Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10721
Peer-review started: May 27, 2022
First decision: June 27, 2022
Revised: July 10, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: October 16, 2022
Processing time: 124 Days and 18.6 Hours
Myocardial bridging is a common anatomical malformation, and the milking effect is a characteristic phenomenon of myocardial bridging in coronary angio
A 63-year-old man was diagnosed with ST-segment elevation myocardial infarction and received primary percutaneous coronary intervention on December 26, 2019. His heart rate was 104 beats per minute, and blood pressure was 15.3/10.3 kPa. A severe milking effect was found in the left anterior descending coronary artery during his index coronary angiography on January 14, 2020. The patient was given intensive medical management, including a β1-adrenergic receptor blocker, during hospitalization and after discharge. Unexpectedly, coronary angiography showed that the previous impressive milking effect was dramatically alleviated (close to normal) at the follow-up on October 13, 2020. At that moment, the patient’s heart rate was 83 beats per minute, and blood pressure was 12.7/8.0 kPa.
The myocardial bridging phenomenon is not invariable and, in certain circumstances, may vary. Furthermore, the autonomic nervous system may be involved in the myocardial bridging phenomenon.
Core Tip: Myocardial bridging is a common anatomical malformation. Coronary angiography is considered the routine method to diagnose myocardial bridging, and the milking effect is a characteristic phenomenon of myocardial bridging in coronary angiography. Myocardial bridging is generally classified as superficial or deep according to anatomical features, which can be manifested by the milking effect. While the milking effect is generally invariable, our case surprisingly showed that the milking effect is not invariable. In certain circumstances, the myocardial bridging phenomenon may vary, which can mislead us into judging the prognosis of myocardial bridging. Therefore, it is necessary to perceive myocardial bridging phenomenon anew.
- Citation: Li HH, Liu MW, Zhang YF, Song BC, Zhu ZC, Zhao FH. Myocardial bridging phenomenon is not invariable: A case report. World J Clin Cases 2022; 10(29): 10721-10727
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10721.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10721
Generally, the coronary artery travels in the connective tissue under the epicardium. When a coronary artery travels in the myocardium, this bundle of myocardial fibers is called myocardial bridging (MB), and this segment of the coronary artery is called the mural coronary artery (MCA). Coronary angio
A 63-year-old man presented with persistent chest pain that had lasted for 1 d.
The patient was admitted to the emergency department of our hospital on December 26, 2019, due to severe chest pain accompanied by dizziness, sweating, vomiting, diarrhea, and frequent urination. His heart rate was 104 beats per minute, and blood pressure was 15.3/10.3 kPa.
The patient had a history of smoking for 30 years, and he had quit 3 years ago. He was newly diagnosed with hyperlipidemia, diabetes mellitus, and acute cerebral infarction. The patient denied an allergy history.
The patient denied relevant familial history.
The patient was conscious. His heart rate was 104 beats per minute, and blood pressure was 15.3/10.3 kPa. There were no significant abnormalities detected upon cardiac auscultation.
The level of cardiac troponin T was high (0.597 ng/mL; normal range: 0.000-0.014 ng/mL), as was that of myoglobin (246.80 ng/mL; normal range: 28.00-72.00 ng/mL) and pro-B-type natriuretic peptide (Pro-BNP) (6828.00 pg/mL; normal level: < 161 pg/mL). In addition, plasma glucose was high (26.51 mmol/L; normal range: 3.90-6.10 mmol/L), as was glycated hemoglobin (HbA1c) (13.60%; normal range: 4.30%-6.10%). The urea level was just above normal range (8.90 mmol/L; normal range: 2.76-8.07 mmol/L) but the creatinine level was normal (66.00 mmol/L; normal range: 59.00-104.00 mmol/L). Total cholesterol was normal (5.34 mmol/L) but low-density lipoprotein (LDL)-cholesterol was high (4.09 mmol/L; desirable range: < 2.59 mmol/L), and high-density lipoprotein (HDL)-cholesterol was satisfactory (9.80 mmol/L; desirable range: > 1.09 mmol/L). The triglycerides level was normal (1.48 mmol/L; normal range: 0.60-1.70 mmol/L). Tests of heart rate variability (HRV) and norms showed the HRV indices standard deviation of RR-intervals (SDNN) to be 19 ms, the root mean squares of successive differences to be 36 ms, and the percentage of RR-intervals with at least 50 ms deviation from the preceding RR-interval (pNN50) to be 0%.
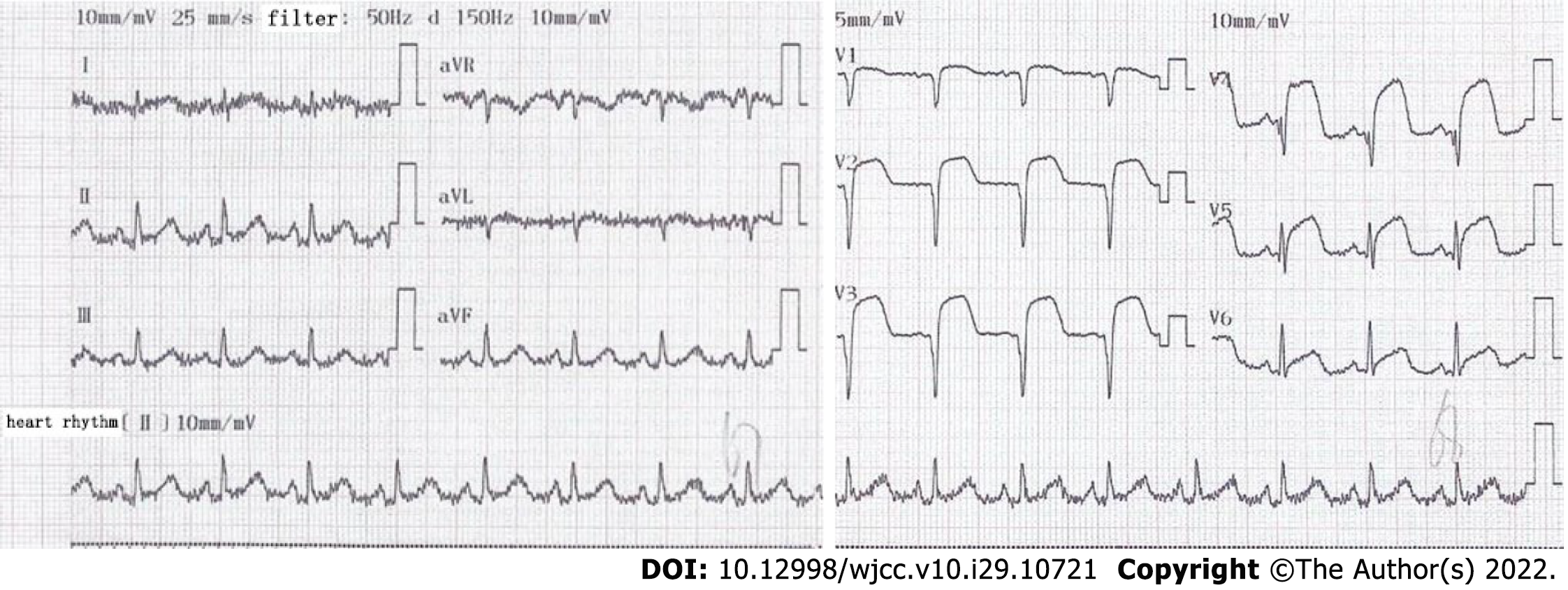
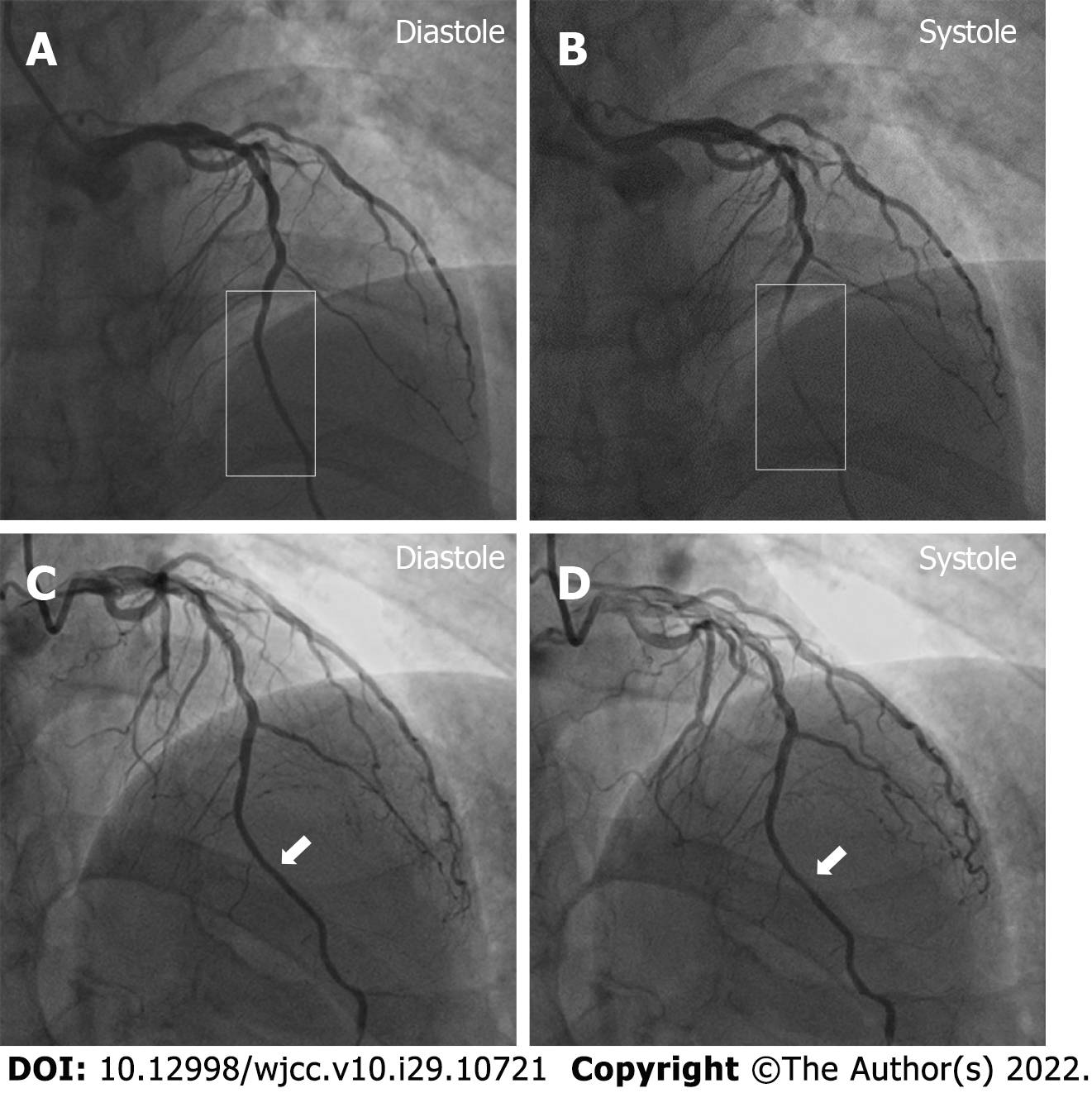
Electrocardiogram indicated an elevation of ST-segment in leads V1–V5 (Figure 1). The patient accepted emergency CAG, which indicated the left anterior descending (LAD) coronary artery as the culprit vessel and 100% occluded. He received emergency primary percutaneous coronary intervention. After one drug eluting stent was implanted, MB was found with 90% stenosis during the systolic phase in the middle segment of the LAD coronary artery. The patient was followed up by CAG on January 14, 2020, and the MB still had 90% stenosis (Figure 2A and B). He was further followed up and received a CAG procedure on October 13, 2020. Incredibly, the previous MBP changed dramatically with a stenosis decrease from 90% to 30% (Figure 2C and D) during heart systole.
The patient was diagnosed with ST-segment elevation myocardial infarction, and his cardiac function was assessed as Killip class Ⅲ. In addition, the patient was diagnosed with hyperlipidemia, diabetes mellitus, and acute cerebral infarction.
In addition to the imaging-consequent surgical interventions described above, the patient was given intensive medical management of bisoprolol fumarate, a type of β1-adrenergic receptor blocker, as well as antiplatelet therapy, anticoagulation, statin and blood glucose control, etc. After discharge, the patient was prescribed orally administered 5 mg qd of bisoprolol fumarate, 0.1 g qd of aspirin, 75 mg qd of clopidogrel, 10 mg qd of rivaroxaban, 10 mg qn of rosuvastatin calcium, 50 mg qd of acarbose, and 100 mg qd of sitagliptin phosphate, and injections with 4 IU to 6 IU of insulin aspart before meals and 12 IU of insulin glargine before sleep.
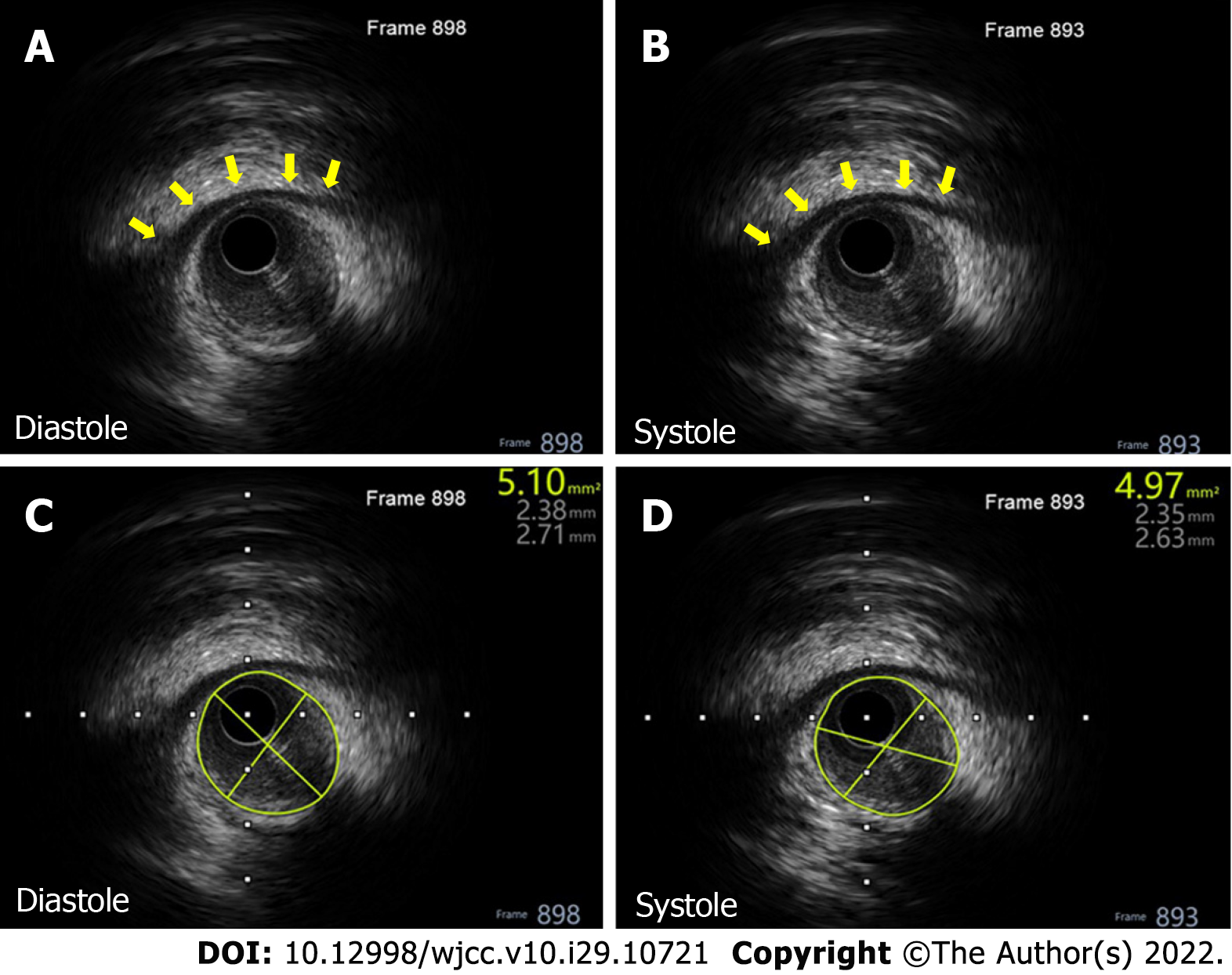
The patient was followed up and accepted a CAG procedure on October 13, 2020. Incredibly, the previous MBP changed dramatically with a stenosis decrease from 90% to 30% (Figure 2C and D) during heart systole. This was very thought-provoking, and further intravascular ultrasound examination showed a distinctive ‘half-moon’ phenomenon of the MB (Figure 3A and B). There was no obvious change of the lumen area during systole (Figure 3C and D) as index CAG had shown. Unfortunately, the intravascular ultrasound procedure was not performed during his index hospitalization on December 26, 2019.
In this follow-up, the patient’s heart rate was 83 beats per minute and blood pressure was 12.7/8.0 kPa. The level of cardiac troponin T lowered substantially to just above normal (0.034 ng/mL) and that of myoglobin normalized (32.53 ng/mL). Pro-BNP also lowered substantially but remained slightly above normal (321.30 pg/mL). The patient’s diabetic markers normalized, with plasma glucose at 5.90 mmol/L and HbA1c at 6.10%. The urea level normalized (5.20 mmol/L) and the creatinine level remained normal (74.00 mmol/L). The total cholesterol remained normal (2.92 mmol/L) and LDL-cholesterol (0.81 mmol/L) and HDL-cholesterol (1.76 mmol/L) were within the desirable range. The triglycerides level was within normal range (1.14 mmol/L).
Between January 2020 and October 2020, the patient was instructed to take a low-salt, low-fat and diabetic diet and avoid exertion and rage. He was also instructed to take drugs for secondary prevention of coronary heart disease (administered orally as 5 mg qd of bisoprolol fumarate, 0.1 g qd of aspirin, 75 mg qd of clopidogrel, 10 mg qd of rivaroxaban, and 10 mg qn of rosuvastatin calcium) and to manage blood glucose (administered orally as 50 mg qd of acarbose and 100 mg qd of sitagliptin phosphate, and administered as subcutaneous injections with 4 IU to 6 IU of insulin aspart before meals and 12 IU of insulin glargine before sleep). The timeline summarized the relevant Information from this case (Table 1).
| Timeline | |
| Patient’s information | A 63-year-old man presented with persistent chest pain that had lasted for 1 d on December 26, 2019. His heart rate was 104 beats per minute, and blood pressure was 15.3/10.3 kPa |
| Clinical findings | The level of cardiac troponin T was high (0.597 ng/mL; normal range: 0.000-0.014 ng/mL), as was that of myoglobin (246.80 ng/mL; normal range: 28.00-72.00 ng/mL) and Pro-BNP (6828.00 pg/mL; normal level: < 161 pg/mL). Tests of HRV and norms showed the HRV indices SDNN to be 19 ms. Myocardial bridging was stenosed about 90% during systole and recovered during diastole on January 14, 2020 |
| Diagnosis | The patient was mainly diagnosed with ST-segment elevation myocardial infarction, and his cardiac function was assessed as Killip class Ⅲ |
| Treatment | The patient accepted PCI and one drug eluting stent was implanted. After PCI, the patient was instructed to take a low-salt, low-fat and diabetic diet and avoid exertion and rage. He was also instructed to take drugs for secondary prevention of coronary heart disease (administered orally as 5 mg qd of bisoprolol fumarate, 0.1 g qd of aspirin, 75 mg qd of clopidogrel, 10 mg qd of rivaroxaban, and 10 mg qn of rosuvastatin calcium) and to manage blood glucose (administered orally as 50 mg qd of acarbose and 100 mg qd of sitagliptin phosphate, and administered as subcutaneous injections with 4 IU to 6 IU of insulin aspart before meals and 12 IU of insulin glargine before sleep) |
| Follow-up | On October 13, 2020, the follow-up manifested that the previous MBP changed dramatically with a stenosis decrease from 90% to 30% during heart systole. In this follow-up, the patient’s heart rate was 83 beats per minute and blood pressure was 12.7/8.0 kPa. The level of cardiac troponin T lowered substantially (0.034 ng/mL) and that of myoglobin normalized (32.53 ng/mL). Pro-BNP also lowered substantially (321.30 pg/mL) |
Although there is no recognized criteria for the classification of MB, it is generally classified as superficial or deep. According to Corban et al[3], the differentiation between superficial MB and deep MB is usually based on anatomical features. The ME is correlated with the depth of the MB, and the depth of the MB is correlated with prognosis[2]. Therefore, the ME is very valuable for judging the prognosis of MB. In traditional opinions, superficial and deep MBs have relatively fixed positions in the myocardium so that the ME is invariable. In this case, however, we found that the MBP changed greatly in a short period, which suggests that it is unadvisable to evaluate the position and prognosis of MB by CAG, considered the gold standard to diagnose the MB[1].
MB typically involves the LAD coronary artery. Watanabe et al[4] dissected 60 hearts, among which 89.4% of MB was found to occur in the LAD coronary artery. MB detection rate varies between 0.8% to 4.9% by CAG compared with 5% to 86% by autopsy[5]. Therefore, it is generally considered benign and does not have clinical significance. However, adverse events caused by MB have still been widely reported and have relevance with the depth of the MB; MB may result in severe cardiovascular events[2,6]. Additionally, sudden cardiac death in young people who lack of any high-risk cardiovascular factors and underlying diseases has received increasing attention in recent years, which reminds us to give attention to congenital coronary malformations, such as MB[7,8].
We speculate that the autonomic nervous system and endocrine system may be involved in the MBP. The sympathetic nervous system would be more excited, and the parasympathetic nervous system would be suppressed after the onset of an acute myocardial infarction[9]. Sympathetic excitation can increase the myocardial contractility, accelerate the heart rate, and quicken the conduction speed. These actions are respectively defined as positive inotropic action, positive chronotropic action, and positive dromotropic action. Furthermore, sympathetic nervous activity would return to normal sooner, along with improvement in cardiac function then parasympathetic nervous activity[9]. Sympathetic nerves can also cause the coronary artery to constrict, even though its action is inconspicuous. Our patient’s sympathetic nervous system was excited just after he experienced ST-segment elevation myocardial infarction and acute cerebral infarction. His heart rate, blood pressure and SDNN were relatively higher. The MBP was severe. When he was followed up after 10 mo, the sympathetic nervous activity was determined to have alleviated because he was gradually recovering and had accepted medical management (especially the β1-adrenergic receptor blocker). The previous stenosis caused by MB was incredibly alleviated. Of course, the role of humoral factors is still not excluded. Vascular function is regulated by both the autonomic nervous system and the endocrine system. As is well-known, pro-BNP can serve to dilate blood vessels. Interestingly, our patient’s pro-BNP was 6828.00 pg/mL when his MBP was significant, compared with a level of 321.30 pg/mL when his MBP was insignificant. As such, we speculate that MBP may be more closely related to the autonomic nervous system rather than to the endocrine system. However, this does not mean that MBP has nothing to do with endocrine factors, because the clinical hormonal assessment that was accepted by this patient was limited and there was not sufficient evidence to deny the effects of other endocrine substances. Based on this dilemma, the MBP is worth exploring further.
Through research, MB was found to be controlled by the cervical ganglia and vagal nerves by dissecting animals[10]. Regrettably, the anatomic evidence of MCA in human is still insufficient, although the autonomic fibers are known to sometimes travel along the coronary artery[4]. Therefore, we conjecture such a possibility that the sympathetic nerve affects the MBP in a manner of acting on the myocardial muscle bands as well as the smooth muscle of the MCA. On the one hand, when sympathetic nervous activity increases, heart contractility increases and the heart rate is faster. As a consequence, the systole is prolonged and the diastole is shortened. The process when the MCA is squeezed is prolonged, and the ME is significant. On the other hand, the MCA is likely distributed with sympathetic fibers. The increased activity that can cause the MCA to shrink results in more significant ME.
This case report describes an inconceivably rare MBP, in which the ME varied greatly during and after follow-up CAG. This case showed that the ME is not invariable, which subverts the traditional deep-rooted views of superficial and deep MB. The MBP may change with activity of the autonomic nerves. Sometimes deep MBP and superficial MBP can change to the other, which can mislead the prognosis of MB. A new perspective of MBP is warranted.
We are extremely grateful for the cooperation of the patient and his family members.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Imed L, Tunisia; Tiwari N, United States S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Vernuccio F, Fazio G, Lo Re G, Grutta G, Insalaco A, Galfano MC, Rabita F, La Grutta L. Diagnosis, prognosis and treatment of "myocardial bridging": state of the art and unresolved issues. Recenti Prog Med. 2013;104:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Elmali M, Soylu K, Gulel O, Bayrak IK, Koprulu D, Diren HB, Celenk C. Correlation between depth of myocardial bridging and coronary angiography findings. Acta Radiol. 2008;49:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Corban MT, Hung OY, Eshtehardi P, Rasoul-Arzrumly E, McDaniel M, Mekonnen G, Timmins LH, Lutz J, Guyton RA, Samady H. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. 2014;63:2346-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 4. | Watanabe Y, Arakawa T, Kageyama I, Aizawa Y, Kumaki K, Miki A, Terashima T. Gross anatomical study on the human myocardial bridges with special reference to the spatial relationship among coronary arteries, cardiac veins, and autonomic nerves. Clin Anat. 2016;29:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Rogers IS, Tremmel JA, Schnittger I. Myocardial bridges: Overview of diagnosis and management. Congenit Heart Dis. 2017;12:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | He X, Ahmed Z, Liu X, Xu C, Zeng H. Recurrent attack of acute myocardial infarction complicated with ventricular fibrillation due to coronary vasospasm within a myocardial bridge: a case report. BMC Cardiovasc Disord. 2020;20:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Belhadj M, Saadi S, Ben Jomaa S, Dhouieb R, Kort I, Marzougui M, Amine Mesrati M, Chadly A, Haj Salem N. Death due to myocardial infarction in young patients: A study of 312 cases of sudden death. Ann Cardiol Angeiol (Paris). 2020;69:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Konduracka E, Piwowarska W, Kitliński M. Myocardial bridge of the coronary arteries and its clinical significance. Pol Merkur Lekarski. 1997;3:86-88. [PubMed] |
| 9. | Oya M, Itoh H, Kato K, Tanabe K, Murayama M. Effects of exercise training on the recovery of the autonomic nervous system and exercise capacity after acute myocardial infarction. Jpn Circ J. 1999;63:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Tangkawattana P, Muto M, Nakayama T, Karkoura A, Yamano S, Yamaguchi M. Prevalence, vasculature, and innervation of myocardial bridges in dogs. Am J Vet Res. 1997;58:1209-1215. [PubMed] |