Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10763
Peer-review started: June 14, 2022
First decision: July 29, 2022
Revised: August 9, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: October 16, 2022
Processing time: 106 Days and 17.2 Hours
Malignant giant cell tumor of the tendon sheath (MGCTTS) is an extremely rare malignant tumor originating from synovial and tendon sheath tissue with highly aggressive biological behavior and a high rate of local recurrence and distant metastasis which should be considered a highly malignant sarcoma and managed aggressively. How to systemically treat MGCTTS remains a challenge. In this case, a patient with MGCTTS suffered a recurrence after 2 surgical resections received adjuvant chemotherapy and radiation therapy, but the treatment outcome remained poor. More clinical trials and better understanding of the biology and molecular aspects of this subtype of sarcoma are needed while novel medicines should be developed to efficiently target particular pathways.
A 52-year-old man presented with persistent dull pain in the right groin accompanied by limited right hip motion starting 6 mo ago. Two months before his attending to hospital, the patient's pain worsened, presenting as severe pain when standing or walking, limping, and inability to straighten or move the right lower extremity. Surgical excision was performed and MGCTTS was confirmed by pathology examination. Two recurrences occurred after surgical resection, moreover, the treatment outcome remained poor after adjuvant chemotherapy and radiation therapy. The patient died only 10 mo after the initial diagnosis.
MGCTTS is characterized by a joint mass with pain and limited motion. It typically grows along the tendons and infiltrated into the surrounding muscle and bone tissue, with a stubborn tendency to relapse, as well as pulmonary metastasis. Radically surgical resection provides a choice of treatment whereas post-operation care should be taken to preserve the function of the joint. Chemotherapy and radiotherapy can be used as alternative treatments when radical resection cannot be performed.
Core Tip: This case demonstrates the highly aggressive biological behavior of Malignant giant cell tumor of the tendon sheath (MGCTTS), which mostly appears in Computed tomography (CT) images as a large mass that grows around the joint with poorly defined borders. CT can show details of bone destruction surrounding the lesion caused by extensive invasion. Magnetic resonance imaging clearly shows the histological features of the tumor and its relationship to the surrounding tissue. There is a stubborn tendency for MGCTTS to relapse and a possibility of pulmonary metastasis. Postoperative follow-up, especially long-term follow-up for evaluating recurrence, is necessary. Meanwhile systemic examination should be undertaken to evaluate distant metastasis.
- Citation: Huang WP, Gao G, Yang Q, Chen Z, Qiu YK, Gao JB, Kang L. Malignant giant cell tumors of the tendon sheath of the right hip: A case report. World J Clin Cases 2022; 10(29): 10763-10771
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10763.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10763
Giant cell tumor of the tendon sheath (GCTTS) is a benign tumor involving the synovial joints, bursae and tendon sheaths. Based on the 2013 edition of the World Health Organization (WHO) Classification of Tumors of Bone and Soft Tissue, it is classified as a "so-called fibrous histiocytic tumor". It can be further classified as limited, diffuse, intra-articular or extra-articular according to its biological behavior and location respectively[1-2]. GCTTS occurs mostly in young people, especially women, with a median age of 40 years[3]. Limited-type GCTTS usually affects small joints, such as fingers and wrists, and is characterized by a low recurrence rate (10%-20%). Rarely, diffuse GCTTS can become malignant, with a highly aggressive biology, such as regional lymph node and distant organ metastases[4].
Here, we report the imaging presentation and treatment of a patient with malignant giant cell tumor of the tendon sheath (MGCTTS) of the right hip who suffered recurrence after two surgical resections and a continuous poor treatment outcome after adjuvant chemotherapy and radiation therapy. The survival time was only 10 mo after initial diagnosis.
A 52-year-old male came to see doctors with a complaint of pain in his right hip for 6 mo.
The patient had developed unexplained right hip pain 6 mo ago, presenting as persistent dull pain accompanied by limited movement of the right hip joint without radiating pain or numbness of the lower limbs. The right hip pain worsened 2 mo prior to this visit, manifested as severe pain when standing or walking, limping, and inability to straighten or move the right lower limb. The patient had developed symptoms of anemia 1 wk ago.
No special circumstances.
The patient had no family history of hereditary diseases.
On admission, the patient suffered normal spinal motion, no pressure or percussion pain in the spinous process, pain in the right hip without radiating, inability to straighten the right lower extremity, limited movement in the angle and range of the right hip, limping, and no erythema found on the skin of the right hip, respectively.
Laboratory tests showed a platelet count of 695 × 109/L (normal range, 125-350 × 109/L); monocyte count of 0.71 × 109/L (normal range, 0.1-0.6 × 109/L); D-dimer of 0.35 mg/L (normal range, 0-0.55 mg/L); C-reactive protein of 176.29 mg/L (normal range, 0-5 mg/L); procalcitonin of 0.25 ng/mL (normal range, 0-0.2 ng/mL); and ESR of 21.00 mm/h (normal range, 0-15 mm/h), respectively.
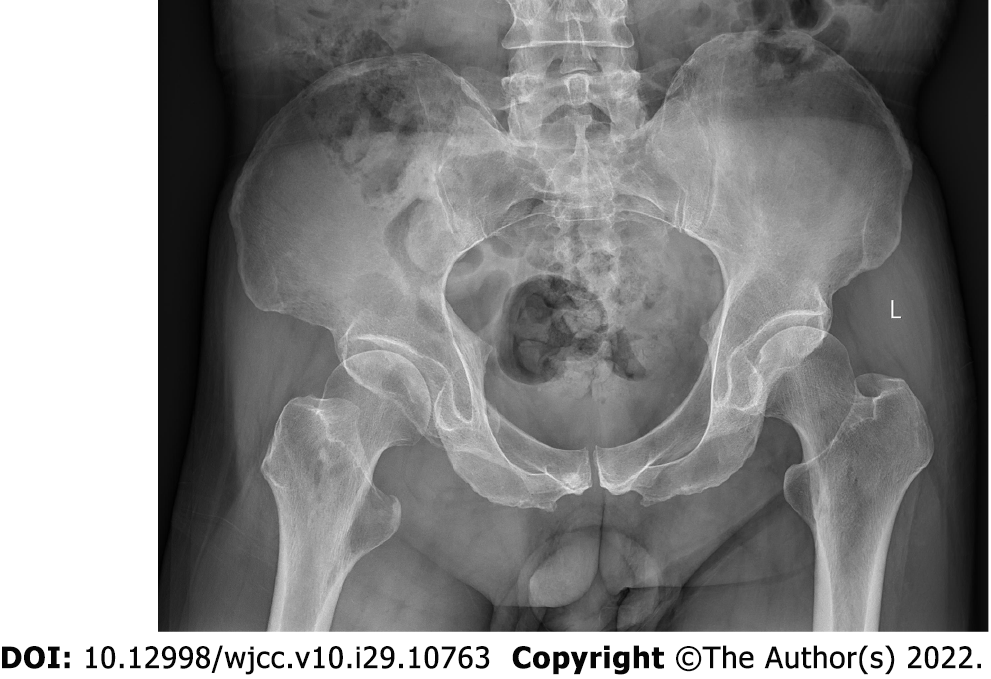
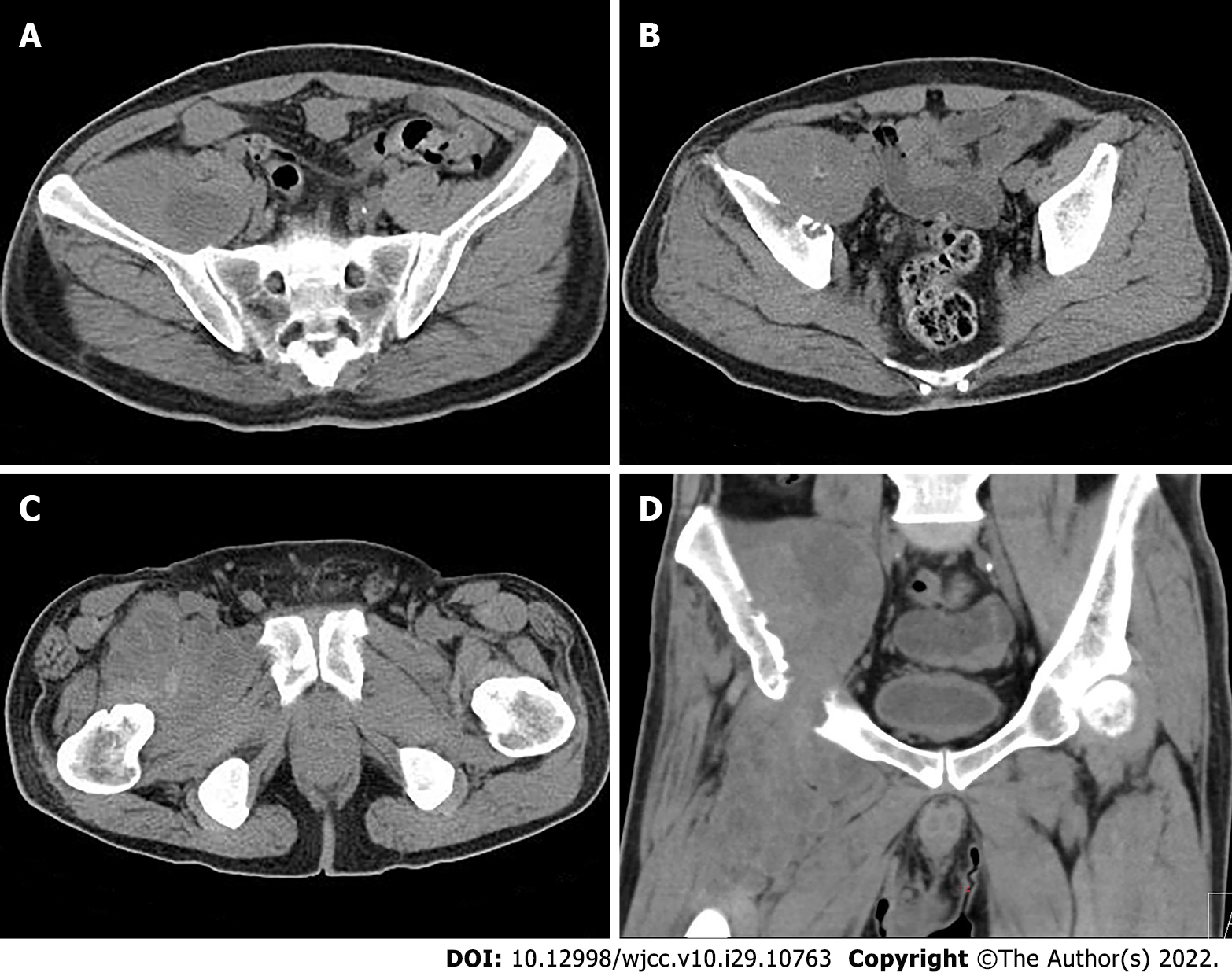
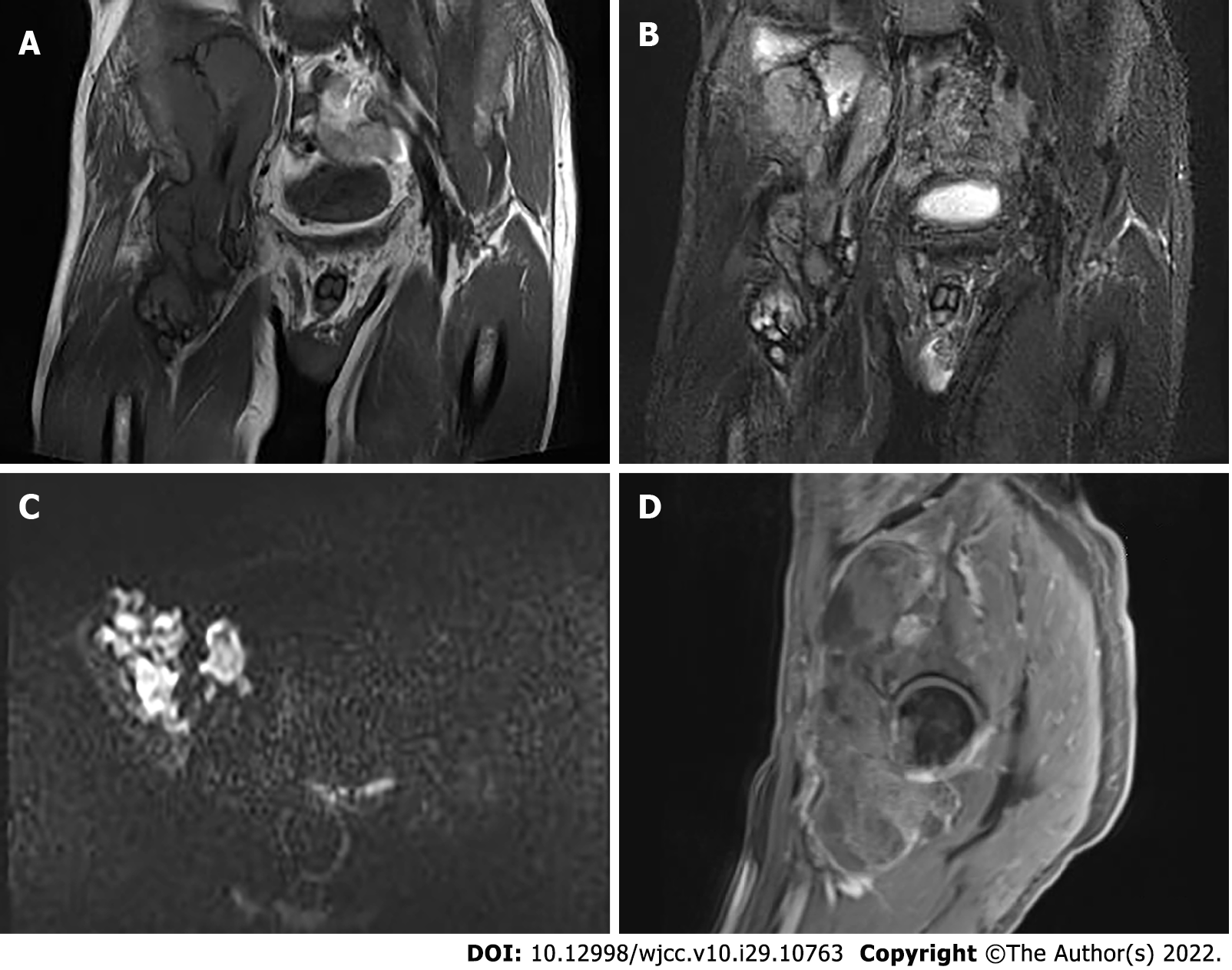
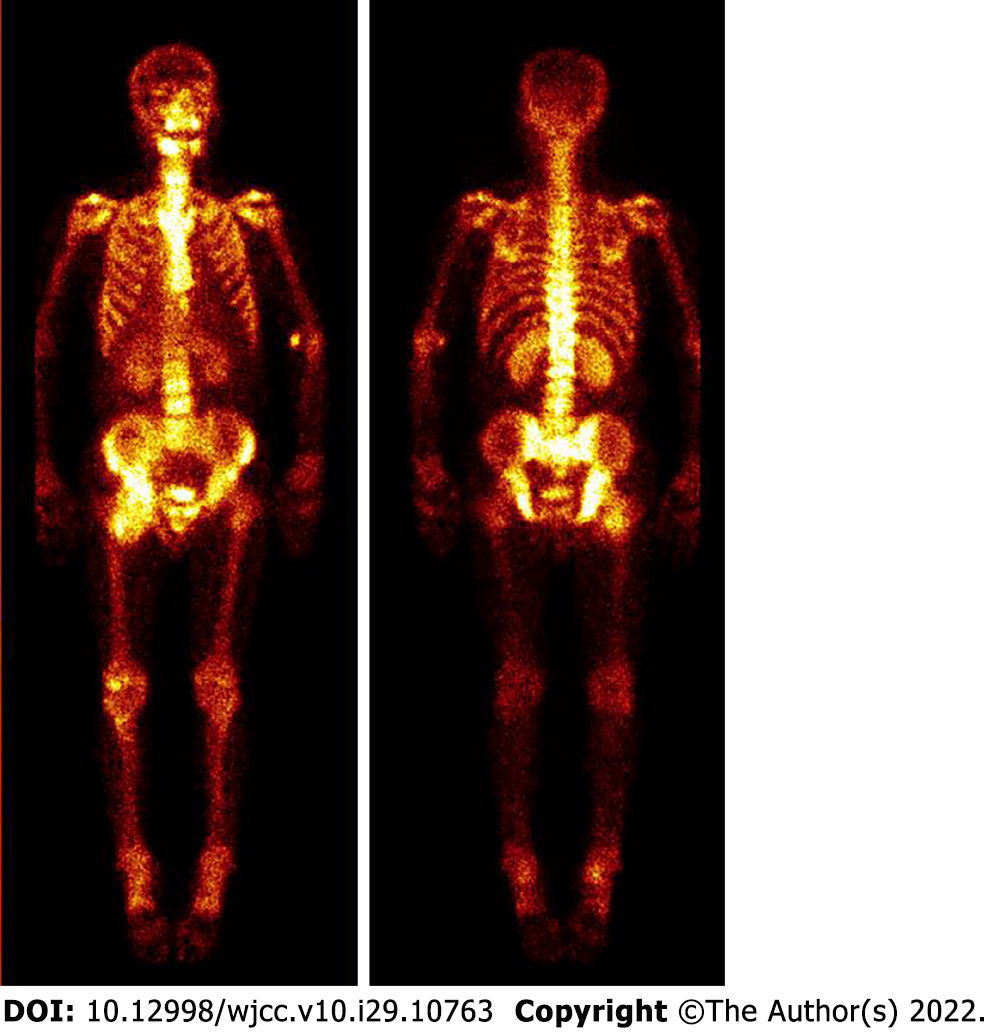
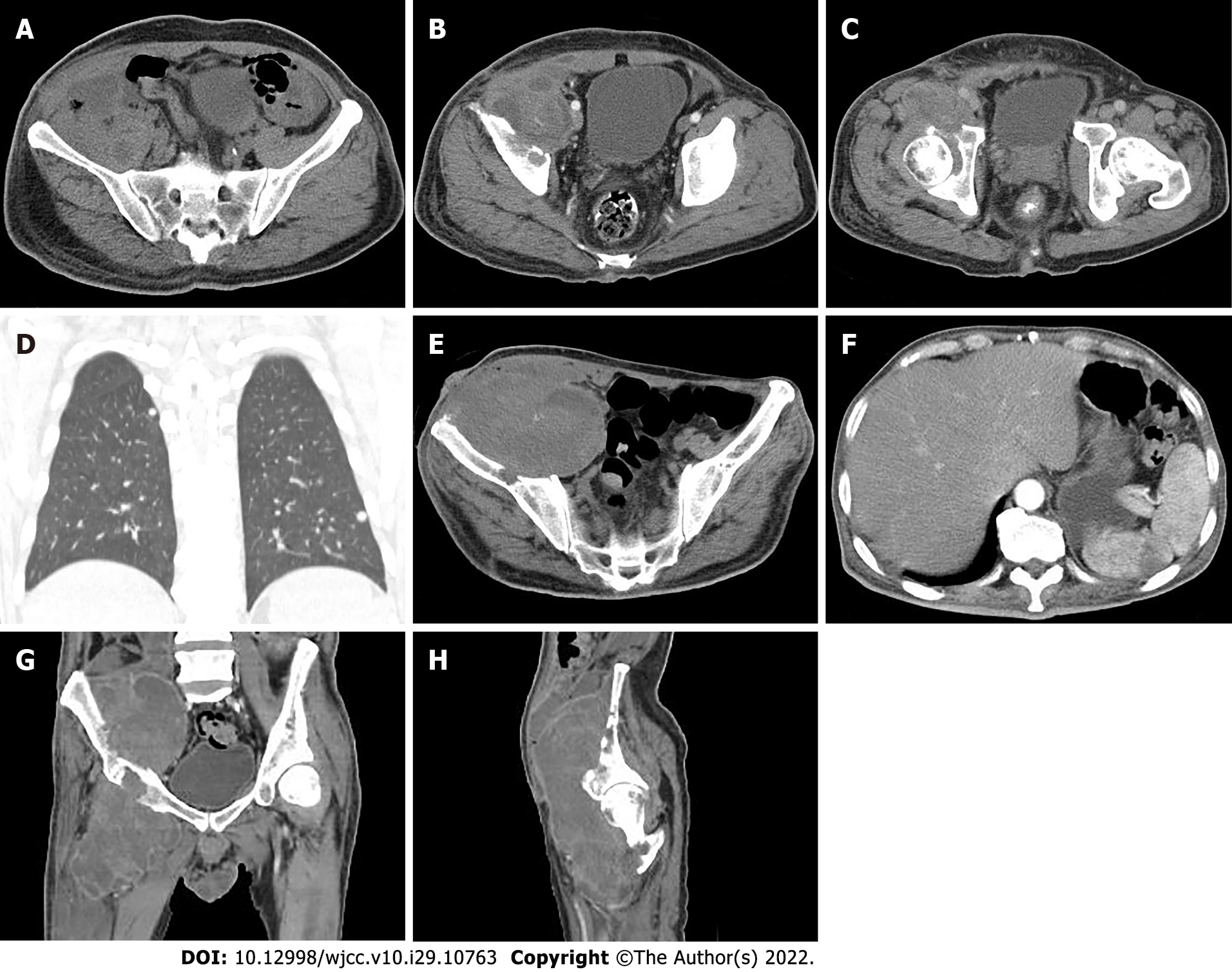
Pelvic radiography showed multiple cystic hypodense shadows in varying sizes, with well-defined borders in the right iliac bone and right upper femur, suggesting osteolytic bone destruction (Figure 1). CT revealed a soft tissue mass with heterogeneous density in the intermuscular space anterior to the right iliopsoas muscle and the right superior femur, with patchy hypodense necrosis within the mass with indistinct borders, at the size of approximately 5.3 cm × 7.5 cm × 21.2 cm (AP × LR × SI). The plain CT attenuation value of the mass was approximately 49 HU and osteolytic bone destruction could be seen in the adjacent iliac bone and acetabulum. The enhanced CT attenuation value of the mass was approximately 55 HU and 58 HU in the arterial and venous phases respectively, representing heterogeneous mild to moderate enhancement (Figure 2). Magnetic resonance imaging (MRI) displayed a mixed-signal mass along the right iliopsoas and the anteromedial muscle gap of the right upper femur, with a heterogeneous low signal on T1-weighted imaging (T1WI), a heterogeneous slightly high signal on fat-saturated T2-weighted imaging (T2WI), a high signal on diffusion weighted image (DWI), and a low signal on apparent diffusion coefficient (ADC) imaging (Figure 3). The patient underwent a whole-body 99mTc-methylene diphosphonate bone scan, which demonstrated a high radioactive accumulation around the areas in the right ilium, acetabulum, proximal femur, and knee (Figure 4).
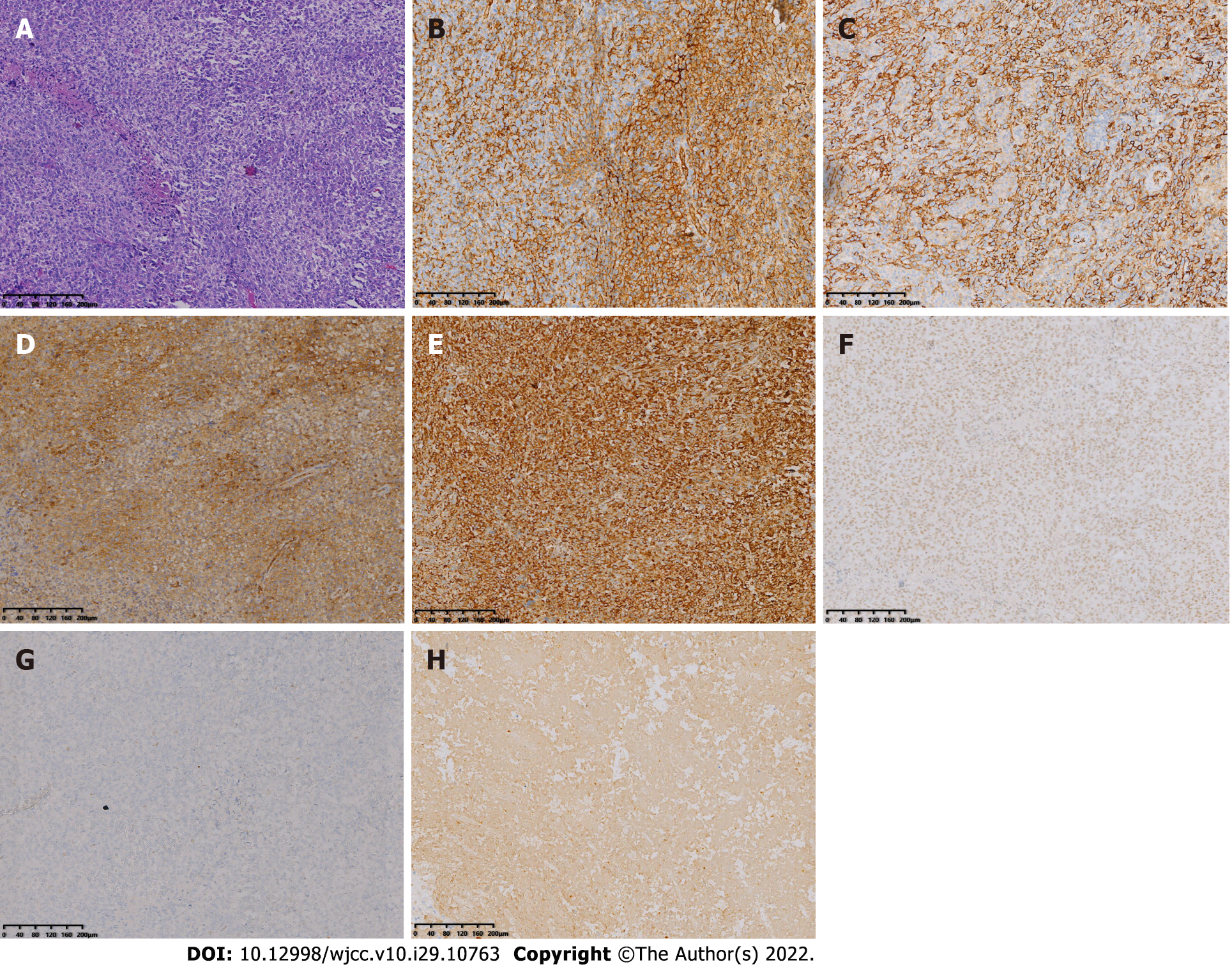
Postoperative histopathology showed that the tumor was composed of mononuclear synovial-like cells and sarcoma cells with lacunar-like lacunae inside. Moreover, tumor cells showed obvious pleomorphism and heterogeneity with pathological nuclear division and collagen matrix between the cells. Immunohistochemical staining was positive for EMA, CK, CD31, CD34, CD99, Vimentin, INI-1, TLE1, and FLI-1 and negative for S-100, SOX-10, GATA-3, CK5/6, CK7, P63, SMA, Desmin, ERG, HMB45, Melan-A, CD21, CD23, and CD35, corresponding to the phenotypic profile of MGCTTS (Figure 5).
The patient underwent resection of the right hip mass under general anesthesia. Intraoperatively, the tumor was found to be grayish-yellow in color and soft in texture with an incomplete local capsule and unclear border from the surrounding tissues. The tumor was rich in blood supply with dark-red bloody fluid inside.
Nineteen days after surgery, the patient suffered pain again in his right hip with a palpated mass measuring approximately 8 cm × 5 cm with a hard texture under the surgical scar, which was considered to be a recurrence of the tumor. Surgical resection was performed again. Fifteen days after the 2nd surgery, a follow-up CT imaging revealed a recurrence of MGCTTS in the right hip with a significantly increased size of approximately 7.7 cm × 7.6 cm × 24.3 cm (AP × LR × SI). Instead of surgical resection, the patient received chemotherapy with isocyclophosphamide (2 g per day fromd1 to d4) and epirubicin (50 mg d1 and 40 mg d2). After 2 cycles of treatment, CT re-examination revealed a decreased CT attenuation value of lesion with expanded necrosis range, and increased tumor size. In addition, the patient had multiple small solid nodulars along the pleura of both lungs with the larger lesion at approximately 0.6 cm in diameter (Figure 6A-D). The treatment was then changed to conformal intensity-modulated radiation therapy (50 Gy/25 F). After 21 sessions of radiation therapy, the patient developed a cutaneous fistula on the ventral side of the right hip with persistent bleeding. Then, CT examination revealed a further enlargement of the lesion, with the multiple, lamellar cystic hypodense lesions around with a mild enhancement of the cyst wall, suggesting the presence of infection. A lamellar hypodense infarcted area of the spleen was observed as well as increased number and sizes of small nodules in both lungs (Figure 6E-F). The patient exhibited low albumin levels with poor nute
MGCTTS was first reported by Castens et al[5]. The site of onset is mostly in the large joints of the extremities, but it can also occur in the myofascia and fascia of the forearms, thighs, and low back[6]. MGCTTS can be divided into primary and secondary lesions, with primary lesions having the typical pattern of GCTTS at first presentation along with areas of malignant sarcoma and secondary lesions having typical GCTTS at first presentation and a malignant sarcoma component at recurrence[4,7]. This case belongs to a primary MGCTS, occurring in the large joint, which affected large angle and range of motion of the hip, susceptibility to injury and chronic strain. It growed rapidly and aggressively with severe destruction of the surrounding bone.
The histological presentation of MGCTTS is comparable to that of a typical mesenchymal sarcoma, both presenting as grayish-yellow and grayish-red soft-textured masses with infiltrative growth, indistinct borders, and large tumor size[6]. Histomorphologically, MGCTTS originates from syno
CT can clearly show the details of bone changes. In CT imaging, MGCTTS mostly appears as a large and irregular mass that grows around a joint, with poorly defined borders. It is accompanied by extensive infiltrative destruction of adjacent bone tissue and shows obvious malignant behavious[9-11]. MRI has better resolution to show soft tissues than CT so that it can accurately demonstrate the histological features of tumors and their relationship with surrounding tissues[12-13]. MGCTTS typically shows heterogeneous signals on MRI images, with a predominantly muscle-like signal, that show a T1WI low signal, a T2WI high signal, or (and) iron-containing heme deposits in T1WI and T2WI low-signal areas due to the existence of necrosis and cystic changes within the tumor[4,6,12,14]. This case presented as a huge mass located in the right side of the proximal iliac, acetabular, and femoral bones, growing long with the tendon and with indistinct borders. It showed isointense with the muscle on CT with lamellar apparent necrosis inside, as well as extensive osteolytic destruction of adjacent bone tissue without periosteal reaction. Moreover, the mass showed a low signal on MRI in T1WI, a slightly high signal in fat-saturated T2WI, and nodular low-signal features in all sequences, suggesting iron-containing heme deposits with inhomogeneous mild enhancement. CT and MRI imaging can reveal the size, shape, location, internal changes such as necrosis and bleeding, and invasion of sur
The complex biology and clinical characteristics of MGCTTS render it difficult to make a definitive diagnosis and a thorough treatment plan preoperatively. MGCTTS is characterized by a high rate of local recurrence and distant metastasis in lungs and lymph nodes, leading to patient death. Therefore, the tumor should be considered a highly malignant sarcoma and managed aggressively[11]. There has been no standard treatment for MGCTTS so far and radical surgical resection remains the first choice for treatment. Surgery was shown to be able to minimize local recurrence and to decrease the occurence of distant metastases[19]. In soft tissue sarcomas of the extremities, producing a careful balance between local control and functional preservation is critical. However, it is important to remember that systemic therapy for MGCTTS is currently limited[11]. Adjuvant radiation therapy is used as an alternative treatment when complete resection is impossible[1,20]. Unfortunately, there is no evidence that these treatments for MGCTTS is totally effective. Some studies concluded that CSF1 of GCTTS is at a high level[21]. In many case reports and a retrospective series of patients with locally progressed or metastatic TGCTs, imatinib against CSF1R, was proven to be effective[22-23]. The patient in this case received multimodal treatments including surgery, radiation therapy, and chemotherapy. Unfortunately, the prognosis was still poor with an overall survival for only 10 mo.
In summary, we report a patient with MGCTTS who experienced recurrence after 2 surgical resections and his treatment outcome remained poor after adjuvant radiotherapy and chemotherapy. The treatment of MGCTTS is still unsatisfied. As a result, MGCTTS remains a difficult illness without enough medical requirements. More clinical trials and better understanding of the biology and molecular aspects of this subtype of sarcoma are needed, as well as the development of new medical treatment that can efficiently target specific pathways. Moreover, CT, MRI, and PET/CT imaging are essential for the staging, management, treatment response assessment, and monitoring of MGCTTS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chrcanovic BR, Sweden; Tangsuwanaruk T, Thailand S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Gouin F, Noailles T. Localized and diffuse forms of tenosynovial giant cell tumor (formerly giant cell tumor of the tendon sheath and pigmented villonodular synovitis). Orthop Traumatol Surg Res. 2017;103:S91-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Coindre JM. [New WHO classification of tumours of soft tissue and bone]. Ann Pathol. 2012;32:S115-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Brahmi M, Vinceneux A, Cassier PA. Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis. Curr Treat Options Oncol. 2016;17:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Kondo R, Akiba J, Hiraoka K, Hisaoka M, Hashimoto H, Kage M, Yano H. Malignant diffuse-type tenosynovial giant cell tumor of the buttock. Pathol Int. 2012;62:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Castens HP, Howell RS. Maglignant giant cell tumor of tendon sheath. Virchows Arch A Pathol Anat Histol. 1979;382:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Li CF, Wang JW, Huang WW, Hou CC, Chou SC, Eng HL, Lin CN, Yu SC, Huang HY. Malignant diffuse-type tenosynovial giant cell tumors: a series of 7 cases comparing with 24 benign lesions with review of the literature. Am J Surg Pathol. 2008;32:587-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Fletcher C, Bridge J, Antonescu C. WHO classification of tumours editorial board[J]. WHO Classification of Tumors Soft Tissue and Bone Tumours, 2020: 403-409. |
| 8. | Fletcher C, Bridge J A, Hogendoorn P C W, Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours, vol. 5[M]. World Health Organization, 2013. |
| 9. | Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 123] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Richman DM, Bresler SC, Rosenthal MH, Howard SA. Malignant tenosynovial giant cell tumor of the leg: a radiologic-pathologic correlation and review of the literature. J Clin Imaging Sci. 2015;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Rebuzzi SE, Grassi M, Catalano F, Buscaglia M, Bertulli R, Satragno C, Belgioia L, Comandini D. Multiple systemic treatment options in a patient with malignant tenosynovial giant cell tumour. Anticancer Drugs. 2020;31:80-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Waldt S, Rechl H, Rummeny EJ, Woertler K. Imaging of benign and malignant soft tissue masses of the foot. Eur Radiol. 2003;13:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Mastboom MJL, Lips W, van Langevelde K, Mifsud M, Ng C, McCarthy CL, Athanasou NA, Gibbons CLMH, van de Sande MAJ. The effect of Imatinib Mesylate in diffuse-type Tenosynovial Giant Cell Tumours on MR imaging and PET-CT. Surg Oncol. 2020;35:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Righi A, Gambarotti M, Sbaraglia M, Frisoni T, Donati D, Vanel D, Dei Tos AP. Metastasizing tenosynovial giant cell tumour, diffuse type/pigmented villonodular synovitis. Clin Sarcoma Res. 2015;5:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Li L, Huang W, Xue K, Feng L, Han Y, Wang R, Gao J. Clinical and imaging features of carcinosarcoma of the uterus and cervix. Insights Imaging. 2021;12:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Trassard M, Le Doussal V, Hacène K, Terrier P, Ranchère D, Guillou L, Fiche M, Collin F, Vilain MO, Bertrand G, Jacquemier J, Sastre-Garau X, Bui NB, Bonichon F, Coindre JM. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol. 2001;19:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Gazendam AM, Popovic S, Munir S, Parasu N, Wilson D, Ghert M. Synovial Sarcoma: A Clinical Review. Curr Oncol. 2021;28:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 18. | Ashikyan O, Bradshaw SB, Dettori NJ, Hwang H, Chhabra A. Conventional and advanced MR imaging insights of synovial sarcoma. Clin Imaging. 2021;76:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, Collini P, Lozza L, Pennacchioli E, Mariani L, Casali PG. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Temponi EF, Barros AAG, Paganini VO, Barbosa VAK, Badet R, Carvalho Júnior LH. Rev Bras Ortop. 217;450-457 Diffuse pigmented villonodular synovitis in knee joint: diagnosis and treatment. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Cassier PA, Gelderblom H, Stacchiotti S, Thomas D, Maki RG, Kroep JR, van der Graaf WT, Italiano A, Seddon B, Dômont J, Bompas E, Wagner AJ, Blay JY. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118:1649-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Blay JY, El Sayadi H, Thiesse P, Garret J, Ray-Coquard I. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT). Ann Oncol. 2008;19:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Stacchiotti S, Crippa F, Messina A, Pilotti S, Gronchi A, Blay JY, Casali PG. Response to imatinib in villonodular pigmented synovitis (PVNS) resistant to nilotinib. Clin Sarcoma Res. 2013;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |