Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10670
Peer-review started: April 27, 2022
First decision: June 27, 2022
Revised: July 11, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: October 16, 2022
Processing time: 155 Days and 0.5 Hours
Infective endocarditis (IE) is a rare disease with a high mortality rate. Leclercia adecarboxylata (L. adecarboxylata) is a movable Gram-negative bacillus of enterobacteriaceae, and it can rarely be a pathogen which often affects immunodeficient patients. There are about three cases of immunocompetent patients with monomi
A 51-year-old man was found to have moderate to severe mitral stenosis on echocardiography. He came to our Cardiothoracic Surgery Department for surgical management. A diastolic murmur was heard on auscultation of the heart in the mitral region. On the second day of hospitalisation, he presented with slurred speech, reduced muscle strength in the left limb, and acute cerebral infarction on cranial computed tomography. Surgical treatment was decided to postpone. On the ninth day of admission, the patient developed a sudden high fever and shock and was transferred to the Cardiac Intensive Care Unit, where echocardiogram revealed an anterior mitral valve leaflet vegetation. After empirical anti-infective treatment with vancomycin (1g q12h), an emergency valve replacement was performed. Bacterial culture identified L. adecarboxylata. Anti-infective treatment with piperacillin-tazobactam (4.5g q8h) was added for 4 wk. Follow-up echocardiography showed normal bioprosthetic valve function after mitral valve replacement.
We report the first case of L. adecarboxylata IE in China, and clinicians should pay attention to this pathogen.
Core Tip: Leclercia adecarboxylata (L. adecarboxylata) is a ubiquitous microorganism often found in water and soil and a rare pathogen can be isolated from specimens such as blood. We present a rare case of infective endocarditis caused by L. adecarboxylata that occurred in an immunocompetent male patient. He presented with clinical symptoms after diarrhoea. L. adecarboxylata was identified to be the only pathogen and the intestine may be the portal of entry for the infection. Immunosuppression is a significant risk factor for L. adecarboxylata infection. L. adecarboxylata is usually the only pathogen cultured in immunosuppressed individuals. Bacteria crossing the intestinal mucosal barrier may cause bacteremia.
- Citation: Tan R, Yu JQ, Wang J, Zheng RQ. Leclercia adecarboxylata infective endocarditis in a man with mitral stenosis: A case report and review of the literature. World J Clin Cases 2022; 10(29): 10670-10680
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10670.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10670
Leclercia adecarboxylata (L. adecarboxylata) is a movable Gram-negative rod-shaped bacterium in the Enterobacteriaceae family, most commonly found in water and soil[1,2] and rarely isolated from clinical specimens, with most of the Enterobacteriaceae characteristics, such as partly anaerobic, oxidase-negative, mesophilic, peripheral flagellate bacilli[3]. Infective endocarditis (IE) caused by L. adecarboxylata is a very rare disease in the clinic. Only three cases of L. adecarboxylata endocarditis have been reported in the existing literature[4-6]. Herein we report a case of L. adecarboxylata endocarditis complicated with severe mitral stenosis, atrial fibrillation, and acute cerebral infarction. L. adecarboxylata strains were sensitive to antibiotics other than amoxicillin, cephalosporin, and compound sulfamethoxazole. After surgery and 4-wk antibiotic therapy, the patient recovered without sequelae.
A 51-year-old male patient presented to the Cardiovascular Surgery Department with a complaint of moderate to severe mitral stenosis combined with mild to moderate regurgitation on echocardiography for 1 mo.
The patient presented to a local hospital 1 mo ago with chest tightness and was diagnosed with “atrial fibrillation”. Echocardiography showed combined valvular disease including moderate to severe mitral stenosis combined with mild to moderate regurgitation, mild aortic valve insufficiency, mild tricuspid valve insufficiency, decreased left ventricular function, and a small amount of pericardial effusion. The patient was admitted to our hospital for mitral valve replacement (MVR).
The patient had a history of atrial fibrillation. Prior to admission to our hospital, he had a short bout of diarrhoea lasting approximately 3 d, which improved with the use of "anti-diarrhoea medication" (not specified).
The patient had no personal and family history.
The patient had a temperature of 36.5°C, heart rate of 91 beats per minute (bpm), respiratory rate of 15 cycles per minute (cpm), and blood pressure of 131/79 mmHg on admission. Cardiac auscultation revealed a severe diastolic murmur in the mitral region and a systolic murmur in the tricuspid valve. Cardiac percussion showed enlargement of the cardiac border to both sides.
Blood analysis, blood biochemistries, prothrombin and partial thromboplastin times, D-dimers, as well as arterial blood gas were normal.
The echocardiogram of the patient at our hospital showed severe mitral stenosis with mild mitral regurgitation, mild and moderate tricuspid regurgitation, mild aortic regurgitation, and mild pulmonary hypertension. A chest computed tomography (CT) scan showed an enlarged cardiac shadow and a small amount of pericardial effusion. The electrocardiogram showed atrial fibrillation.
On the second day of admission, the patient was found to have slurred speech, a shallow left nasolabial fold, and grade 4 muscle strength in the left limb.
Cranial CT showed acute cerebral infarction in the right frontotemporal occipital lobe with a small amount of post-infarct blood leakage. Considering the patient's sudden acute cerebral infarction complicated by cerebral haemorrhage, we discussed and decided to postpone the surgical treatment.
On the 9th day of hospitalization, the patient developed a sudden high fever with a temperature of 38.6°C. The examination showed a heart rate of 170 bpm, blood pressure of 90/60 mmHg, and respiratory rate of 25 cpm. The patient was transferred to the Cardiac Intensive Care Unit (CICU) for further treatment.
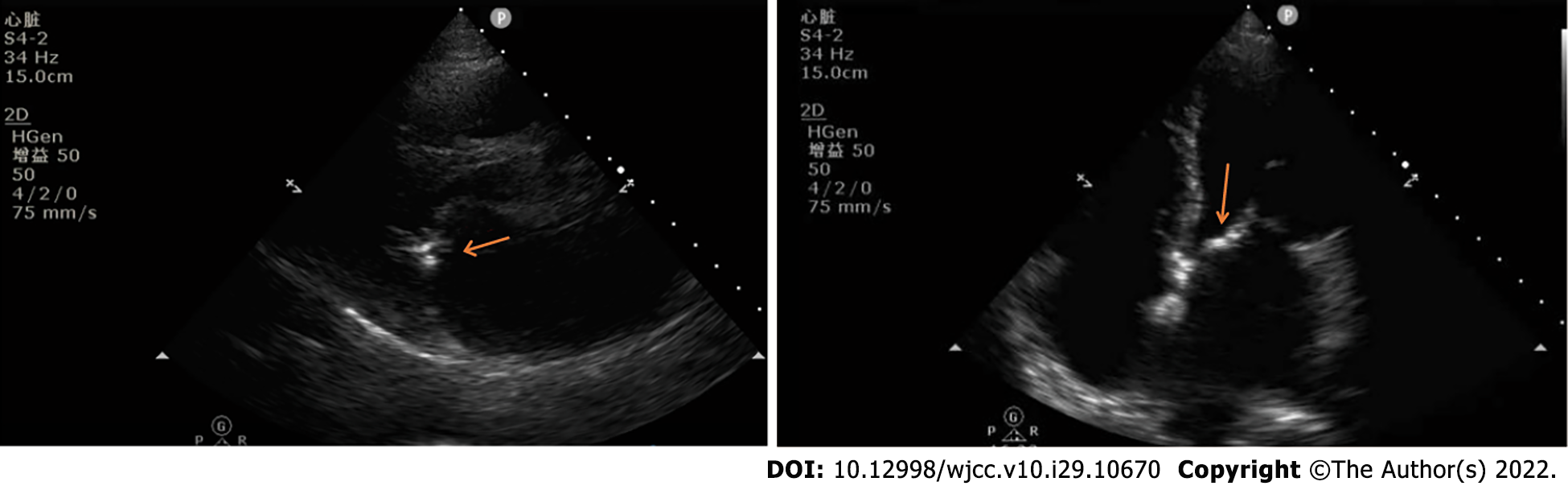
After admission to the CICU, blood count analysis showed a white blood cell count of 7010/mm3 (85.6% neutrophils), and blood gas analysis showed a lactate level of 2.8 mmol/L and a calcitonin level of 0.98 Pg/L. Bedside echocardiogram showed a vegetation attaching to the anterior leaflet of mitral valve (Figure 1).
The initial diagnosis was severe mitral stenosis with mild regurgitation, moderate tricuspid regurgitation, mild aortic regurgitation, mild pulmonary hypertension, pericardial effusion, atrial fibrillation, acute cerebral infarction, acute cerebral haemorrhage, shock, and acute IE.
Appropriate microbiological analysis was performed to determine the etiology and susceptibility to antibiotics in this patient with acute IE. Gram staining, antacid bacillus staining, and culture for aerobic and anaerobic bacteria were performed on blood and intraoperatively resected valve vegetation. Samples were first enriched in thioglycolic acid broth, incubated overnight, and then inoculated on blood agar plates, McConkey agar plates, Sabouraud agar plates, anaerobic growth media, and Lö wenstein-Jensen medium. Blood cultures and superfluous cultures were suggestive of positive Gram-negative bacilli and L. adecarboxylata infection. The organism was sensitive to a variety of antibiotics, including ampicillin, amikacin, aztreonam, ciprofloxacin, cefazolin, cefepime, cefotaxime, gentamicin, imipenem, meropenem, and piperacillin.
The final diagnosis was severe mitral stenosis with mild regurgitation, moderate tricuspid regurgitation, mild aortic regurgitation, mild pulmonary hypertension, pericardial effusion, atrial fibrillation, acute cerebral infarction, acute cerebral haemorrhage, septic shock, and acute IE due to L. adecarboxylata. The patient's IE diagnosis was based on the two main criteria (echocardiography finding vegetations in the anterior lobe of the mitral valve, and positive cultures of blood and vegetation) and three minor criteria (history of mitral stenosis, fever of 38.6 °C, and embolic infarction) of modified Duke criteria.
Immediately after admission to the CICU, fluid replacement, volume expansion, and norepinephrine (18 μg/min) was given to maintain blood pressure and correct shock, and vancomycin (1 g q12h) was administered empirically after blood cultures were retained for anti-infective treatment. Considering that the patient had acute IE with shock, which is an indication for emergency surgery, emergency surgery was performed. The patient underwent cardiac lesion resection, mitral bioprosthetic valve replacement, tricuspid valvuloplasty, radiofrequency ablation of atrial fibrillation, and left atrial auricular resection under general anaesthesia. Postoperatively, levosimendan (1 mg/h) was given to enhance cardiac function, norepinephrine (24 μg/min) to maintain blood pressure and circulatory stability, vancomycin (1 g q12h) for experienced anti-infection, and warfarin (2.5 mg qd) for anticoagulation. After 24 h of these treatments, the patient's circulation and respiration were relatively stable, but he was still febrile. Levosimendan and norepinephrine were discontinued. Two tubes of blood were obtained for pre-operative blood cultures, suggesting L. adecarboxylata infection, and anti-infective therapy with piperacillin tazobactam (4.5 g q8h) in combination with vancomycin (1 g q12h) was administered on the second day after surgery. The patient's fever resolved and the neutrophil percentage decreased to normal within 48 h of the combined anti-infective treatment. Blood cultures were performed on the first, second, third, fifth, and seventh postoperative days, respectively (2 tubes of blood per day). Blood cultures were positive on the first 3 d after surgery, and negative on the fifth and seventh postoperative days. On the 17th day of hospitalization, the patient returned to the general ward of the Cardiacvascular Surgery Department and continued anti-infective treatment with vancomycin (1g q12h) and piperacillin tazobactam (4.5 g q8h) for a total of 4 wk of the dosing cycle.
The patient's vital signs were stable and blood cultures were negative after surgery and anti-infective therapy. At the follow-up visit 1 mo after surgery, the patient was asymptomatic, and a new echocardiography showed that the patient had normal biologic valve function after MVR, and no abnormal echogenicity was detected around the valve.
The first case of aortic valve disease combined with endocarditis was reported by Lazare Riviére[7] in 1646, and in 1806, Jean-Nicolas Corvisart[8] introduced the term 'vegetation'. Jean Baptiste Bouillaud[9] called the inner layer of the heart the “endocardium", and the term” endocarditis" came into use in 1835[10]. IE is a rare disease with a high mortality rate, ranging from 1.5 to 11.6 per 100000 people worldwide each year. However, despite significant advances in antibiotic therapy and surgery, the in-hospital mortality rate for IE still exceeds 20%, with 1-year mortality rates still as high as 30%[11] and 5-year mortality rates as high as 45%[12]. In the real clinic, these figures may be underestimated[13].
Although the incidence varies little numerically between countries, there are significant differences in patterns. Historically, rheumatic heart disease and dental surgery were considered to be the major contributing factors to IE. In recent years, the incidence of rheumatic heart disease has declined in high-income countries, and the incidence of heart disease and cardiac surgery has increased as the average human age increases[14], with invasive procedures, prosthetic valves, and cardiac implant devices emerging as risk factors for IE. Long-term intravenous drug use (IDU-IE) is also an increasingly common cause of IE. Degenerative valve disease and congenital heart disease are also risk factors for IE[15,16]. Currently, the most common susceptibility disease in IE is valvular heart disease[17], and with an ageing population, there is an increasing incidence of degenerative valve disease such as mitral and aortic regurgitation[18]. Of these, mitral valve prolapse is the predominant structural abnormality in native valve endocarditis. Nosocomial infections are also an important factor in the disease, with hospital-acquired staphylococci being the main source of infection in high-income countries, mainly affecting the elderly[11].
As a typical lesion of IE, vegetation is composed of a large number of platelets, fibrin, microorganisms, and a small number of inflammatory cells. The pathogenesis of IE usually begins with damage to the endothelium[19]. Endothelial damage triggers thrombosis caused by fibrin and platelet deposits[20]. When bacteraemia occurs, bacteria can reach and colonise these damaged areas. After colonisation, the surface is rapidly covered with another layer of platelets and fibrin, suitable for further colonisation, leading to progressive bacterial infection. In general, the endothelial layer and valves of the heart are resistant to infection. However, very deadly microorganisms such as Staphylococcus aureus are able to infect normal heart valves[21].
IE includes both natural valve endocarditis and IE caused by intracardiac prosthetic materials. The latter includes prosthetic valve endocarditis[22] and IE associated with pacemakers, implantable cardiac defibrillators, and ventricular assist devices. Early and accurate diagnosis of IE is essential, as delayed treatment can affect the prognosis of the disease[23,24]. The diagnosis of IE is widely made using microbiological techniques and imaging techniques (echocardiography, CT, and nuclear imaging). Histological findings from postoperative excised tissue can also be used for diagnosis. The clinical diagnosis of IE is based on the modified Duke criteria, which are included in the guidelines for IE[22]. The 2000 modified Duke criteria classify the diagnosis into 'major' and 'minor' criteria and classify cases as "definite", "possible", or "rejected"[25]. A positive blood culture is one of the main diagnostic criteria. When atypical infection is clinically suspected, routine serological screening should be performed. If all serology is negative, the sample should be checked for antinuclear and antiphospholipid antibodies. The cornerstone of the modified Duke criteria is echocardiography.
In any case of suspected IE, echocardiography is the imaging technique of choice[26]. Echocardiography is the most important tool for the detection of endocardial lesions as it can reveal vegetations, abscesses, perforations, fistulas, valvular aneurysms, and pseudoaneurysms.
The clinical features of IE are highly variable and depend largely on the infecting organism and the medical history of heart disease or not[27,28]. The clinical presentation may be acute, subacute, or chronic[29]. Approximately 90% of infected patients present with fever, and IE is also an important cause of unexplained fever[30-32]. It is accompanied by various systemic symptoms such as appetite loss and weight loss[33]. About 85% of patients have heart murmurs[34]. Clinical features also include neurological signs and symptoms[35]. IE is a risk factor for stroke[36]. Intravenous drug use, HIV, chronic liver disease, elevated CRP, microorganisms such as Staphylococcus aureus, the presence of vegetations on echocardiography, multiple movable mitral valve vegetations, vegetation > 10 mm in size, and prosthetic materials are risk factors for a clinically significant increased risk of embolism[37-40].
Antibiotics are the basis of treatment for IE and appropriate antibiotic therapy is important to control local and systemic infection, eradicate microorganisms from the vegetation, and reduce the risk of complications such as septic embolism[41]. There is no standardisation in the use of antibiotics[42]. Most infections are caused by streptococci, staphylococci, or enterococci, and the microbial profile of pathogenic microorganisms has changed over time. Staphylococcus species now account for an increasing proportion of IE cases[43]. The most common of these pathogens is Staphylococcus aureus[43]. Successful treatment of IE relies on the eradication of microorganisms with antimicrobial drugs. Many antimicrobial drugs are used alone or in combination to treat IE, including beta-lactams (β-lactams), aminoglycosides, glycopeptides, oxazolidinones, complex macrocyclic compounds, and quinolones[44]. Antibiotics are administered immediately after blood cultures are performed. The choice of antibiotics should be specific to the organism isolated from the blood culture and will depend primarily on the antibiotic sensitivity of the pathogen and whether the valve is natural or prosthetic[29].
Medical treatment of IE has a mortality rate of 60%-90% and a high proportion of patients with active IE are at high risk of death without cardiovascular surgery[45]. Effective surgical treatment can reduce mortality by 8%-16%[46,47]. Heart failure, persistent or uncontrolled infection, IE caused by fungal or highly drug-resistant strains, heart block, and circumferential or aortic root abscesses caused by IE are indications for Class I surgery[26]. Of these, heart failure is the most common indication for surgery in patients with IE, accounting for 60% of patients undergoing surgery, followed by uncontrolled infection (48%) and embolic episodes (18%)[48]. Surgery is usually recommended for this group of patients early in the disease, during hospitalization, and before the end of antibiotic therapy[41]. Patients with refractory pulmonary oedema or cardiogenic shock due to IE should undergo emergency surgery within 24 h[26].
In IE, the mitral valve is often affected[49]. The prognosis for IE with mitral valve lesions is often poor, and extensive excision of the infected focus and MVR are considered to be the better surgical approach. However, the graft can easily become another source of infection, and mitral valve repair (MVP) can overcome this disadvantage. A meta-analysis has shown that the in-hospital mortality rate of MVP is lower than that of MVR in the surgical treatment of active native mitral IE. The efficacy and follow-up of MVP are comparable to those of MVR, with a lower mortality rate at 5 years after surgery, suggesting a lower risk of reoperation or recurrence of infection after long-term follow-up MVP[50]. A systematic review found that in cases of mitral valve surgery, valve repair resulted in lower in-hospital and long-term mortality, lower recurrence rates, fewer repeat surgeries after mitral valve surgery, and fewer cerebrovascular events compared with valve replacement[51].
Gram-positive bacteria account for more than 70% of IE cases, and are the main pathogen of community-acquired and hospital-acquired IE cases[52]. Gram-negative bacteria usually do not cause IE[53]. Gram-negative IE accounts for less than 5% of IE cases[54]. In the past 50 years, the incidence of Gram-negative endocarditis has been increasing[55,56]. In addition to the traditional risk factors of structural heart disease (congenital or acquired)[57,58], most cases of Gram-negative endocarditis are mainly caused by medical related events[59]. Patients with indwelling catheters, invasive lines, and intracardiac devices are at higher risk[60,61]. Among patients with Gram-negative endocarditis, 60% had renal insufficiency[17], and about 52% had embolic symptoms[60]. Embolic symptoms can be divided into the presence or absence of central nervous system involvement, including neurological complications such as stroke or transient ischemic attack, and other complications such as metastatic infection, mesenteric ischemia, and peripheral terminal artery occlusion[41,62]. A large prospective study found that the incidence of new murmurs was 48%-60%, and 30%-40% of patients lost weight[17,59]. Studies have reported that about 11%-33% of patients have splenomegaly[59,63].
L. adecarboxylata was first described by H. Leclerc[64] in 1962 and was previously known as "Enteric group 410 or Escherichia adecarboxylata". The name L. adecarboxylata was officially given by Tamura et al[65,66] in 1987. The true frequency of infections caused by L. adecarboxylata has been underestimated and underreported for decades. The underestimation of L. adecarboxylata infections is mainly due to the fact that several strains of the bacterium are often misidentified as Escherichia coli, as the two species share a number of morphological and metabolic characteristics[67].
L. adecarboxylata is a ubiquitous microorganism, most commonly found in water and soil[1,2]. Cases of L. adecarboxylata infection in patients with corneal abscesses combined with keratitis have been reported with a history of exposure to the aquatic environment[68]. L. adecarboxylata also exists in the intestinal symbiotic flora of some animals[69], which is an active Gram-negative rod-shaped bacterium in the Enterobacteriaceae family, with most of the Enterobacteriaceae characteristics, such as partly anaerobic, oxidase-negative, mesophilic, peripheral flagellate bacilli[3].
L. adecarboxylata is a rare emerging pathogen and immunosuppression is the most significant risk factor for infection with L. adecarboxylata, which often affects immunodeficient patients, including those with immunomodulators and haematological malignancies or receiving chemotherapy[70], and is the only isolate cultured from blood, sputum, urine, and peritoneal fluid in immunosuppressed individuals[71-73]. There are currently about three reported cases of immunocompetent patients with monomicrobial L. adecarboxylata infection[74-76]. The remaining cases are all immunosuppressed patients[70]. L. adecarboxylata is usually isolated as part of a polymicrobial infection in immunocompetent patients[66,67,70]. Because of its low virulence, L. adecarboxylata is often overlooked in the context of polymicrobial infections, and asymptomatic carriage or colonization is common. Among the polymicrobial infections, the most common co-infecting pathogen of L. adecarboxylata is Enterococcus. The others are Acinetobacter, Fusarium, Staphylococcus epidermidis, Klebsiella, and Pseudomonas aeruginosa[77,78].
L. adecarboxylata has been isolated mainly from food, water, and environmental sources or from animal specimens. Knowledge of the route of transmission of L. adecarboxylata is limited, and the possible sources of infection are unknown. In the available case reports, L. adecarboxylata can be isolated from specimens including blood, wound pus, faeces, urine, gallbladder, peri-ciliary and ciliary abscesses, synovial fluid, peritoneal fluid from peritoneal dialysis, sputum, cerebrospinal fluid, and catheters[79,80]. A few cases with diabetes as the only risk factor have L. adecarboxylata infection of the skin and soft tissues[81,82]. L. adecarboxylata has been implicated in endocarditis[6], catheter-associated bacteremia[78], bacteremia, sepsis[3,67], septic arthritis, meningitis[83], cellulitis[2,84], urinary tract infections[85], pneumonia[70], and bacterial peritonitis, especially in patients on peritoneal dialysis[86]. Of these, the most common clinically are catheter-associated male urinary tract infections (translocation through the genitourinary tract), followed by ventilator-associated pneumonia, peritoneal dialysis peritonitis, corneal abscesses, vascular graft infections (entry into the infected host via catheter or wound)[87], and intestinal translocation (translocation of bacteria through the mucosal barrier of the gastrointestinal tract, presumably gastrointestinal bacteremia)[2,70].
Bacteremia may be due to bacterial translocation across the intestinal mucosal barrier as a result of megacolon, antibiotic use, and altered dietary habits. To assess the relationship between gastrointestinal pathology and L. adecarboxylata bacteraemia, four cases of L. adecarboxylata associated with gastrointestinal pathology were reviewed. Three of these reported cases presented with only intestinal mucosal disturbances with L. adecarboxylata as a possible source of infection[74]. These patients did not undergo any invasive intervention prior to isolation of the bacteria from their blood. In the fourth reported case, the bacterial translocation could have been the result of mucosal alterations due to invasive interventions to the gastrointestinal tract[88]. It can therefore be hypothesised that the gastrointestinal pathology causing the alteration of the mucosal barrier leads to a higher risk of this L. adecarboxylata bacteraemia[76]. Our case presented with clinical signs associated with IE following diarrhoea, and we hypothesize that the intestine may have been the entry point for this L. adecarboxylata infection. Our patient had severe mitral stenosis and other underlying organic heart disease, with sudden acute cerebral infarction, high fever, high white blood cell count, and shock during the course of the disease. Echocardiography showed formation of a vegetation in the anterior mitral leaflet, and cultures of blood and vegetation showed L. adecarboxylata infection. Of the reported cases of endocarditis in the world, only three were caused by L. adecarboxylata[4-6]. To our knowledge, this is the first case of L. adecarboxylata endocarditis in China. In our case, the diagnosis was based on one main criterion for IE (echocardiographic finding of a vegetation) and three minor criteria (positive cultures of blood and vegetation, fever of 38.6°C and embolic infarction)[25]. After mitral valve replacement and 4-wk piperacillin-tazobactam anti-infection treatment, the patient's cardiac function returned to normal. The optimal regimen and duration of antibiotic therapy for L. adecarboxylata endocarditis remain imperfect due to the low number of case reports. As an emerging pathogen, further research into the pathogenesis and risk factors of L. adecarboxylata is needed.
There are few cases of L. adecarboxylata isolated from environmental and clinical specimens, and few cases of L. adecarboxylata infection have been reported in the literature. In previous cases, it was most commonly isolated as part of a mixture of microorganisms that could be the only pathogen cultured in immunosuppressed patients. In our case, L. adecarboxylata was the only pathogen isolated from bacterial cultures in an immunocompetent patient, and there are only three existing case reports of monomicrobial L. adecarboxylata infection in immunocompetent patients[74]. Of the case reports of endocarditis found worldwide, only three cases of endocarditis were caused by L. adecarboxylata[4-6]. We report the first case of IE caused by L. adecarboxylata in China. In this case, symptoms of IE developed after diarrhoea, and we speculate that the intestine may be the portal of entry for L. adecarboxylata infection. Clinicians should be aware of the gastrointestinal translocation of this pathogen and the possibility of causing IE.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Emergency medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra AK, United States; Park J, South Korea S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | Osaili TM, Alaboudi AR, Al-Quran HN, Al-Nabulsi AA. Decontamination and survival of Enterobacteriaceae on shredded iceberg lettuce during storage. Food Microbiol. 2018;73:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Shaikhain T, Al-Husayni F, Al-Fawaz S, Alghamdi EM, Al-Amri A, Alfares M. Leclercia adecarboxylata Bacteremia without a Focus in a Non-Immunosuppressed Patient. Am J Case Rep. 2021;22:e929537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Nelson MU, Maksimova Y, Schulz V, Bizzarro MJ, Gallagher PG. Late-onset Leclercia adecarboxylata sepsis in a premature neonate. J Perinatol. 2013;33:740-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Dudkiewicz B, Szewczyk E. [Etiology of bacterial endocarditis in materials from Cardiology and Cardiac Surgery Clinics of the Lodz Academy]. Med Dosw Mikrobiol. 1993;45:357-359. [PubMed] |
| 5. | Lee B, Sir JJ, Park SW, Kwak CH, Kim SM, Kim SB, Kwak YG, Whang DH, Cho WH, Choi SK. A case of Leclercia adecarboxylata endocarditis in a woman with endometrial cancer. Am J Med Sci. 2009;337:146-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Malik K, Davie R, Withers A, Faisal M, Lawal F. A case of Leclercia adecarboxylata endocarditis in a 62-year-old man. IDCases. 2021;24:e01091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Rivière L. Lazari Riverii, Opera medica universa, quibus continentur. Montpellier, France: Cellier, 1663.. |
| 8. | Corvisart JN. Essai sur les Maladies et les Lasions Organiques du Cceur et des gros Vaisseaux. Paris: Mequigon Marvis, 1806. |
| 9. | Bouillaud JB. Traité Clinique Des Maladies Du Coeur J. Paris: Librairie J.B. Baillière, 1841.. |
| 10. | Geller SA. Infective endocarditis: a history of the development of its understanding. Autops Case Rep. 2013;3:5-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 652] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 12. | Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B; AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 430] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 13. | Bin Abdulhak AA, Tleyjeh IM. Indications of Surgery in Infective Endocarditis. Curr Infect Dis Rep. 2017;19:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Kucukates E, Gultekin N, Bagdatli Y. Cases of active infective endocarditis in a university hospital during a 10-year period. J Pak Med Assoc. 2013;63:1163-1167. [PubMed] |
| 15. | Chambers J, Sandoe J, Ray S, Prendergast B, Taggart D, Westaby S, Arden C, Grothier L, Wilson J, Campbell B, Gohlke-Bärwolf C, Mestres CA, Rosenhek R, Pibarot P, Otto C. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100:524-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Slipczuk L, Codolosa JN, Davila CD, Romero-Corral A, Yun J, Pressman GS, Figueredo VM. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One. 2013;8:e82665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 17. | Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1628] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 18. | Theologou T, Harky A, Shaw M, Harrington D, Kuduvalli M, Oo A, Field M. Mitroflow and Perimount Magna 10 years outcomes a direct propensity match analysis to assess reintervention rates and long follow-up mortality. J Card Surg. 2019;34:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Thiene G, Basso C. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovasc Pathol. 2006;15:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Shannon O, Mörgelin M, Rasmussen M. Platelet activation and biofilm formation by Aerococcus urinae, an endocarditis-causing pathogen. Infect Immun. 2010;78:4268-4275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Melehani JH, Duncan JA. Inflammasome Activation Can Mediate Tissue-Specific Pathogenesis or Protection in Staphylococcus aureus Infection. Curr Top Microbiol Immunol. 2016;397:257-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherapy. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2012;67:269-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 23. | Tanis W, Scholtens A, Habets J, van den Brink RB, van Herwerden LA, Chamuleau SA, Budde RP. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2014;63:186-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med. 2011;52:1673-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2699] [Cited by in RCA: 2809] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 26. | Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2661] [Cited by in RCA: 3323] [Article Influence: 332.3] [Reference Citation Analysis (0)] |
| 27. | Song JK. Infective endocarditis involving an apparently structurally normal valve: new epidemiological trend? Korean J Intern Med. 2015;30:434-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Sun BJ, Choi SW, Park KH, Jang JY, Kim DH, Song JM, Kang DH, Kim YS, Song JK. Infective endocarditis involving apparently structurally normal valves in patients without previously recognized predisposing heart disease. J Am Coll Cardiol. 2015;65:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, Popescu BA, Prendergast B, Tornos P, Sadeghpour A, Oliver L, Vaskelyte JJ, Sow R, Axler O, Maggioni AP, Lancellotti P; EURO-ENDO Investigators. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40:3222-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 475] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 30. | Sipahi OR, Senol S, Arsu G, Pullukcu H, Tasbakan M, Yamazhan T, Arda B, Ulusoy S. Pooled analysis of 857 published adult fever of unknown origin cases in Turkey between 1990-2006. Med Sci Monit. 2007;13:CR318-CR322. [PubMed] |
| 31. | Zhai YZ, Chen X, Liu X, Zhang ZQ, Xiao HJ, Liu G. Clinical analysis of 215 consecutive cases with fever of unknown origin: A cohort study. Medicine (Baltimore). 2018;97:e10986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Vahabi A, Gül F, Garakhanova S, Sipahi H, Sipahi OR. Pooled analysis of 1270 infective endocarditis cases in Turkey. J Infect Dev Ctries. 2019;13:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Ba DM, Mboup MC, Zeba N, Dia K, Fall AN, Fall F, Fall PD, Gning SB. Infective endocarditis in Principal Hospital of Dakar: a retrospective study of 42 cases over 10 years. Pan Afr Med J. 2017;26:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Damasco PV, Correal JCD, Cruz-Campos ACD, Wajsbrot BR, Cunha RGD, Fonseca AGD, Castier MB, Fortes CQ, Jazbick JC, Lemos ERS, Rossen JW, Leão RS, Hirata Junior R, Guaraldi ALM. Epidemiological and clinical profile of infective endocarditis at a Brazilian tertiary care center: an eight-year prospective study. Rev Soc Bras Med Trop. 2019;52:e2018375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Sotero FD, Rosário M, Fonseca AC, Ferro JM. Neurological Complications of Infective Endocarditis. Curr Neurol Neurosci Rep. 2019;19:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Cantier M, Sabben C, Adle-Biassette H, Louedec L, Delbosc S, Desilles JP, Journé C, Diallo D, Ou P, Klein I, Chau F, Lefort A, Iung B, Duval X, Olivot JM, Ho-Tin-Noe B, Michel JB, Sonneville R, Mazighi M. Neurologic Complications of Infective Endocarditis: A Joint Model for a Septic Thromboembolism and Inflammatory Small Vessel Disease. Crit Care Med. 2019;47:e685-e692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 791] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 38. | Durante Mangoni E, Adinolfi LE, Tripodi MF, Andreana A, Gambardella M, Ragone E, Precone DF, Utili R, Ruggiero G. Risk factors for "major" embolic events in hospitalized patients with infective endocarditis. Am Heart J. 2003;146:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Leitman M, Dreznik Y, Tyomkin V, Fuchs T, Krakover R, Vered Z. Vegetation size in patients with infective endocarditis. Eur Heart J Cardiovasc Imaging. 2012;13:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Seminari E, De Silvestri A, Ravasio V, Ludovisi S, Utili R, Petrosillo N, Castelli F, Bassetti M, Barbaro F, Grossi P, Barzaghi N, Rizzi M, Minoli L. Infective endocarditis in patients with hepatic diseases. Eur J Clin Microbiol Infect Dis. 2016;35:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 2077] [Article Influence: 207.7] [Reference Citation Analysis (1)] |
| 42. | Martí-Carvajal AJ, Dayer M, Conterno LO, Gonzalez Garay AG, Martí-Amarista CE. A comparison of different antibiotic regimens for the treatment of infective endocarditis. Cochrane Database Syst Rev. 2020;5:CD009880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Vogkou CT, Vlachogiannis NI, Palaiodimos L, Kousoulis AA. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis. 2016;35:1227-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Pabilona C, Gitler B, Lederman JA, Miller D, Keltz TN. Prosthetic valve endocarditis with valvular obstruction after transcatheter aortic valve replacement. Tex Heart Inst J. 2015;42:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Varela Barca L, Fernández-Felix BM, Navas Elorza E, Mestres CA, Muñoz P, Cuerpo-Caballero G, Rodríguez-Abella H, Montejo-Baranda M, Rodríguez-Álvarez R, Gutiérrez Díez F, Goenaga MA, Quintana E, Ojeda-Burgos G, de Alarcón A, Vidal-Bonet L, Centella Hernández T, López-Menéndez J; Spanish Collaboration on Endocarditis—Grupo de Apoyo al Manejo de la Endocarditis infecciosa en ESpaña (GAMES). Prognostic assessment of valvular surgery in active infective endocarditis: multicentric nationwide validation of a new score developed from a meta-analysis. Eur J Cardiothorac Surg. 2020;57:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Cabell CH, Wang A. Current Treatment Options for Patients with Endocarditis: The Evolving Indications for Cardiac Surgery. Curr Treat Options Cardiovasc Med. 2004;6:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | David TE, Gavra G, Feindel CM, Regesta T, Armstrong S, Maganti MD. Surgical treatment of active infective endocarditis: a continued challenge. J Thorac Cardiovasc Surg. 2007;133:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Tornos P, Iung B, Permanyer-Miralda G, Baron G, Delahaye F, Gohlke-Bärwolf Ch, Butchart EG, Ravaud P, Vahanian A. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 49. | Toyoda N, Itagaki S, Egorova NN, Tannous H, Anyanwu AC, El-Eshmawi A, Adams DH, Chikwe J. Real-world outcomes of surgery for native mitral valve endocarditis. J Thorac Cardiovasc Surg. 2017;154:1906-1912.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Liu JZ, Li XF, Miao Q, Zhang CJ. Surgical treatment of active native mitral infective endocarditis: A meta-analysis of current evidence. J Chin Med Assoc. 2018;81:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Feringa HH, Shaw LJ, Poldermans D, Hoeks S, van der Wall EE, Dion RA, Bax JJ. Mitral valve repair and replacement in endocarditis: a systematic review of literature. Ann Thorac Surg. 2007;83:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Mishra AK, Sahu KK, Baddam V, Sargent J. Stroke and infective endocarditis. QJM. 2020;113:515-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Raza SS, Sultan OW, Sohail MR. Gram-negative bacterial endocarditis in adults: state-of-the-heart. Expert Rev Anti Infect Ther. 2010;8:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Tagliari AP, Steckert GV, da Silveira LMV, Kochi AN, Wender OCB. Infective endocarditis profile, prognostic factors and in-hospital mortality: 6-year trends from a tertiary university center in South America. J Card Surg. 2020;35:1905-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Sahu KK, Tsitsilianos N, Moselle L, Mishra AK. Septic arthritis of hip joint and its devastating complications. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Mishra AK, Lahiri A. Aspergillosis following bronchial artery embolization. QJM. 2021;114:63-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Michel PL, Acar J. Native cardiac disease predisposing to infective endocarditis. Eur Heart J. 1995;16 Suppl B:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Mishra AK, Sahu KK, George AA, Lal A. Safety and Efficacy of Thrombolysis and Mechanical Thrombectomy in Infective Endocarditis. J Stroke Cerebrovasc Dis. 2020;29:104784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Thomas VV, Mishra AK, Jasmine S, Sathyendra S. Gram-negative infective endocarditis: a retrospective analysis of 10 years data on clinical spectrum, risk factor and outcome. Monaldi Arch Chest Dis. 2020;90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Siegman-Igra Y, Koifman B, Porat R, Porat D, Giladi M. Healthcare associated infective endocarditis: a distinct entity. Scand J Infect Dis. 2008;40:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Carrel T, Schaffner A, Vogt P, Laske A, Niederhäuser U, Schneider J, Turina M. Endocarditis in intravenous drug addicts and HIV infected patients: possibilities and limitations of surgical treatment. J Heart Valve Dis. 1993;2:140-147. [PubMed] |
| 62. | Mishra AK, Sahu KK, Lal A, Sujata M. Systemic embolization following fungal infective endocarditis. QJM. 2020;113:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Durante-Mangoni E, Bradley S, Selton-Suty C, Tripodi MF, Barsic B, Bouza E, Cabell CH, Ramos AI, Fowler V Jr, Hoen B, Koneçny P, Moreno A, Murdoch D, Pappas P, Sexton DJ, Spelman D, Tattevin P, Miró JM, van der Meer JT, Utili R; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med. 2008;168:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 64. | Leclerc H. [Biochemical study of pigmented Enterobacteriaceae]. Ann Inst Pasteur (Paris). 1962;102:726-741. [PubMed] |
| 65. | Tamura K, Sakazaki R, Kosako Y, Yoshizaki E. Leclercia adecarboxylata gen. nov. comb. nov. formerly known asescherichia adecarboxylata [J]. Current Microbiology. 1986;13:179-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Zayet S, Lang S, Garnier P, Pierron A, Plantin J, Toko L, Royer PY, Villemain M, Klopfenstein T, Gendrin V. Leclercia adecarboxylata as Emerging Pathogen in Human Infections: Clinical Features and Antimicrobial Susceptibility Testing. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Makanera A, Conde M, Diallo MA. A multi-drug resistance pattern of a Leclercia adecarboxylata strain isolated from a urinary tract infection of a patient at China-Guinea friendship hospital of Kip/Conakry [J]. J Biol Chem. 2018;12:1550. [DOI] [Full Text] |
| 68. | Broderick A, Lowe E, Xiao A, Ross R, Miller R. Leclercia adecarboxylata folliculitis in a healthy swimmer-An emerging aquatic pathogen? JAAD Case Rep. 2019;5:706-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Hess B, Burchett A, Huntington MK. Leclercia adecarboxylata in an immunocompetent patient. J Med Microbiol. 2008;57:896-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Spiegelhauer MR, Andersen PF, Frandsen TH, Nordestgaard RLM, Andersen LP. Leclercia adecarboxylata: a case report and literature review of 74 cases demonstrating its pathogenicity in immunocompromised patients. Infect Dis (Lond). 2019;51:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Forrester JD, Adams J, Sawyer RG. Leclercia adecarboxylata bacteremia in a trauma patient: case report and review of the literature. Surg Infect (Larchmt). 2012;13:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Kim HM, Chon CY, Ahn SH, Jung SJ, Han KH, Moon BS, Moon YM. Fatal spontaneous bacterial peritonitis by Leclercia adecarboxylata in a patient with hepatocellular carcinoma. Int J Clin Pract. 2008;62:1296-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Sawamura H, Kawamura Y, Yasuda M, Ohkusu K, Takahashi Y, Ishihara S, Deguchi T, Ezaki T. [A clinical isolate of Leclercia adecarboxylata from a patient of pyelonephritis]. Kansenshogaku Zasshi. 2005;79:831-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Sethi K, Barker EM, Metlay LA, Caserta MT, Daugherty LE. Leclercia adecarboxylata Sepsis and Cerebral Herniation. J Pediatric Infect Dis Soc. 2014;3:e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Marina VP, Abidi S, Malhotra D. Leclercia adecarboxylata, an unusual hemodialysis catheter-related infection. Int Urol Nephrol. 2011;43:1257-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Aarab A, Saddari A, Noussaiba B, Ayyad A, Messaoudi S, Amrani R, Benaissa E, Ben Lahlou Y, Maleb A, Elouennass M. Leclercia adecarboxylata invasive infection in a patient with Hirschsprung disease: A case report. Ann Med Surg (Lond). 2021;71:102927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Gajdács M, Ábrók M, Lázár A, Terhes G, Burián K. Leclercia adecarboxylata as an emerging pathogen in human infections: a 13-year retrospective analysis in Southern Hungary. J Infect Dev Ctries. 2020;14:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Alosaimi RS, Muhmmed Kaaki M. Catheter-Related ESBL-Producing Leclercia adecarboxylata Septicemia in Hemodialysis Patient: An Emerging Pathogen? Case Rep Infect Dis. 2020;2020:7403152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Fattal O, Deville JG. Leclercia adecarboxylata peritonitis in a child receiving chronic peritoneal dialysis. Pediatr Nephrol. 2000;15:186-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | GHOSH R, MISRA R, PRASAD K, Prasad N. Peritonitis by Leclercia adecarboxylata in a patient with continuous ambulatory peritoneal dialysis: the first case report from India [J]. J Res Med Sci. 2016;1254-1256. [DOI] [Full Text] |
| 81. | Gomez CH, Bravo JS, CA Botero-García, Pescador LN. Leclercia adecarboxylata, a rare cause of soft tissue infections in immunocompromised patients, case report and review of the literature[J]. Infection. 2018;22. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Beltrán A, Molinero AV, Capilla S, Polo AM. [Isolation of Leclercia adecarboxylata from wound exudate of a diabetic patient]. Med Clin (Barc). 2004;122:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 83. | Denis JP. Leclercia adecarboxylata: The First Reported Infection of Cerebrospinal Fluid and a Systematic Review of the Literature[J]. J Neu Dis. 2015;6. |
| 84. | Sng ECY, Goh KCM, Tan SH, Tan AL, Oh HML. Leclercia adecarboxylata bacteraemia: Clinical features and antibiotic susceptibilities in 2 hospitals in Singapore. Ann Acad Med Singap. 2021;50:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Li J, Park A, Fulmer BR, Garg T. Leclercia adecarboxylata urinary tract infection in a patient with bladder cancer and recurrent hematuria. Urol Case Rep. 2021;36:101579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Hassan I, Gupta P, Ray P, Tiewsoh K. Leclercia adecarboxylata Causing Spontaneous Bacterial Peritonitis in a Child with Nephrotic Syndrome: A Case Report and Review of Literature. J Lab Physicians. 2020;12:222-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Jean SS, Lee WS, Bai KJ, Lam C, Hsu CW, Chen RJ, Hsueh PR. Leclercia adecarboxylata bacteremia in a patient with long-term use of nonsteroidal anti-inflammatory drugs. J Microbiol Immunol Infect. 2016;49:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Kashani A, Chitsazan M, Che K, Garrison RC. Leclercia adecarboxylata Bacteremia in a Patient with Ulcerative Colitis. Case Rep Gastrointest Med. 2014;2014:457687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |