Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10755
Peer-review started: July 4, 2022
First decision: August 1, 2022
Revised: August 15, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 86 Days and 18 Hours
Hydrodissection is a widely used technique during radiofrequency ablation (RFA) for benign thyroid nodules. Although it could effectively avoid thermal injury to the surrounding critical structures and achieve complete treatment, routine operation of the remaining needle could cause perithyroidal hemorrhage. In this report, we present 2 cases of perithyroidal hemorrhage during RFA caused by a hydrodissection needle, which have not been reported before.
A 21-year-old female and a 45-year-old male were admitted for RFA for benign thyroid nodules. Considering that their nodules were adjacent to the recurrent laryngeal nerve, the needle used for hydrodissection was placed and remained between the dorsal capsule of the lateral lobe and the recurrent laryngeal nerve. During the procedure, active bleeding near the needle appeared on ultrasonography (US). Although moderate pressure was quickly applied to the neck for several minutes, contrast-enhanced US (CEUS) still showed an active hemorrhage. A radiofrequency electrode was placed at the bleeding point under the guidance of CEUS to stop the bleeding, and the procedure was finally confirmed to be successful by CEUS, without other complications.
Hydrodissection during RFA of benign thyroid nodules was associated with a risk of perithyroidal hemorrhage. The timely recognition of this acute hemorrhage could help in the timely control of the bleeding, and CEUS-guided ablation of the bleeding point could be useful.
Core Tip: Hydrodissection is a widely-used technique during radiofrequency ablation on benign thyroid nodules to avoid thermal injury to the surrounding critical structures. Though it is widely regarded as safe, its routine performing of the remaining needle could cause perithyroidal hemorrhage. We presented 2 cases of perithyroidal hemorrhage during radiofrequency ablation caused by the hydrodissection needle, and additional intervention was used to stop the bleeding, which has no yet been reported. We believe that clinicians should be aware of the possible risk, and the improvement of hydrodissection is needed.
- Citation: Zheng BW, Wu T, Yao ZC, Ma YP, Ren J. Perithyroidal hemorrhage caused by hydrodissection during radiofrequency ablation for benign thyroid nodules: Two case reports. World J Clin Cases 2022; 10(29): 10755-10762
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10755.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10755
Currently, radiofrequency ablation (RFA) has been widely used in the treatment of benign thyroid nodules (BTNs)[1-3]. It is safe and extremely well tolerated, and no life-threatening complications have been reported[4-6]. Although a hemorrhage or a hematoma is one of the most common complications, the reported incidence is still relatively low (0.9%-17.0%)[6]. It includes perithyroidal, subcapsular, and intranodular hemorrhage[4-6]. Although the mechanical injury due to the electrode because of the rich blood supply to the BTNs, or the sudden reduction in intranodular pressure due to fluid evacuation primarily contributes to hemorrhage susceptibility[4-7], hydrodissection is an additional causative factor that should not be ignored.
Hydrodissection is a widely used technique that can help improve the efficacy and minimize the complications of RFA for BTNs[2,8]. It mostly involves using a needle, which is inserted through the skin and placed between the nodule and the adjacent critical structures to inject fluids to separate the nodule and the adjacent structures[8]. This technique could effectively avoid thermal injury to the surrounding critical structures and achieve complete treatment. The injection needle should remain in place[8] so that the fluids can be injected continuously to achieve a sufficient safety margin during the procedure because the injected fluid may spread to other cervical spaces[9]. In that case, the remaining needle has a possibility of causing perithyroidal hemorrhage if the nodule has a rich blood supply. Here, we encountered 2 cases of perithyroidal hemorrhage during RFA caused by a hydrodissection needle, which has not been reported before. We presented the cases to make clinicians aware of the possible risk of hydrodissection.
Case 1: A 21-year-old female presented with a left neck mass that existed for 2 mo with cosmetic concerns and abnormal sensation.
Case 2: A 45-year-old male presented with a left neck mass that existed for 4 mo with cosmetic concerns and abnormal sensation.
Case 1: Her report showed no compressive symptoms of the neck mass, a symptom score of 5/10 on the visual analog scale, and a cosmetic score of 3/4.
Case 2: His report showed no compressive symptoms of the neck mass, a symptom score of 5/10 on the visual analog scale, and a cosmetic score of 3/4.
Case 1: She stated that she did not have a past medical history of thyroid diseases, allergies, or a family history of any thyroid diseases.
Case 2: He was infected with hepatitis B virus but did not have a coagulation disorder, allergies, thyroid diseases, or a family history of any thyroid diseases.
Case 1: She had a normal body mass index (20.32 kg/m2; weight, 54 kg; height, 163 cm), blood pressure (106/72 mmHg), and electrocardiography (sinus rhythm with 70 bpm).
Case 2: He had a normal body mass index (24.6 kg/m2; weight, 63 kg; height, 160 cm), blood pressure (106/72 mmHg), and electrocardiography (sinus rhythm with 73 bpm).
Case 1: She had normal thyroid function (thyroid-stimulating hormone, 2.92 μIU/mL; normal, 0.55-4.78 μIU/mL), but both antithyroglobulin antibody and anti-thyroid peroxidase antibody were positive.
Case 2: He had normal thyroid-stimulating hormone (1.08 μIU/mL, normal, 0.55-4.78 μIU/mL) and negative antithyroglobulin antibody and anti-thyroid peroxidase antibody.
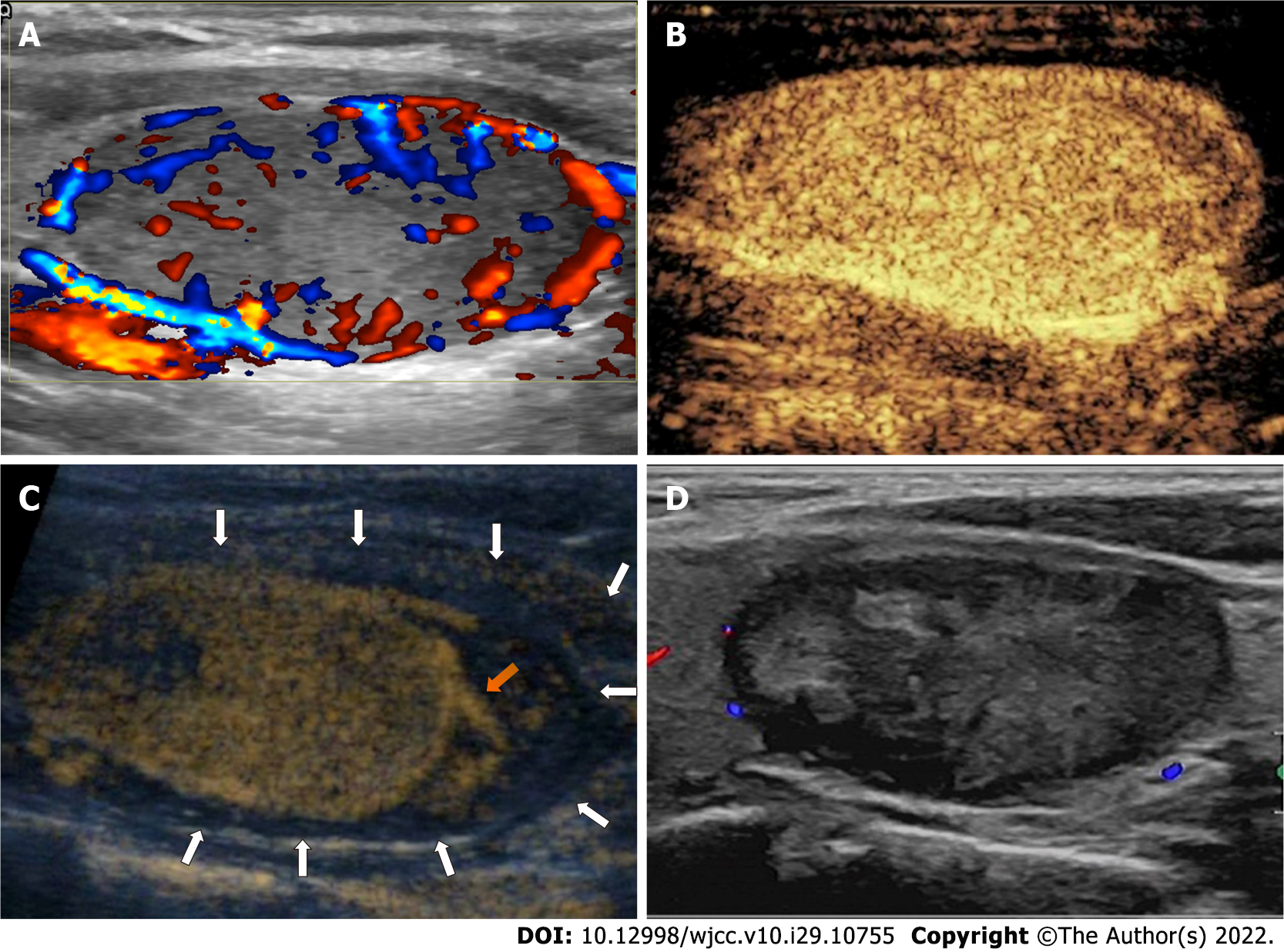
Case 1: Ultrasonography (US) of the thyroid showed a single nodule measuring 25 mm × 17 mm × 40 mm (volume, 8.9 mL) in the left lobe. The nodule was determined as level 4 based on the American College of Radiology’s Thyroid Imaging, Reporting and Data System, with a completely solid composition, hypoechogenicity, a wider-than-tall shape, and smooth margins. The nodule also showed marked vascularity and the presence of peripheral blood supply on color Doppler US (CDUS) (Figure 1A).
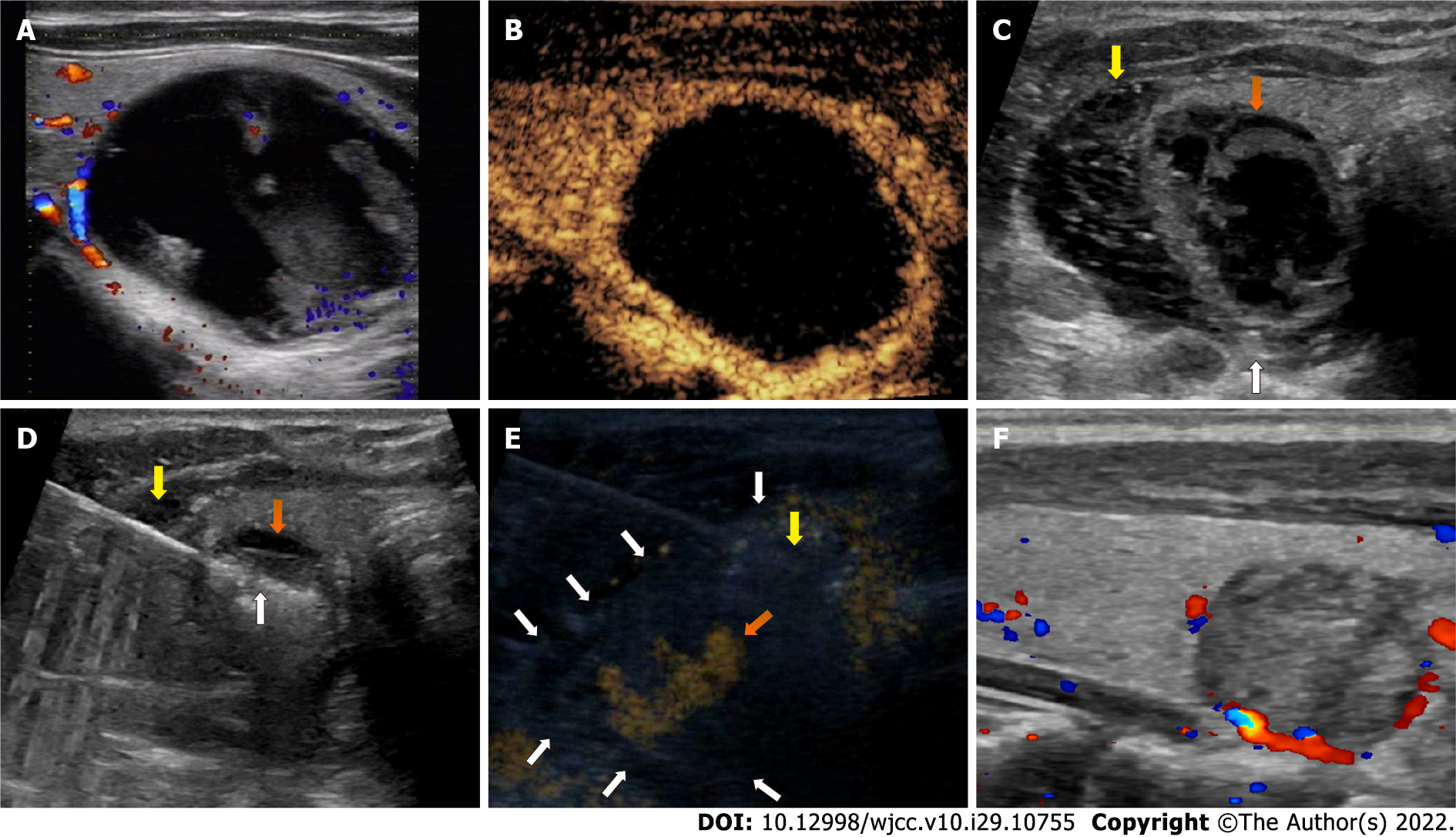
Case 2: US showed a single nodule, measuring 20 mm × 21 mm × 31 mm (volume, 6.8 mL) in the left lobe. The nodule was determined to be level 2 based on the American College of Radiology’s Thyroid Imaging, Reporting and Data System, with almost cystic composition, iso-echogenicity, a wider-than-tall shape, and smooth margins. The nodule also showed minimal vascularity and limited peripheral blood supply on CDUS (Figure 2A).
Case 1: The patient underwent US-guided fine needle aspiration, and the cytological diagnosis was benign (The Bethesda System for Reporting Thyroid Cytopathology II).
Case 2: The patient underwent US-guided fine needle aspiration, and the cytological diagnosis was benign (The Bethesda System for Reporting Thyroid Cytopathology II).
Case 1: The patient was admitted to our institute for RFA treatment. A detailed preoperative assessment was performed, and an operative plan was created, which included local anesthesia, a hydrodissection approach (5% glucose), lateral approach, a moving-shot technique, and contrast-enhanced US (CEUS). Considering that the nodule was adjacent to the recurrent laryngeal nerve (RLN), the needle used for hydrodissection was planned to be placed between the dorsal capsule of the lateral lobe and the RLN and to stay in place for continuous injection.
During the procedure, preoperative CEUS was used to determine the range of RFA, and the nodule showed homogenous hyperenhancement and a peripheral blood supply (Figure 1B). Following local anesthesia, the needle was inserted and placed in the intended location based on the hydrodissection plan. After 40 mL of 5% glucose was injected, an irregular iso-echogenicity area around the needle, nodule, and lobe appeared on US. Perithyroidal hemorrhage was the first consideration, and CDUS showed active hemorrhage near the needle. The needle was quickly withdrawn, and moderate pressure was applied on the neck for several minutes. Another CEUS still showed active hemorrhage near the location where the needle was placed (Figure 1C). The radiofrequency electrode was inserted into the location under the guidance of CEUS, and RFA was used to stop the bleeding. A third CEUS confirmed its success and showed that the hematoma had surrounded the nodule. Since a perithyroidal hematoma (range, 14 mm × 21 mm × 40 mm; volume, 6.2 mL) separated the nodule and the RLN, further hydrodissection was not performed. The electrode was inserted into the nodule, and the moving-shot technique was used during the ablation, with a delivered energy of 29.3 kJ (3.3 kJ/mL). Complete ablation was confirmed by CEUS.
Case 2: The patient was admitted for RFA. A detailed preoperative assessment was performed, and the operative plan was created, which included local anesthesia, aspiration of the intranodular hemorrhage, hydrodissection (5% glucose), lateral approach, moving-shot technique, and CEUS. Considering that the nodule was adjacent to the RLN, the needle used for hydrodissection was planned to be placed between the dorsal capsule of the lateral lobe and the RLN and to be kept in place for continuous injection.
During the procedure, preoperative CEUS was used to determine the range of RFA, and the nodule showed only a peripheral blood supply and almost no enhancement inside (Figure 2B). Following local anesthesia, a needle was first inserted into the nodule, and 5 mL intranodular hemorrhage was withdrawn. The nodule showed little shrinkage but was still adjacent to the RLN. Then, another needle was inserted and placed in the intended location based on the hydrodissection plan, and the nodule was successfully separated from the surrounding structures (Figure 2C). The needle remained in place as planned, the radiofrequency electrode was inserted into the nodule, and the moving-shot technique was performed for ablation (Figure 2D). During the ablation, an irregular hyperechogenicity area around the nodule appeared on US. Perithyroidal hemorrhage was diagnosed, and CDUS showed active hemo
Case 1: The patient showed stable vital signs during the procedure and did not experience other complications, such as a voice change or an upper airway obstruction. At the 1-mo follow-up, the irregular iso-echogenicity area had disappeared on US, and the volume reduction ratio of the nodule was 44.1% (Figure 1D).
Case 2: The patient showed stable vital signs during the procedure and did not experience other complications, such as a voice change or an upper airway obstruction. At the 1-mo follow-up, the irregular hyperechogenic area had disappeared on US, and the volume reduction ratio of the nodule was 86.4% (Figure 2F).
This is the first report of 2 cases of active perithyroidal hemorrhage induced by hydrodissection during RFA of BTNs. Both cases were controlled by the ablation, and the RFA procedures were completed without other complications.
Hydrodissection is an important technique that is performed to ensure the success and safety of percutaneous US-guided RFA for a wide range of tumors throughout the body[8,10-12]. It is widely regarded as safe, and few studies have shown reports of its related complications or side effects. Regarding RFA for BTNs, only one study showed that large-volume hydrodissection might cause more patients to suffer pain and chest tightness[9], but whether hydrodissection causes perithyroidal hemorrhage has not been reported. We supposed that the possible causes of perithyroidal hemorrhage secondary to hydrodissection during RFA were as follows: (1) Most BTNs that need treatment are large and hypervascular. They are usually closely adjacent to the capsule of the thyroid and usually have a peripheral blood supply. Therefore, most of them have large blood vessels that run over the dorsal capsule; (2) The needle used for hydrodissection is usually placed between the RLN and the dorsal capsule of the thyroid to separate the RLN and the BTNs since the RLN is one of the most important adjacent critical structures. The unintentional movement of the needle tip during the entire procedure could easily cause unexpected displacement or injury to the blood vessels on the dorsal capsule; and (3) Hydrodissection sometimes tears the surrounding blood vessels, causing hemorrhage, which has been reported in some patients undergoing thermal ablation for liver tumors[13]. Therefore, for BTNs, hydrodissection is associated with a risk of causing unwanted bleeding, and clinicians should increase their awareness and should be concerned about needle placement during the entire procedure.
The management of hemorrhage during the RFA procedure is not difficult. It can usually be controlled by moderate pressure on the neck or the ablation of the bleeding point[5,6]. For hemorrhage induced by hydrodissection, clinicians should also focus on the following points: (1) The timely recognition of hemorrhage during hydrodissection or caused by hydrodissection during the RFA procedure should be important since it could help with timely management. Previous studies showed that perithyroidal hemorrhage could be found immediately on US as a hypoechoic thin layer surrounding the thyroid[4,6], but it should be noted that an acute hemorrhage could be iso- or hyperechoic, which is similar to the thyroid or the nodule, and may be difficult to distinguish. In this situation, clinicians should stay alert to the abnormally increased range of hydrodissection. In addition, CEUS could also play a role in the recognition of active bleeding[14] and the bleeding point[15]; (2) If the needle remains inserted within the same place, compression on the neck may have the risk of causing a secondary injury of the blood vessels because of the movement of the needle tip. Withdrawing the needle before moderate pressure is applied would be safer; and (3) Since the bleeding point is usually deeply located in the dorsal capsule, achieving hemostasis by compression may be less effective. If active bleeding persists, timely CEUS-guided RFA on the bleeding point could be performed. Surgical treatment should always be available; however, there have not been any cases of hemorrhage that need further hospital or surgical treatment in any of the studies.
Some precautions could help. First, clinicians should preoperatively assess whether patients with coagulation disorders or who are taking drugs that have a risk of hemorrhage should take necessary measures, i.e. stop the related drugs for a period before RFA[16]. In addition, a replacement could be considered for needles, as the needle tip can easily injure the blood vessels. Catheters would be a possible solution. Some studies have performed the Seldinger technique to insert catheters[17-21] or have directly inserted catheters[22] for hydrodissection during RFA of liver tumors. However, this approach had a reported technical failure rate of 11.1%-12.0%[17,22], especially in patients with postoperative adhesion formation because of previous surgery in the corresponding region[18]. This is likely due to increased difficulty in using catheters to open or establish spaces in different layers of tissue to reach the predetermined site. Improving the approaches for successful and safe hydro
In conclusion, hydrodissection during RFA of BTNs has the risk of perithyroidal hemorrhage. The timely recognition of this acute hemorrhage helps to achieve timely control of the bleeding, and CEUS-guided ablation of the bleeding point is useful. Further studies that focus on the improvement of hydrodissection are needed for safer and more effective RFA for BTNs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chrcanovic BR, Sweden; Ghimire R, Nepal; Moshref RH, Saudi Arabia S-Editor: Wang DM L-Editor: Filipodia P-Editor: Wang DM
| 1. | Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, Chung SR, Ha EJ, Hahn SY, Jung SL, Kim DS, Kim SJ, Kim YK, Lee CY, Lee JH, Lee KH, Lee YH, Park JS, Park H, Shin JH, Suh CH, Sung JY, Sim JS, Youn I, Choi M, Na DG; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 437] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 2. | Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J. 2020;9:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 3. | Dobnig H, Zechmann W, Hermann M, Lehner M, Heute D, Mirzaei S, Gessl A, Stepan V, Höfle G, Riss P, Simon A. Radiofrequency ablation of thyroid nodules: "Good Clinical Practice Recommendations" for Austria : An interdisciplinary statement from the following professional associations: Austrian Thyroid Association (ÖSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (ÖGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med Wochenschr. 2020;170:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, Baek SM, Kim YS, Shin JH, Park JS, Kim DW, Kim JH, Kim EK, Jung SL, Na DG; Korean Society of Thyroid Radiology. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Kim C, Lee JH, Choi YJ, Kim WB, Sung TY, Baek JH. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27:3128-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Wang JF, Wu T, Hu KP, Xu W, Zheng BW, Tong G, Yao ZC, Liu B, Ren J. Complications Following Radiofrequency Ablation of Benign Thyroid Nodules: A Systematic Review. Chin Med J (Engl). 2017;130:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Miller JM, Zafar SU, Karo JJ. The cystic thyroid nodule. Recognition and management. Radiology. 1974;110:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Park HS, Baek JH, Park AW, Chung SR, Choi YJ, Lee JH. Thyroid Radiofrequency Ablation: Updates on Innovative Devices and Techniques. Korean J Radiol. 2017;18:615-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Ma Y, Wu T, Yao Z, Zheng B, Tan L, Tong G, Lian Y, Baek JH, Ren J. Continuous, Large-Volume Hydrodissection to Protect Delicate Structures around the Thyroid throughout the Radiofrequency Ablation Procedure. Eur Thyroid J. 2021;10:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, Hur YH, Park CH, Jeong YY, Kang HK. Ultrasound-Guided Percutaneous Radiofrequency Ablation of Liver Tumors: How We Do It Safely and Completely. Korean J Radiol. 2015;16:1226-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Uppot RN, Gervais DA. Imaging-guided adrenal tumor ablation. AJR Am J Roentgenol. 2013;200:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Gunn AJ, Gervais DA. Percutaneous ablation of the small renal mass-techniques and outcomes. Semin Intervent Radiol. 2014;31:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Wang CS, Sun J, Shi J. Effect of water isolation technology in MWA treatment for primary liver cancer on the 3-year prognosis of patients. Ganzang. 2021;527-529. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Kummer T, Oh L, Phelan MB, Huang RD, Nomura JT, Adhikari S. Emergency and critical care applications for contrast-enhanced ultrasound. Am J Emerg Med. 2018;36:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Naganuma H, Funaoka M, Fujimori S, Niwa M, Hirano H, Ishida H, Komatsuda T, Yamada M. Rupture of liver metastasis: report of a case with an emphasis on contrast-enhanced US. J Med Ultrason (2001). 2007;34:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, Kim EK, Kim DW, Park JS, Kim KS, Baek SM, Lee Y, Chong S, Sim JS, Huh JY, Bae JI, Kim KT, Han SY, Bae MY, Kim YS, Baek JH; Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol. 2013;68:e641-e651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Zhang L, Li Y, Wang W. Computed tomography-guided percutaneous microwave ablation with artificial ascites for problematic hepatocellular tumors. Int J Hyperthermia. 2020;37:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Park SJ, Lee DH, Han JK. Reducing Pain by Artificial Ascites Infusion During Radiofrequency Ablation for Subcapsular Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2021;44:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia. 2014;30:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |