Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10772
Peer-review started: June 23, 2022
First decision: July 12, 2022
Revised: July 22, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: October 16, 2022
Processing time: 98 Days and 7.2 Hours
Takotsubo cardiomyopathy (TS) is a rare acute cardiac disease with clinical features, symptoms, and electrocardiographic manifestations similar to those of acute myocardial infarction. We present the case of a patient with TS caused by a pheochromocytoma, which was confirmed by the postoperative pathology. Furthermore, we present the patient's subsequent management, treatment, and outcome.
A 64-year-old woman was admitted to the hospital with episodic chest pain and palpitations, electrocardiogram (ECG) findings suggestive of high lateral wall myocardial infarction, echocardiogram showing left ventricular wall segmental motion abnormalities, and elevated levels of the myocardial marker troponin. The patient underwent coronary angiography, which revealed unobstructed blood flow without obvious stenosis. During their hospitalization, the patient had paro
Cardiologists should consider pheochromocytoma in patients with TS. Early detection allows timely intervention, benefiting patients.
Core Tip: Pheochromocytomas are a common class of endocrine tumors that can cause cardiovascular pathology, often resulting in stress cardiomyopathy due to the intermittent or sustained release of catecholamines. We report a patient with a postoperatively confirmed diagnosis of Takotsubo cardiomyopathy caused by pheochromocytoma.
- Citation: Wang ZH, Fan JR, Zhang GY, Li XL, Li L. Atypical Takotsubo cardiomyopathy presenting as acute coronary syndrome: A case report. World J Clin Cases 2022; 10(29): 10772-10778
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10772.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10772
Takotsubo cardiomyopathy (TS) is a rare acute cardiac disease that was first reported by a Japanese physician in 1991[1] but has not been widely recognized. TS mainly occurs in women, with a higher incidence in postmenopausal women[2], which may be related to the loss of estrogen protection[3]. Because TS is closely related to emotions and external stimuli, it is also called “broken heart syndrome”. At present, more attention has been paid to the increase of blood-derived catecholamines in the pathogenesis of TS. Pheochromocytoma is a common type of endocrine system tumor[4] that can cause cardiovascular pathology due to the intermittent or persistent release of catecholamines, which can lead to the development of TS. There are few previous reports in the literature on this secondary stress cardiomyopathy, especially caused by pheochromocytoma. Here we report a case in which atypical TS occurred due to the presence of a pheochromocytoma with massive release of catecholamines.
A 64-year-old Chinese woman was admitted to the Department of Integrative Cardiology of the China-Japan Friendship Hospital with severe chest pain.
The patient presented with left-sided chest pain confined to the precordial region, accompanied by panic, shoulder and back pain, dizziness, and profuse sweating for several hours after receiving acupuncture treatment on the scapula 12 d prior.
The patient had hyperlipidemia and homocysteinemia but was not taking medications. She denied a history of hypertension and diabetes mellitus.
The patient did not smoke or drink alcohol. She became menopausal at age 50 without the use of chemotherapy drugs. She also confirmed no family history of genetic disorders.
General examination revealed no cardiopulmonary or abdominal abnormalities. The patient had a heart rate of 60 beats/min and a BMI of 31.6. At the time of admission, her blood pressure was elevated to 20.0/14.7 kPa; however, when she was at rest, a stable blood pressure of 16.0/10.7 kPa was measured. During the patient's hospitalization, we found that her blood pressure was unstable, occasionally up to 26.7/14.7 kPa.
Blood test results were as follows: Creatine kinase-MB 15.67 ng/mL (reference range: < 5.00); cardiac troponin I 5.31 ng/mL (reference range: < 1.00); myoglobin 78.6 ng/mL (reference range: < 70.0); and N-terminal pro-brain natriuretic peptide 3578 pg/mL (reference range: < 300). Further examinations revealed a fasting blood-glucose of 8.73 mmol/L (reference range: 3.90-6.10), normal C-reactive protein, normal renal function, and negative tumor markers. Importantly, catecholamine levels were significantly elevated during episodes of elevated blood pressure, with epinephrine of 11.60 pmol/L (reference range: 0.164-0.519) and norepinephrine of 56.19 pmol/L (reference range: 1.182-2.364).
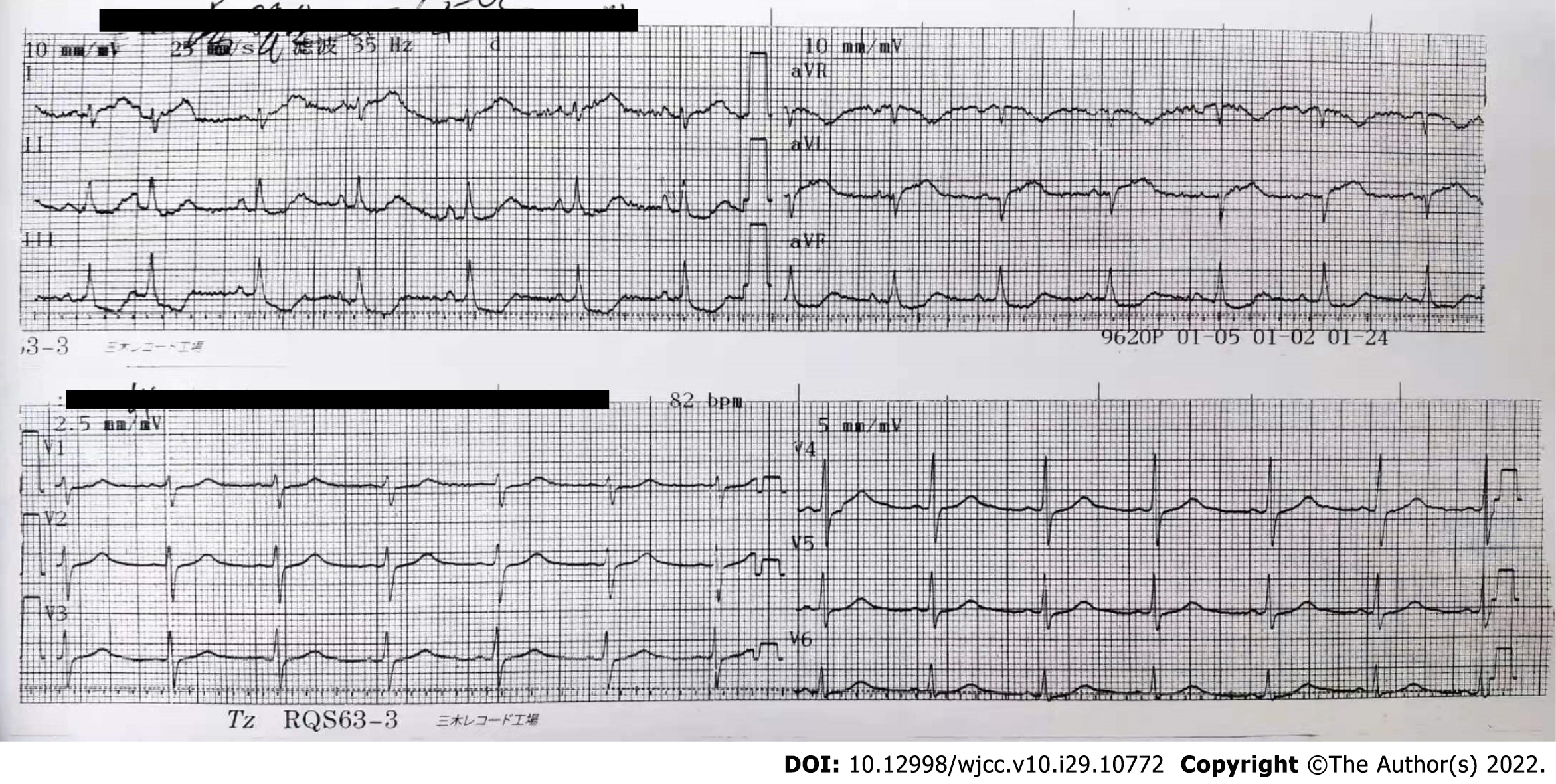
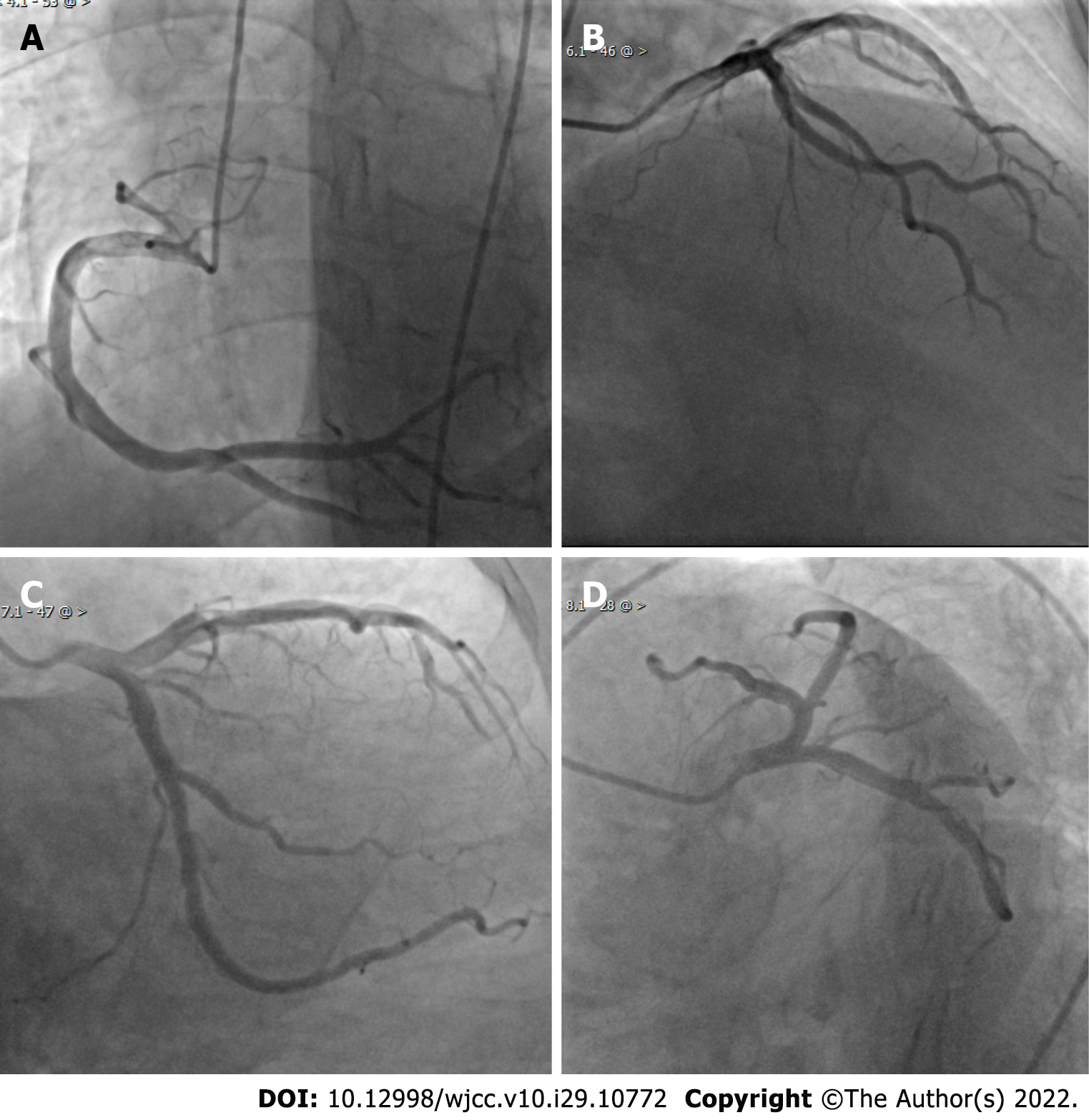
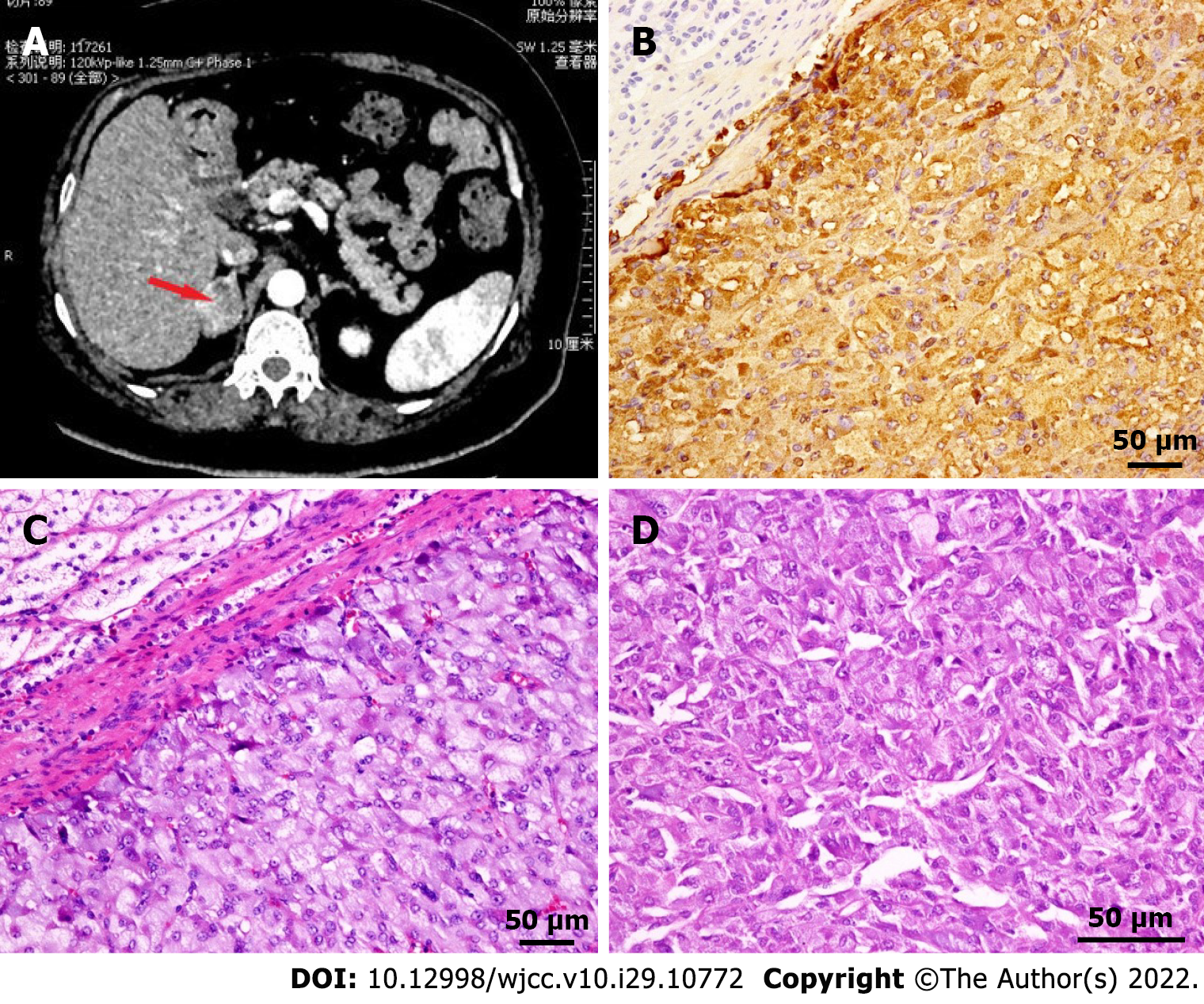
The electrocardiogram (ECG) showed ST-segment elevation in lead I and AVL and ST-segment depression in leads II, III, and AVF (Figure 1). Echocardiogram findings showed reverse motion of the posterior wall of the left ventricle and septum, normal motion of the posterior septum and posterior ventricular wall, and loss of motion of the rest of the ventricular wall, with an ejection fraction of 50%. Re-examination of the ECG showed T-wave inversion in leads I and AVL, while the echocardiogram showed normal wall motion in each compartment. Imaging results showed no meaningful stenosis in the coronary arteries and unobstructed blood flow (Figure 2). Computed tomography scan of the adrenal glands showed the presence of nodules in the right adrenal gland, on the basis of which we suspected the presence of a pheochromocytoma (Figure 3A).
Considering the patient’s history, laboratory examinations, and imaging results, we determined that she had developed atypical TS with involvement of the lateral wall caused by a pheochromocytoma.
At the time of admission, we gave the patient aspirin (100 mg, qd, po), clopidogrel (75 mg, qd, po), metoprolol (47.5 mg, qd, po), and atorvastatin (20 mg, qn, po) to prevent recurrence of cardiovascular events, isosorbide mononitrate (60 mg, qd, po) to improve cardiac circulation, and acarbose (50 mg, tid, po) to lower blood glucose. The patient underwent laparoscopic surgical resection of the pheochromocytoma, and doxazosin mesylate (4 mg, qd, po) was applied. The postsurgical pathologic diagnosis was right adrenal pheochromocytoma (size: 3.5 cm + ACo-3.5 cm + ACo-3 cm) (Figure 3B-D).
After tumor resection, the patient had a smooth postoperative recovery without adverse events and was treated only with atorvastatin 10 mg qd for lipid-lowering. Repeat ECG examination showed no ST-segment abnormality, and the echocardiogram showed normal motion of all ventricular walls. We followed the patient for one year, and she did not have any further cardiac-related diseases, while the ECG and echocardiogram were normal.
The electrocardiographic changes and clinical presentation of TS are similar to acute coronary syndromes (ACS) without the presence of coronary occlusion. The typical patient with TS has a distinctive abnormal contour of ventricular annular contraction with a peculiar circumferential pattern and apical ballooning of the left ventricle extending beyond the coronary blood supply region[5]. In our present report, the heart showed abnormal activity of the lateral wall and was judged to be an atypical case of TS. This abnormal motion is reversible, and ventricular dysfunction returns to normal within hours to weeks. Coronary angiogram is the key test to distinguish ACS from TS[6].
The pathogenesis of TS is unclear, and pathophysiological mechanisms such as myocardial ischemia, left ventricular outlet tract obstruction, blood-borne catecholamine myocardial toxicity, adrenergic-induced signal transduction abnormalities, and autonomic nervous system dysfunction have been proposed[7]. More attention is currently focused on elevated blood-borne catecholamine levels and excessive activation of cardiac sympathetic nerves. The rapid elevation of plasma catecholamine levels leads to vasospasm and activation of cardiac sympathetic nerves, inducing TS and acute reversible myocardial dysfunction[8]. Pheochromocytomas produce large amounts of catecholamines when stimulated, which have been reported to induce TS[9] and were the final etiologic diagnosis in this case. Previously, many guidelines for the diagnosis of TS required the exclusion of pheochromocytoma, such as the Mayo diagnostic criteria[10] that had been used for many years; however, the European Journal of Heart Failure introduced new diagnostic principles[7] in recent years, and pheochromocytoma is no longer an exclusion criterion for TS. Typical apical balloon-like changes account for approximately 80% of all TS, and the current literature is dominated by these cases, while the remaining atypical subtypes are rarely reported. Epidemiologic studies show[11] that the incidence of secondary forms of TS is about 3.4 hospitalizations per 100000 person-years, and TS caused by pheochromocytoma is rarely reported.
TS is broadly classified into four subtypes according to the site of involvement, apical, midven
In this case, the patient had the classical symptoms of myocardial infarction, such as episodes of chest tightness and breathlessness, ST-T segment abnormalities on ECG, wall dyskinesia on echocardiogram, and elevated troponin levels, as well as newly discovered unexplained paroxysmal palpitations, sweating, and elevated blood pressure and blood glucose levels. Although cardiac imaging did not reveal typical apical spherical changes, the diagnosis of TS could be made based on the absence of significant abnormalities on coronary angiogram, echocardiographic findings suggesting a basic loss of proximal apical lateral wall motion and abnormal segmental ventricular wall motion, and a repeat echocardiogram showing normal motion, which could further be classified as an atypical focal type of TS. The postoperative pathology clearly showed the presence of pheochromocytoma, which was the underlying cause of TS.
Pheochromocytomas are a significant pathogenic factor in TS. The sudden production of large doses of catecholamines is directly toxic to the myocardium, producing catecholamine-induced myocarditis, diffuse myocardial fibrosis, and induced heart failure[15]; most patients can recover normal cardiac function after removal of the primary tumor[16].
In conclusion, we report a case of TS caused by pheochromocytoma. The leading causes of death in patients with TS are the sudden onset of illness and failure to receive prompt emergency treatment; therefore, the patient's prognosis depends on timely detection of the cause of the illness. Pheochromocytoma as the causative factor should not be overlooked in the management of TS, since early intervention can effectively improve myocardial remodeling, and clinicians should pay attention to this.
This report was supported by doctors working in the Department of Integrative Cardiology, China-Japan Friendship Hospital in acquisition, analysis and interpretation of data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Faraji N, Iran; Lin MS, Taiwan; Shrestha AB, Bangladesh; Ullah K, Pakistan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Tofield A. Hikaru Sato and Takotsubo cardiomyopathy. Eur Heart J. 2016;37:2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Hurst RT, Prasad A, Askew JW 3rd, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Ueyama T, Hano T, Kasamatsu K, Yamamoto K, Tsuruo Y, Nishio I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc Pharmacol. 2003;42 Suppl 1:S117-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Nölting S, Bechmann N, Taieb D, Beuschlein F, Fassnacht M, Kroiss M, Eisenhofer G, Grossman A, Pacak K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr Rev. 2022;43:199-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 206] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 5. | Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1694] [Article Influence: 169.4] [Reference Citation Analysis (1)] |
| 7. | Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 790] [Article Influence: 79.0] [Reference Citation Analysis (1)] |
| 8. | Kato K, Lyon AR, Ghadri JR, Templin C. Takotsubo syndrome: aetiology, presentation and treatment. Heart. 2017;103:1461-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 9. | Kido K, Guglin M. Drug-Induced Takotsubo Cardiomyopathy. J Cardiovasc Pharmacol Ther. 2017;22:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1288] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 11. | Y-Hassan S, Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res. 2018;28:53-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O'Gara P, Stuckey DJ, Nikolaev VO, Diakonov I, Pannell L, Gong H, Sun H, Peters NS, Petrou M, Zheng Z, Gorelik J, Lyon AR, Harding SE. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 594] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 13. | Bonnemeier H, Demming T, Weidtmann B, Ortak J, Burgdorf C, Reppel M, Mäuser W, Rosenberg M, Frey N. Differential heart rate dynamics in transient left ventricular apical and midventricular ballooning. Heart Rhythm. 2010;7:1825-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Lüscher TF, Templin C; International Takotsubo (InterTAK) Registry. Differences in the Clinical Profile and Outcomes of Typical and Atypical Takotsubo Syndrome: Data From the International Takotsubo Registry. JAMA Cardiol. 2016;1:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 15. | Ferreira VM, Marcelino M, Piechnik SK, Marini C, Karamitsos TD, Ntusi NAB, Francis JM, Robson MD, Arnold JR, Mihai R, Thomas JDJ, Herincs M, Hassan-Smith ZK, Greiser A, Arlt W, Korbonits M, Karavitaki N, Grossman AB, Wass JAH, Neubauer S. Pheochromocytoma Is Characterized by Catecholamine-Mediated Myocarditis, Focal and Diffuse Myocardial Fibrosis, and Myocardial Dysfunction. J Am Coll Cardiol. 2016;67:2364-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Falhammar H, Kjellman M, Calissendorff J. Treatment and outcomes in pheochromocytomas and paragangliomas: a study of 110 cases from a single center. Endocrine. 2018;62:566-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |