients. To avoid confusion, iMCD patients with HV-like histopathological characteristics are defined as HyperV histopathological subtypes. On the other hand, some iMCD patients have lamellar plasmacytosis, increased number of follicles and proliferation of GCs. These cases represent the "plasmacytic" histopathological subtypes of iMCD[5]. The patient's pathological biopsy showed that some of the follicles were proliferated in an 'onion skin' -like structure with increased blood vessels, swelling of endothelial cells and partial hyaline changes which were in line with the hypervascular type.
The most common type of CD is UCD. The most common manifestation is an asymptomatic mass. Symptoms will appear only after the mass is large and compresses adjacent tissues. It can be diagnosed by imaging examination. Generally, the prognosis is good and the 5-year survival rate can be greater than 90%. The disease progression of MCD is slow, often showing universality and invasiveness. It is characterized mainly by systemic symptoms, such as fever, fatigue, night sweats, weight loss, and even monoclonal gammopathy, skin lesions syndrome (Grow–Fukase syndrome), which is characterized by multiple neuropathies, organ swelling, endocrine disease, monoclonal γ globulinopathy and skin changes[6] and usually accompanied by splenomegaly, with a 5-year survival rate of approximately 50%-77%. MCD has also been found to coexist and overlap with human immunodeficiency virus. At the same time, the incidence of Kaposi's sarcoma in CD is significantly increased[7].
In this case, the patient had enlarged lymph nodes in many areas, accompanied by weight loss, massive pleural effusion and ascites, splenomegaly, and increased CRP and ESR, and the pathological biopsy of lymph nodes showed Castleman’s disease-like changes, without tuberculosis, autoimmune diseases and lymphoma. It was considered that the patient had MCD, and the patient was HHV-8 negative. It was proposed according to the diagnostic guidelines of Castleman’s disease (Table 2). HHV-8-negative patients need to meet two main criteria and two secondary criteria for the diagnosis of iMCD (at least one is a laboratory criterion)[5]. The pathological biopsy results of lymph nodes, in this case, are consistent with CD findings. Multiple CT and color Doppler ultrasound suggested that lymph nodes in multiple regions of the body were swollen, which met the main diagnostic criteria. The ESR in laboratory examination was 29 mm/h > 20 mm/h, and albumin was 32.6 g/L (< 35 g/L). The clinical symptoms and signs were weight loss, splenomegaly and malignant ascites, which met the two secondary diagnostic criteria. Combined with the patient's laboratory examination, imaging results and clinical symptoms, the patient was considered to have iMCD after excluding autoimmune diseases, lymphoma and tuberculosis.
iMCD is a rare nonclonal lymphoproliferative hematological disease with significant heterogeneity. According to the different manifestations of iMCD, it is further divided into the iMCD-thrombocytopenia, fever, anasarca, organomegaly, reticulin fibrosis (TAFRO) and iMCD nonspecific type. The diagnosis of iMCD-TAFRO needs to meet the main criteria and more than one secondary criterion (Table 3)[8]. The patient has multiple swollen lymph nodes, and the volume of enlarged lymph nodes is not high (approximately 0.4-1.6 cm). Lymph node biopsy showed the transformation of the germinal center, obvious hyperplasia of the mantle area, an "onion skin" structure, and vascular proliferation between and within follicles. Abdominal CT showed hepatosplenomegaly with a large amount of ascites. Bone marrow examination showed that megakaryocytes were slightly higher than the normal value and there was no obvious abnormality in the delivered IgG4. However, the patient had no fever or thrombocytopenia, and the patient did not undergo a bone marrow biopsy; thus, it was not clear whether the patient had bone marrow fibrosis, so it was not clear whether the patient could be diagnosed with iMCD-TAFRO.
| Diagnostic criteria | Contents |
| Main diagnostic criteria | Lymph node pathology conforms to iMCD spectrum; Enlargement of lymph nodes in 2 or more lymph nodes (short axis ≥ 1 cm) |
| Secondary diagnostic criteria | Laboratory standards: (1) C-reactive protein > 10 mg/L. ESR > 20 mm/h (female) or 15 mm/h (male); (2) Anemia (Hgb < 100 g/L). Thrombocytopenia (PLT < 100) × 109/L) or increased (PLT > 350 × 109/L); (3) Serum albumin < 35 g/L; and (4) Estimated glomerular filtration rate < 60 mL/(min· 73 m2) or proteinuria (total urinary protein > 150 mg/24 h or 100 mg/L), serum IgG > 17 g/L |
| Clinical criteria: (1) Systemic symptoms: night sweats, fever (> 38 °C), weight loss (≥ 10% after 6 mo) or fatigue (affecting instrumental activities of daily living); (2) Hepatomegaly and/or splenomegaly; (3) Edema or serous effusion; and (4) Cherry or violet skin rash. Lymphocytic interstitial pneumonia | |
| Exclusion criteria | (1) Infection-related diseases (such as EBV, HIV, tuberculosis); (2) Autoimmune diseases (such as rheumatoid arthritis, systemic lupus erythematosus, adult still disease, adolescent idiopathic arthritis, autoimmune lymphoproliferative syndrome); and (3) Neoplastic diseases (such as multiple myeloma, lymphoma, primary lymph node plasmacytoma, follicular dendritic cell sarcoma, POEMS syndrome) |
| Diagnostic criteria | Contents |
| Histopathological criteria | (1) The pathological results of lymph nodes are consistent with the characteristic manifestations of TAFRO lymph nodes1; and (2) Negative LANA-1 for HHV-8 |
| Main diagnostic criteria | (1) Three of the five TAFRO related symptoms were met: Thrombocytopenia, fever (body temperature ≥ 38 ℃), anasarca, organomegaly, reticulin fibrosis; (2) Absence of hypergammaglobulinemia; and (3) Small volume lymphadenopathy |
| Secondary diagnostic criteria | (1) Normal or proliferative of megakaryocytes in bone marrow; and (2) The level of serum alkaline phosphatase increased, but the level of transaminase did not increase significantly |
IL-6 is a B-cell differentiation factor that plays a key role in the pathogenesis of iMCD in some patients. Excessive IL-6 induces the release of inflammatory factors and the secretion of vascular endothelial growth factor, leading to inflammatory storms, angiogenesis and vascularization in lymph nodes. The biological activity of IL-6 occurs mainly through glycoprotein 130 (gp130) and IL-6R. IL-6 first forms a heterodimer with IL-6R and then forms a hexamer complex with gp130 to activate the intracellular signal transduction pathway on the target cell, thereby activating the JAK-signal transducer and activator of transcription (STAT) signaling pathway[7,9]. Its signal transduction pathway is strictly regulated. Dysregulated IL-6 signal transduction causes chronic inflammation and autoimmune diseases, among other effects. IL-6 activates STAT3 through JAKs. Small-molecule inhibitors targeting IL-6/STAT3 may become potential drugs for treating inflammatory diseases. The elevated level of IL-6 in CD is the main cause of various clinical symptoms of CD[10]. At present, the IL-6 chimeric monoclonal antibody siltuximab is usually the first choice for the treatment of iMCD[11]. It typically has a significant therapeutic effect on patients with high IL-6 Levels, but in some iMCD patients with normal or low IL-6 Levels, the response rate is low or zero. Some experiments have found that siltuximab treatment is ineffective in 50%-60% of patients[11,12]. Glucocorticoids have been used to treat CD before the appearance of siltuximab and can improve the symptoms of acute exacerbation of iMCD[13]; however, the response level is low. The failure rate of glucocorticoids alone in the treatment of CD is as high as 50%[14]. At present, siltuximab is not listed in China and the patient's IL-6 Level is low, so its use is considered as an alternative treatment. The failure rate of glucocorticoid therapy alone was high. Therefore, after referring to the relevant literature, this patient was treated with the JAK inhibitor tofacitinib combined with hormones.
At present, treatment of the IL-6 upstream pathway has been reported in iMCD. The formation of IL-6 classical or cross-signal ligand-receptor complexes leads to the activation of a variety of intracellular signaling pathways, including the JAK/STAT pathway, RAS MAPK pathway, p38 and JNK-MAPK pathway, PI3K/Akt/mTOR pathway and MEK-ERK5 pathway[15]. The IL-6/JAK/STAT3 pathway is an important pathway by which IL-6 is associated with a variety of diseases. JAK 1 is the main kinase activated by IL-6 in vivo[16]. Inhibiting STAT activation can block the recruitment of STAT3 to the tail of the IL-6 receptor by blocking the formation of complexes between IL-6 and receptors or inhibiting the phosphorylation of JAKs. Pierson et al[17] assessed the activation index pSTAT3 of the JAK-STAT3 pathway and found that JAK-STAT3 is activated in the lymphoid tissue of iMCD patients, resulting in a significant increase in the expression of pSTAT3 in follicles compared with normal people, but the expression is not obvious in germinal centers. At the same time, they also found that JAK 1/2 inhibitors can reduce the hypersensitivity of peripheral blood monocytes in patients with iMCD to IL-6 stimulation, indicating that the imbalance of the IL-6-JAK-STAT3 signaling pathway may play a key role in the pathogenesis of iMCD. Some literature has pointed out that the IL-6-JAK-STAT3 signal is enriched in the serum protein tissue of iMCD patients and that JAK1/2 inhibitors can inhibit the hypersensitivity caused by cytokine stimulation. The expression of pSTAT3 in the follicular space of lymph nodes in patients with iMCD is significantly higher than that in normal people, indicating that the JAK-STAT3 pathway may be involved in the pathogenesis of iMCD. Therefore, inhibition of the JAK-STAT pathway in the treatment of iMCD may be effective for patients who cannot use siltuximab[15]. Tofacitinib directly or indirectly inhibits the production of proinflammatory cytokines by reversibly and competitively inhibiting the ATP binding sites of JAK1 and JAK2, inhibiting the JAK signal transduction pathway thereby blocking the activation of key inflammatory cytokine signal transduction and activator of transcription. Blocking the cascade amplification of inflammation reduces the synthesis of downstream inflammatory factors and then promotes the level of related inflammatory factors to decline exponentially[18]. When we could not use siltuximab, we chose tofacitinib combined with glucocorticoids as the treatment plan. After 1 wk of routine treatment, the CT Reexamination of the patient showed that ascites and pericardial effusion were lower than they had been previously and laboratory indicators such as ESR and CRP were also reduced to the normal range; After discharge, the patient continued to take tofacitinib orally until now with the stable condition and no recurrence. It indicates that tofacitinib plus glucocorticoid is effective in the treatment of iMCD.
This case reported a case of iMCD with malignant ascites as the main clinical symptom, accompanied by multiple lymphadenopathies and splenomegaly. Laboratory examination showed that the ESR and CRP were accelerated. After regular treatment with tofacitinib combined with hormones, CT reexamination showed that pleural effusion and pericardial effusion were reduced compared with those before treatment and the ESR and CRP fell to the normal range suggesting that tofacitinib combined with hormones may be a potentially effective means to treat iMCD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gunduz E, Turkey; Masaki Y, Japan; Wang H, China S-Editor: Wang DM L-Editor: Filipodia P-Editor: Wang DM
| 1. | Aljubran SA, Khan BF, Alqahtani MM, Shaikh AY, Alghamdi RA. Unicentric Castleman's Disease with an Unusual Clinical Behavior. Cureus. 2020;12:e10973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. N Engl J Med. 1978;298:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135:1353-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 282] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 4. | Dispenzieri A, Armitage JO, Loe MJ, Geyer SM, Allred J, Camoriano JK, Menke DM, Weisenburger DD, Ristow K, Dogan A, Habermann TM. The clinical spectrum of Castleman's disease. Am J Hematol. 2012;87:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Fajgenbaum DC, Uldrick TS, Bagg A, Frank D, Wu D, Srkalovic G, Simpson D, Liu AY, Menke D, Chandrakasan S, Lechowicz MJ, Wong RS, Pierson S, Paessler M, Rossi JF, Ide M, Ruth J, Croglio M, Suarez A, Krymskaya V, Chadburn A, Colleoni G, Nasta S, Jayanthan R, Nabel CS, Casper C, Dispenzieri A, Fosså A, Kelleher D, Kurzrock R, Voorhees P, Dogan A, Yoshizaki K, van Rhee F, Oksenhendler E, Jaffe ES, Elenitoba-Johnson KS, Lim MS. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129:1646-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 6. | van Rhee F, Voorhees P, Dispenzieri A, Fosså A, Srkalovic G, Ide M, Munshi N, Schey S, Streetly M, Pierson SK, Partridge HL, Mukherjee S, Shilling D, Stone K, Greenway A, Ruth J, Lechowicz MJ, Chandrakasan S, Jayanthan R, Jaffe ES, Leitch H, Pemmaraju N, Chadburn A, Lim MS, Elenitoba-Johnson KS, Krymskaya V, Goodman A, Hoffmann C, Zinzani PL, Ferrero S, Terriou L, Sato Y, Simpson D, Wong R, Rossi JF, Nasta S, Yoshizaki K, Kurzrock R, Uldrick TS, Casper C, Oksenhendler E, Fajgenbaum DC. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132:2115-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 282] [Article Influence: 40.3] [Reference Citation Analysis (1)] |
| 7. | Srivastava H, Reddy DS, Shah SN, Shah V. Castleman's disease. J Oral Maxillofac Pathol. 2020;24:593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Iwaki N, Fajgenbaum DC, Nabel CS, Gion Y, Kondo E, Kawano M, Masunari T, Yoshida I, Moro H, Nikkuni K, Takai K, Matsue K, Kurosawa M, Hagihara M, Saito A, Okamoto M, Yokota K, Hiraiwa S, Nakamura N, Nakao S, Yoshino T, Sato Y. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 9. | Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 330] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 10. | Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122:143-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 622] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 11. | van Rhee F, Casper C, Voorhees PM, Fayad LE, Gibson D, Kanhai K, Kurzrock R. Long-term safety of siltuximab in patients with idiopathic multicentric Castleman disease: a prespecified, open-label, extension analysis of two trials. Lancet Haematol. 2020;7:e209-e217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Casper C, Chaturvedi S, Munshi N, Wong R, Qi M, Schaffer M, Bandekar R, Hall B, van de Velde H, Vermeulen J, Reddy M, van Rhee F. Analysis of Inflammatory and Anemia-Related Biomarkers in a Randomized, Double-Blind, Placebo-Controlled Study of Siltuximab (Anti-IL6 Monoclonal Antibody) in Patients With Multicentric Castleman Disease. Clin Cancer Res. 2015;21:4294-4304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123:2924-2933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Ebisawa K, Shimura A, Honda A, Masamoto Y, Nakahara F, Kurokawa M. Hemoglobin and C-reactive protein levels as predictive factors for long-term successful glucocorticoid treatment for multicentric Castleman's disease. Leuk Lymphoma. 2021;62:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Sumiyoshi R, Koga T, Kawakami A. Candidate biomarkers for idiopathic multicentric Castleman disease. J Clin Exp Hematop. 2022;62:85-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 462] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 17. | Pierson SK, Shenoy S, Oromendia AB, Gorzewski AM, Langan Pai RA, Nabel CS, Ruth JR, Parente SAT, Arenas DJ, Guilfoyle M, Reddy M, Weinblatt M, Shadick N, Bower M, Pria AD, Masaki Y, Katz L, Mezey J, Beineke P, Lee D, Tendler C, Kambayashi T, Fosså A, van Rhee F, Fajgenbaum DC. Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease. Blood Adv. 2021;5:3445-3456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, Menon S, Lamba M, Zwillich S. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:318-328. [PubMed] |