Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10681
Peer-review started: April 27, 2022
First decision: June 27, 2022
Revised: July 9, 2022
Accepted: September 8, 2022
Article in press: September 8, 2022
Published online: October 16, 2022
Processing time: 154 Days and 21.6 Hours
Cerebrotendinous xanthomatosis is an autosomal recessive disorder of lipid meta
Here we report the clinical, biochemical, and molecular characterization of a 33-year-old female patient with cerebrotendinous xanthomatosis. The patient deve
Cerebrotendinous xanthomatosis requires a multidisciplinary diagnosis that must be made early to avoid progressive neurological degeneration. c.1263+1G>T is a known mutation, but c.255+1G>T is a rare mutation site.
Core Tip: This study identified a case of delayed diagnosis of cerebrotendinous xanthomatosis (CTX) that resulted in severe neurological impairment. CTX is caused by CYP27A1 gene mutations, and one rare mutation and one known mutation were identified in our patient. CTX diagnosis must be made early to avoid neurologic injury and worsening. This finding also provides new data for further revealing the pathogenesis of CTX, enriching the pathogenic mutation spectrum of the CYP27A1 gene and molecular diagnosis of the disease, which is of great significance for fertility guidance and prenatal diagnosis of this patient in the future.
- Citation: Chang YY, Yu CQ, Zhu L. Progressive ataxia of cerebrotendinous xanthomatosis with a rare c.255+1G>T splice site mutation: A case report . World J Clin Cases 2022; 10(29): 10681-10688
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10681.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10681
Cerebrotendinous xanthomatosis (CTX) is an autosomal recessive disorder of lipid metabolism caused by mutations of the CYP27A1 gene encoding sterol 27-hydroxylase. Sterol 27-hydroxylase is vital to the rate-limiting step in bile acid synthesis, which when deficient results in insufficient production of chenodeoxycholic acid[1]. Therefore, cholesterol accumulates in plasma and precipitates into various lipophilic tissues, such as the brain (with a preference for the cerebellum), eyes, and tendons. This results in the characteristic cataracts and tendon xanthomata. CTX can occur in infants, adolescents, and adults. The clinical symptoms are diverse, with 85% of patients developing bilateral cataracts in childhood, 90% presenting with intelligence lower than other children of the same age, 80% with pyramidal tract abnormalities, 73% with the typical signs of cerebellar ataxia, and about 50% of the patients having seizures. Tendon xanthomata is the most common symptom, present in 90% of patients. Other symptoms include osteoporosis, arterial disease, and early atherosclerosis. The rarest symptoms include Parkinson’s disease and xanthoma of the spine[2]. CTX is easily misdiagnosed as a treatable disease. While patients with early detection show good treatment effects[3], patients with severely impaired neurological function have poor treatment effects and are prone to sequelae. In this article, we share the clinical misdiagnosis and genotypic manifestations of a CTX patient and report a rare pathogenic mutation site for clinical reference.
The patient, a female with non-consanguineous parents, came to clinical attention at age 33.
She reported having poor stress tolerance, attention deficits, a “weird personality,” and poor learning ability during childhood. When she was 9, she was diagnosed with cataracts in both eyes and sub
There was no significant history of past illnesses other than those described.
There were no known consanguineous marriages in the family.
In a neurological examination at the age of 33, the patient presented with spastic paraparesis, progressive gait ataxia, mild dysarthria, clonic knee reflexes, bilateral Babinski sign, and abnormal proprioception, vibration, and temperature sensation in both feet.
Her laboratory data were as follows: triglyceride, 0.83 mmol/L (normal range: 0-1.7); total cholesterol, 3.92 mmol/L (normal range: 0-5.2); high-density lipoprotein, 2.02 mmol/L (normal range: 1.04-1.90); and low-density lipoprotein, 1.77 mmol/L (normal range: 1.63-3.81).
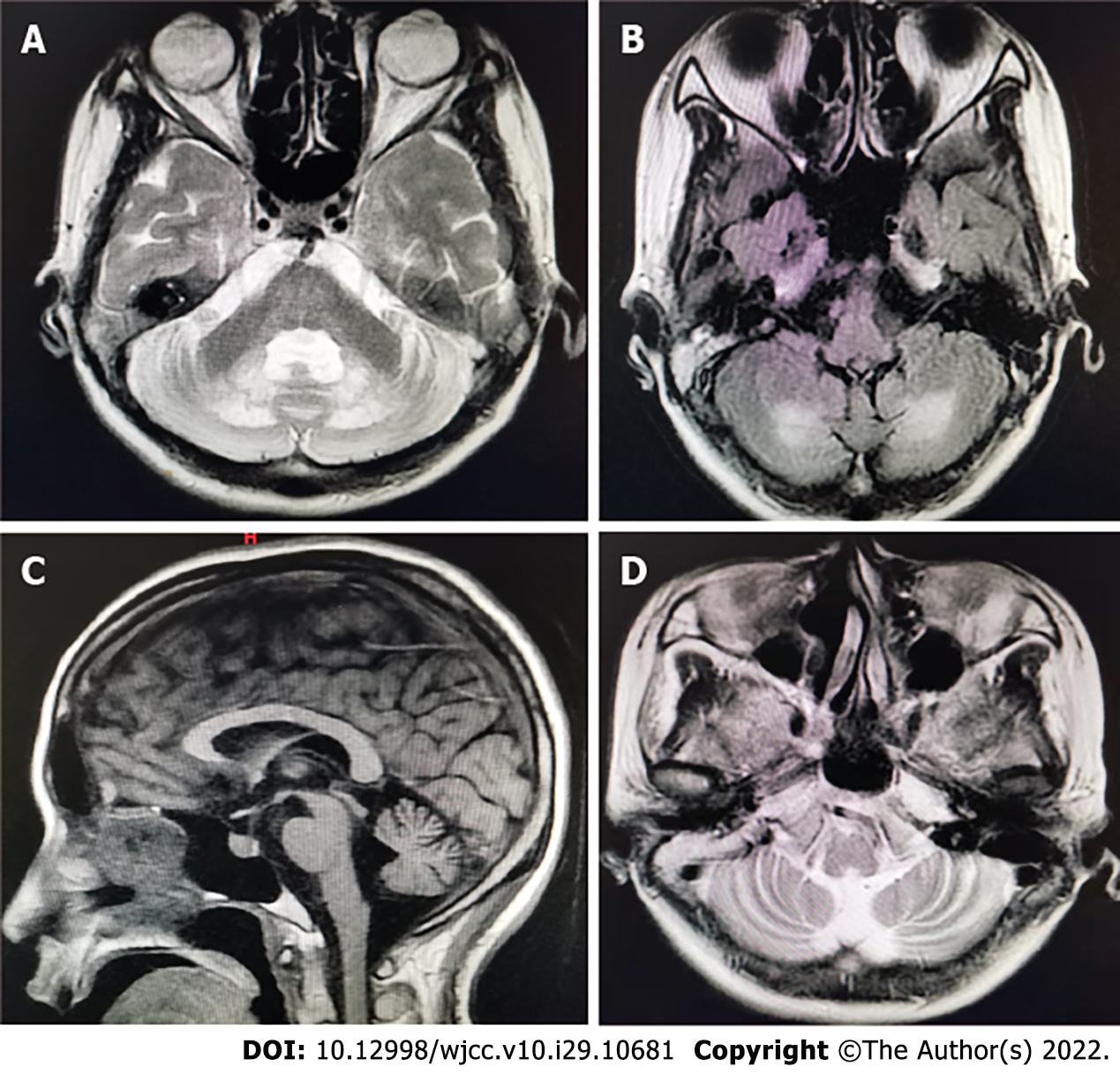
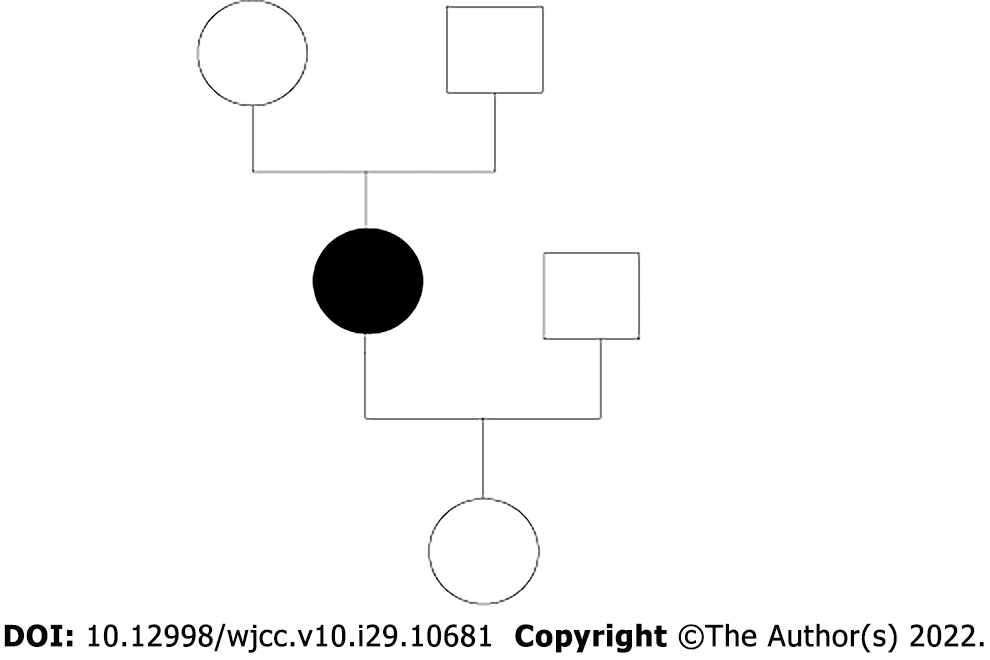
The characteristic magnetic resonance imaging (MRI; 1.5 T) features of CTX (bilateral cerebellar lesions of dentate nuclei) were particularly prominent. On T2 weighted and FLAIR sequences, an MRI of the brain clearly revealed hyperintensity of the dentate nuclei (Figure 1). The CTX index family pedigree is shown in Figure 2. There were no known consanguineous marriages in the family.
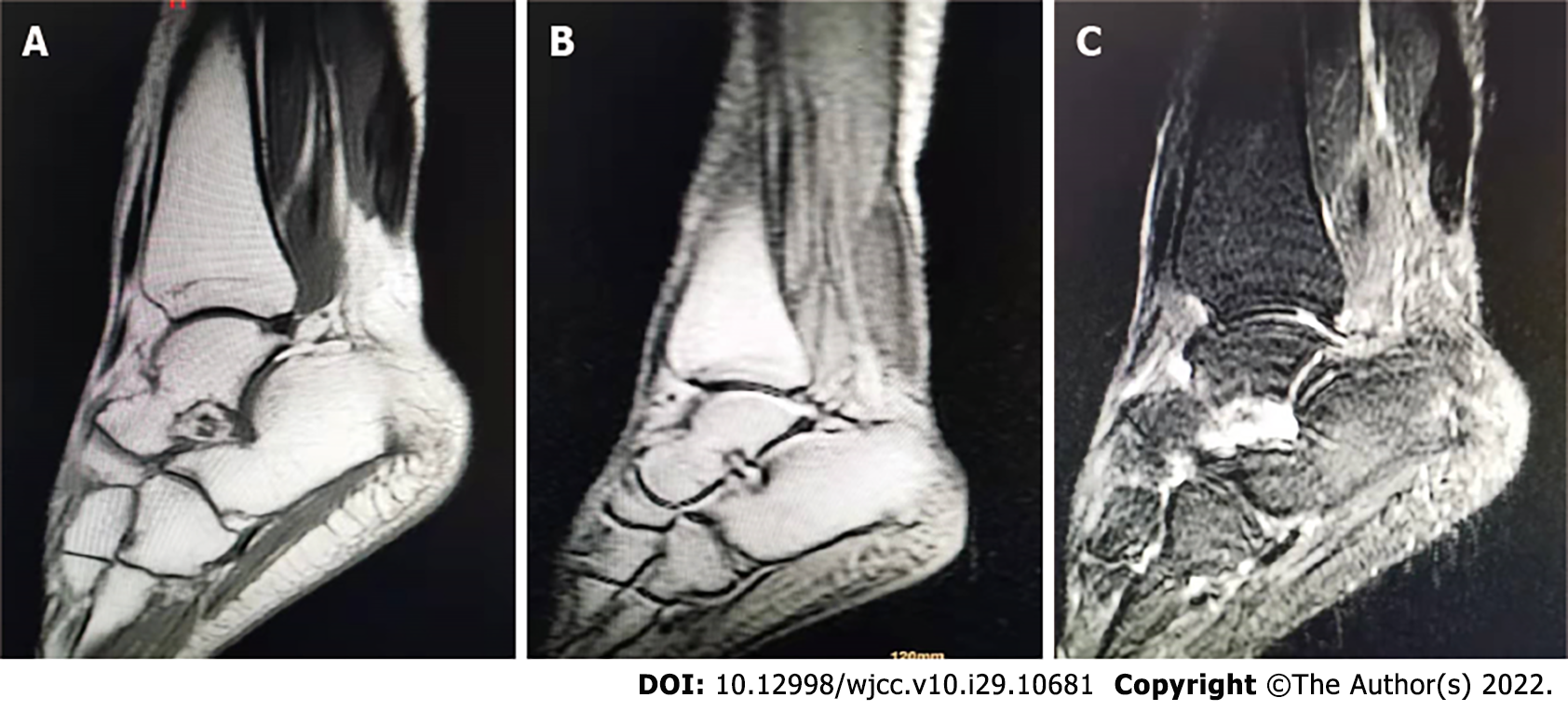
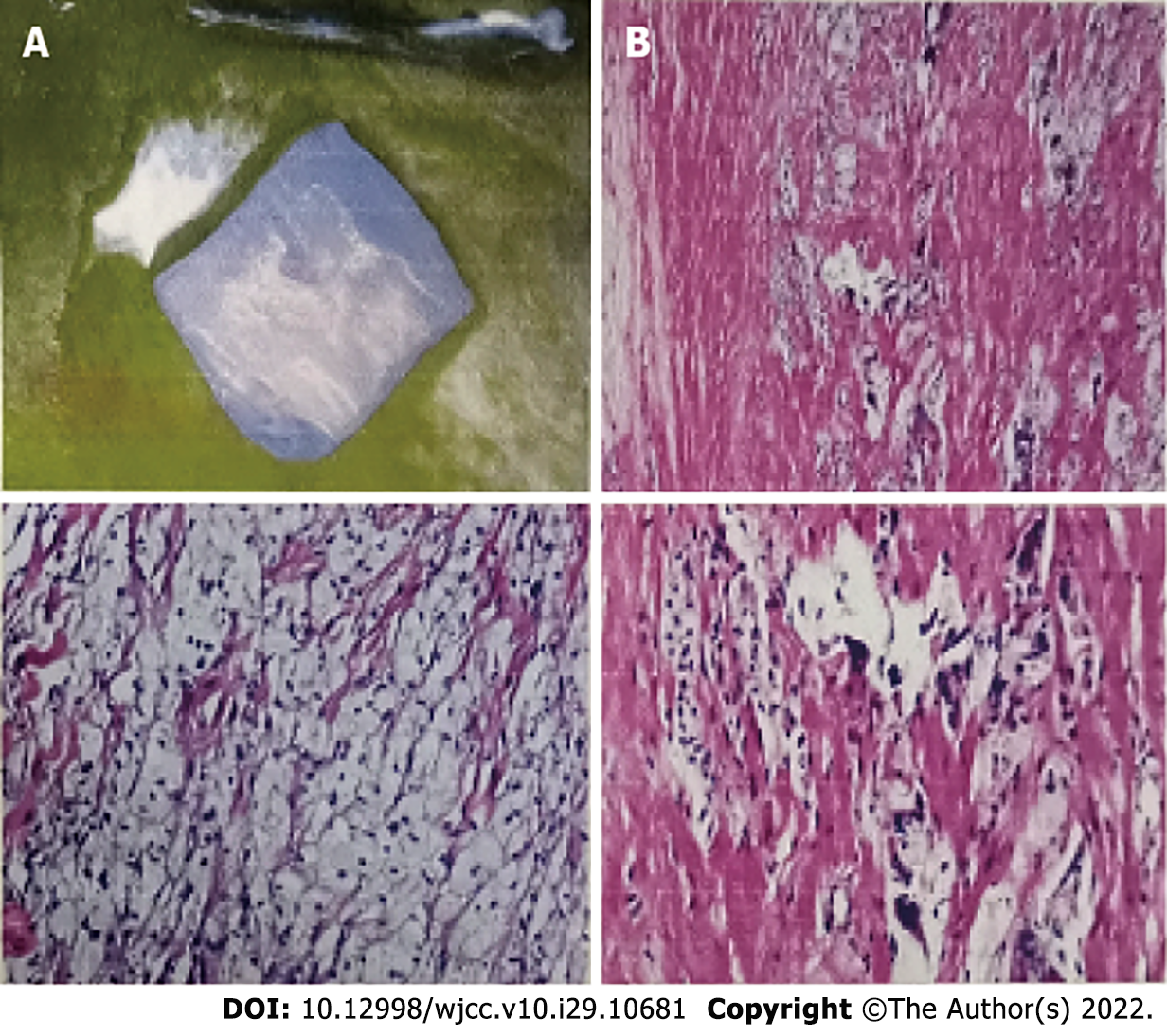
The right Achilles tendons had a fusiform expansion with a convex anterior border, and on MRI the ankles appeared isointense to muscle. The axial image revealed low signal intensity patches across the muscle, giving it a distinctive speckled look (Figure 3). An MRI of the right tendon showed a 56 mm × 16 mm × 21 mm mass in the middle and upper segments of the Achilles tendon. A biopsy of this mass showed mostly foam cells and a few multinucleated giant cells in the fibrous tissue (Figure 4). At the age of 33, she started the chenodeoxycholic acid (CDCA) regimen.
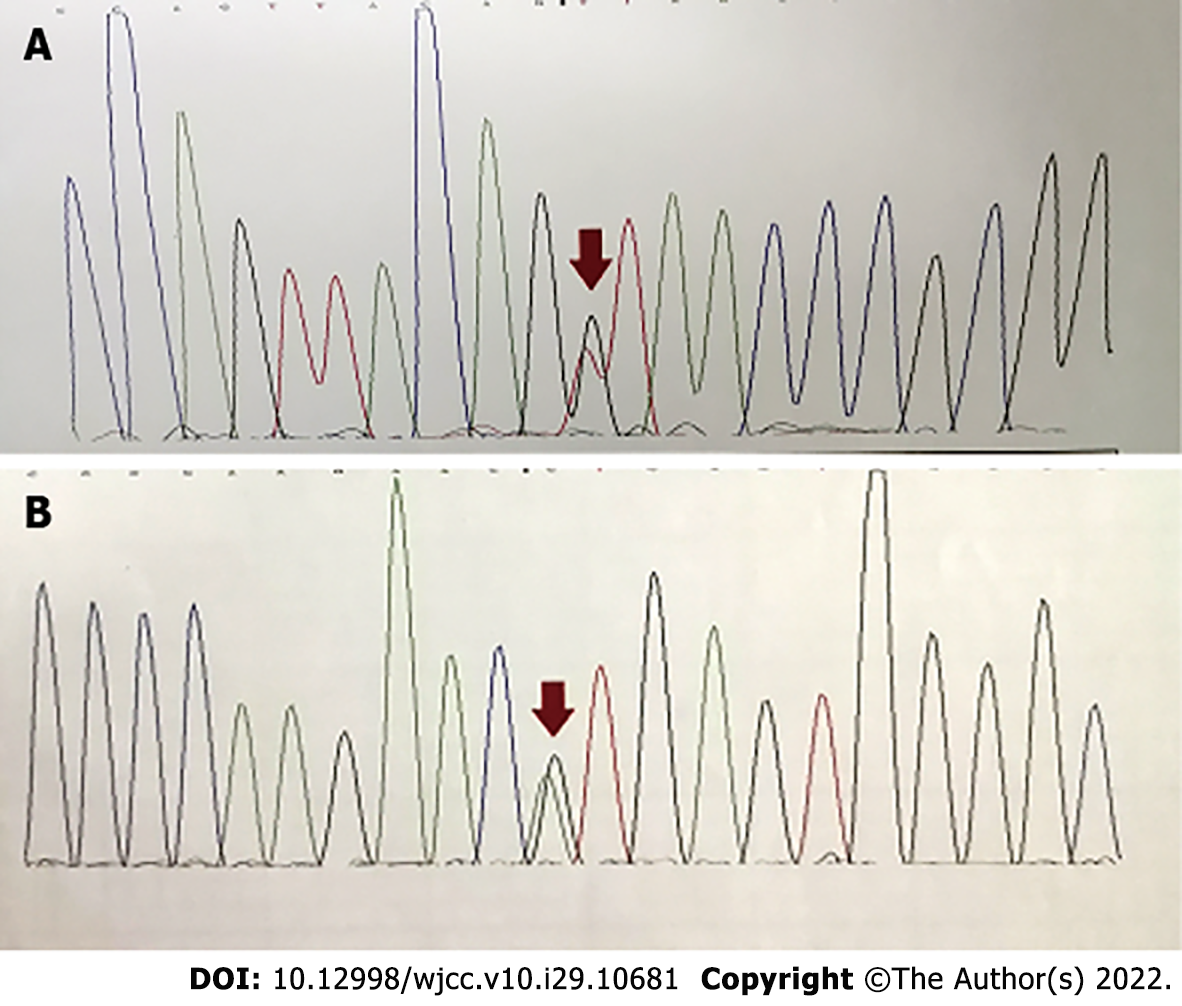
Genetic confirmation of the diagnosis was made, showing two known pathological variants in the CYP27A1 gene: c.255+1G>T and c.1263+1G>T (Figure 5). The mutation c.1263+1G>T has been reported previously, but the pathogenicity of c.255+1G>T has rarely been reported. There were clinical features of the c.255+1G>T mutation of CTX as reported in the literature (Table 1).
| Patients | Patient 1[11] | Patient 2[11] | Patient 3[11] | Patient 4[11] | Patient 5[12] | Patient 6 |
| Country | South Africa | South Africa | South Africa | South Africa | China | China |
| CYP27A1 mutations | c.2T>C, c.255+1G>A | c.2T>C, c.255+1G>A | c.2T>C, c.255+1G>A | c.2T>C, c.255+1G>A | c.1263+1G>A, c.255+1G>T | c.1263+1G>A, c.255+1G>T |
| Sex | M | F | F | M | F | F |
| Age at diagnosis in yr | 50 | 47 | 47 | 46 | 34 | 33 |
| Diarrhea | - | - | - | - | - | - |
| Tendon xanthomata | + | + | + | + | + | + |
| Cataracts | - | - | - | - | + | + |
| Neurological symptoms | - | - | - | - | Ataxia, dysarthria, pyramidal signs/spasticity, cognitive impairment | Ataxia, dysarthria, pyramidal signs/spasticity |
| Psychiatric symptoms | - | Depression + | - | - | + | - |
| Brain MRI | - | Cerebellar atrophy, involvement of basal ganglia, dentate nuclei | NP | NP | Cerebellar atrophy, involvement of basal ganglia, dentate nuclei | Cerebellar atrophy, involvement of basal ganglia, dentate nuclei |
CTX.
At the age of 33, she started the CDCA regimen.
She had been treated with CDCA (750 mg/d) orally for ≥ 2 years but still presented with spastic paraparesis, progressive gait ataxia, mild dysarthria, clonic knee reflexes, and tendon xanthomata.
CTX is a rare disease of lipid deposition that characteristically deposits lipids in the tendons, resulting in tendinous xanthomatosis. Typical clinical manifestations of CTX include diarrhea, bilateral cataracts in childhood, progressive neurologic dysfunction, tendon xanthomata, and atherosclerosis in adolescence and early adulthood. There are also some cases where the spinal form has a milder clinical manife
The CTX median age at diagnosis is 24.5-years-old[7]. The onset of symptoms for the present patient began at 9-years-old with cataracts, and by the age of 27, she reported symptoms of nervous system damage such as ataxia, with her symptoms progressively worsening. She repeatedly visited doctors but did not receive a clear diagnosis. On May 28, 2019, she was admitted to our department with ataxia and finally diagnosed with CTX at the age of 33 with serious neurological involvement. She had been treated with CDCA (750 mg/d) orally for ≥ 2 year but still presented with spastic paraparesis, progressive gait ataxia, mild dysarthria, clonic knee reflexes, and tendon xanthomata. While CTX can be treated, it is very easy to misdiagnose. Once there is severe nervous system damage, it is likely to leave serious disabilities, affecting the patient’s quality of life.
The CYP27A1 gene contains nine exons and eight introns. Of the 108 variants of CYP27A1 that have been reported, over 50 are considered pathogenic or likely pathogenic according to the Human Gene Mutation Database. Several studies have reported CYP27A1 mutations including nonsense (22%), splice site (20%), and small deletion and insertion (18%) mutations[8]. According to a recent nationwide survey on CTX in Japan, the most frequent mutations in the CYP27A1 gene were c.1214G>A, c.1421G>A, and c.435G>T[9]. In the Chinese population, we found that the most frequent mutations were c.410G>A, c.379C>T, and c.1435C>T. The pathogenic mutations (c.389 T>A and c.571C>T, c.379C>T, c.435G>T, c.1016C>T, c.1214G>A, c.1263+1G>A, c.1420C>T, and c.1435C>T) were also identified[10]. The genotype of the patient included c.435+1G>T and c.1263+1G>T as well as the rare mutation site c.255+1G>T.
Mutation c.1263+1G>T has been reported previously, but the pathogenicity of c.255+1G>T has rarely been reported. Stelten et al[11] reported 4 cases of CTX in a South African family that showed multiple xanthomata on the Achilles tendons. Despite this, their neurological examinations were all normal, their brain MR spectroscopies were unremarkable, and even their ophthalmology evaluations showed no signs of cataracts. However, the diagnosis of CTX was confirmed through genetic analysis, identifying the mutations c.2T>C and c.255+1G>A[11,12]. The latter mutation is shared with the current case, suggesting that the same genotype but different clinical manifestations may be caused by different races.
Our paper reports a case of delayed CTX diagnosis resulting in severe neurological impairment. The CTX diagnosis was confirmed by detection of CYP27A1 gene mutations, which comprised one rare mutation (c.255+1G>T) and one known mutation (c.1263+1G>A). CTX requires a multidisciplinary diagnosis and must be made early to avoid progressive neurological impairment.
We would like to thank the patient for her cooperation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Jordan; Pitton Rissardo J, Brazil; S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991;266:7779-7783. [RCA] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 318] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Duell PB, Salen G, Eichler FS, DeBarber AE, Connor SL, Casaday L, Jayadev S, Kisanuki Y, Lekprasert P, Malloy MJ, Ramdhani RA, Ziajka PE, Quinn JF, Su KG, Geller AS, Diffenderfer MR, Schaefer EJ. Diagnosis, treatment, and clinical outcomes in 43 cases with cerebrotendinous xanthomatosis. J Clin Lipidol. 2018;12:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Islam M, Hoggard N, Hadjivassiliou M. Cerebrotendinous Xanthomatosis: diversity of presentation and refining treatment with chenodeoxycholic acid. Cerebellum Ataxias. 2021;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Gelzo M, Di Taranto MD, Bisecco A, D'Amico A, Capuano R, Giacobbe C, Caputo M, Cirillo M, Tedeschi G, Fortunato G, Corso G. A case of Cerebrotendinous Xanthomatosis with spinal cord involvement and without tendon xanthomas: identification of a new mutation of the CYP27A1 gene. Acta Neurol Belg. 2021;121:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Chen C, Zhang Y, Wu H, Sun YM, Cai YH, Wu JJ, Wang J, Gong LY, Ding ZT. Clinical and molecular genetic features of cerebrotendinous xanthomatosis patients in Chinese families. Metab Brain Dis. 2017;32:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Abdel-Hamid MS, Issa MY, Otaify GA, Zaki MS. A novel frameshift mutation in the sterol 27-hydroxylase gene in an Egyptian family with cerebrotendinous xanthomatosis without cataract. Metab Brain Dis. 2017;32:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Degos B, Nadjar Y, Amador Mdel M, Lamari F, Sedel F, Roze E, Couvert P, Mochel F. Natural history of cerebrotendinous xanthomatosis: a paediatric disease diagnosed in adulthood. Orphanet J Rare Dis. 2016;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Di Taranto MD, Gelzo M, Giacobbe C, Gentile M, Marotta G, Savastano S, Dello Russo A, Fortunato G, Corso G. Cerebrotendinous xanthomatosis, a metabolic disease with different neurological signs: two case reports. Metab Brain Dis. 2016;31:1185-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Sekijima Y, Koyama S, Yoshinaga T, Koinuma M, Inaba Y. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet. 2018;63:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Tao QQ, Zhang Y, Lin HX, Dong HL, Ni W, Wu ZY. Clinical and genetic characteristics of Chinese patients with cerebrotendinous xanthomatosis. Orphanet J Rare Dis. 2019;14:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Stelten BML, Raal FJ, Marais AD, Riksen NP, Roeters van Lennep JE, Duell PB, van der Graaf M, Kluijtmans LAJ, Wevers RA, Verrips A. Cerebrotendinous xanthomatosis without neurological involvement. J Intern Med. 2021;290:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Jiang J, Chen G, Wu J, Luan X, Zhou H, Liu X, Zhu Z, Song X, Wang S, Qian X, Du J, Huang X, Zhang M, Xu W, Cao L. c.1263+1G>A Is a Latent Hotspot for CYP27A1 Mutations in Chinese Patients With Cerebrotendinous Xanthomatosis. Front Genet. 2020;11:682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |