Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10647
Peer-review started: April 14, 2022
First decision: June 16, 2022
Revised: June 26, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 167 Days and 15.8 Hours
Spinal gout (SG) is a rare condition. So far, a limited number of cases have been reported. Herein, we reported a single case of a 42-year-old male patient with SG involving the cervicothoracic and lumbar spine who underwent cervicothoracic segmental surgery.
The patient presented to the hospital with neck pain and limb weakness lasting for one month. He had a history of gout for more than 10 years. Clinical and imaging findings indicated bone and joint tophus erosion, and the patient underwent standard tophi excision and internal fixation with a nail-and-rod system. Histopathological examination suggested gout-like lesions. After the operation, the patient’s spinal nerve symptoms disappeared, and muscle strength gradually returned to normal. The patient maintained a low-purine diet and was recommended to engage in healthy exercises. The patient recovered well.
Clinicians should highly suspect SG when patients with chronic gout presented with low back pain and neurological symptoms. Early decompression and debridement surgery are important to relieve neurological symptoms and prevent severe secondary neurological deficits.
Core Tip: Spinal gout (SG) is a rare condition. This case report described the full course of surgical management of a SG patient. After surgical treatment, the patient’s symptoms were alleviated and the radiological findings improved. The case report highlighted the importance of surgical management of SG with neurological symptoms and presented a rare case of whole-spinal tophus deposition.
- Citation: Chen HJ, Chen DY, Zhou SZ, Chi KD, Wu JZ, Huang FL. Multiple tophi deposits in the spine: A case report. World J Clin Cases 2022; 10(29): 10647-10654
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10647.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10647
Spinal gout (SG) is a rare type of inflammatory arthritis that affects areas of the spine. It is often caused by urate crystal deposition in the spinal midshaft bones, causing pain and inflammation. The first case of SG was reported by Kersley et al[1] in 1955. Later, a cross-sectional study at the Washington Hospital Center showed that SG mainly affected the lumbar spine, followed by the cervical spine and sacroiliac joints[2]. However, most of these crystal deposits are centered in the facet joints of the spine, while they rarely accumulate in the spinal canal and even more rarely spread throughout the spine. In addition, some studies have suggested that the actual incidence of SG is higher than the current estimate.
The diagnosis of SG can be challenging due to the lack of specific symptoms. Histopathology test remains the diagnostic gold standard, while imaging, such as dual-energy computed tomography (DECT), may also be helpful. For treatment, regular use of uric acid-controlling medications is necessary, and early surgical intervention is important when symptoms of nerve injury and spinal cord compression develop.
Herein, we reported a single case of a patient with SG affecting the cervicothoracic and lumbar spine who underwent cervicothoracic segmental surgery.
On April 1, 2021, a 42-year-old man was admitted to our hospital for neck pain and limb weakness that persisted for 1 mo.
The patient’s family found him delirious and the medicine bottle used to store quetiapine [20 tablets (200 mg)] was empty. The patient was suspected of taking high doses of quetiapine. Gastric lavage was performed and he was admitted to the intensive care unit for further treatment.
He had no history of smoking or alcoholism, nor a history of drug or food allergies. He had a history of gout for more than 10 years and did not take medication regularly. No history of chronic diseases such as hypertension and diabetes were reported. In June 2020, he underwent right 1st toe amputation at another hospital for gouty arthritis, and recovered well after the surgery.
No abnormalities were found in the patient’s personal and family history.
The patient’s physical signs were as follows: Sensory numbness in the limbs, lumbosacral pain, pain and swelling in both elbows, interphalangeal joints of both hands, knees, and ankles, and limited mobility. The muscle strength of the extremities was classified as grade IV; the tendon reflexes of both upper extremities were hyperactive, the Hoffmann sign was positive, and the straight leg raise test was negative. Scattered multiple tophus were seen in both elbows, interphalangeal joints, knees, and ankles, with the size of a soybean. The first toe of the right foot was absent. The patient was 170 cm tall and weighed 60 kg (BMI: 20.8).
The test results showed C-reactive protein 7.73 mg/L, leukocytes 10.61 × 109/L, neutrophils (%) 79.20%, and calcitoninogen 0.02 ng/mL.
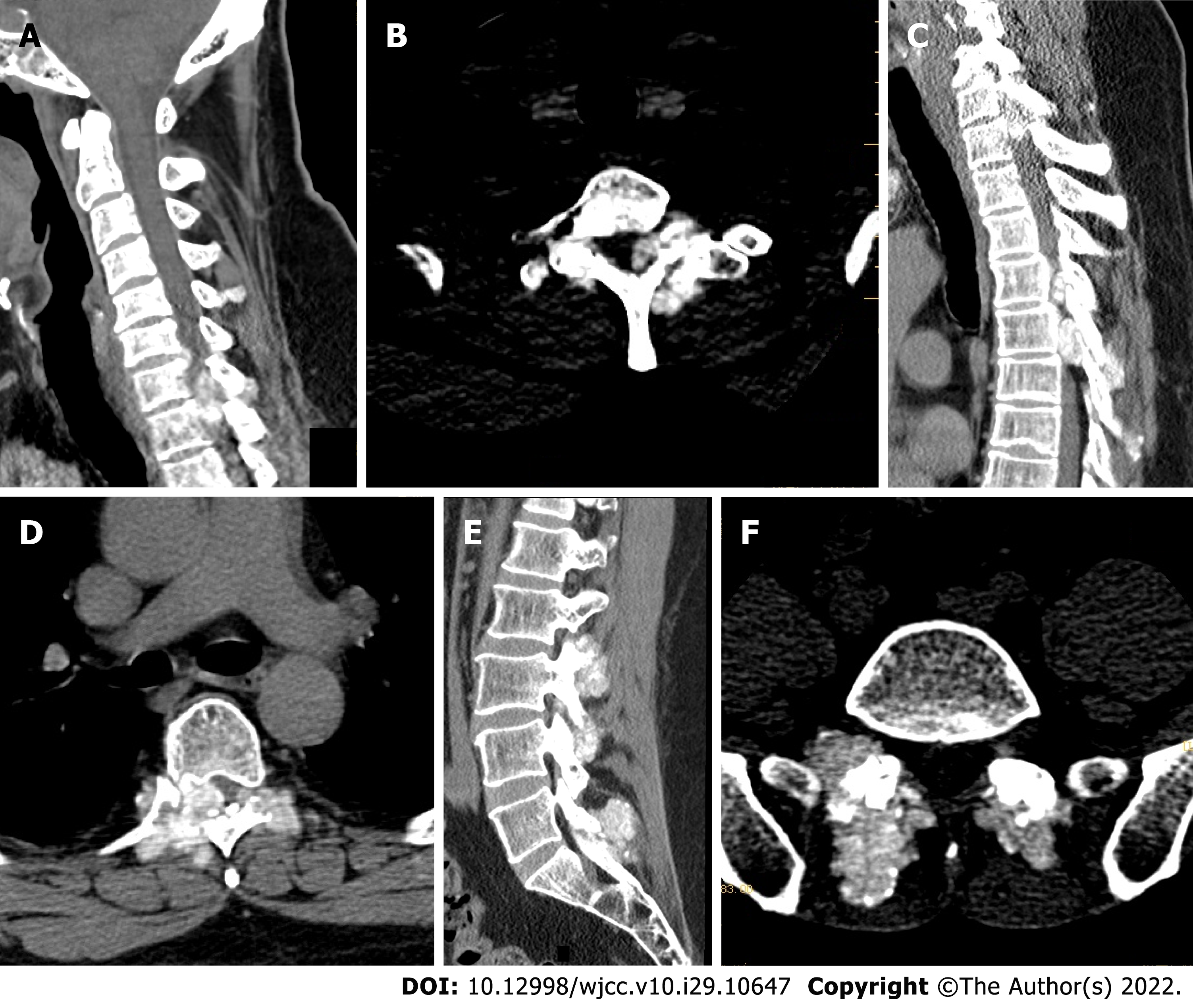
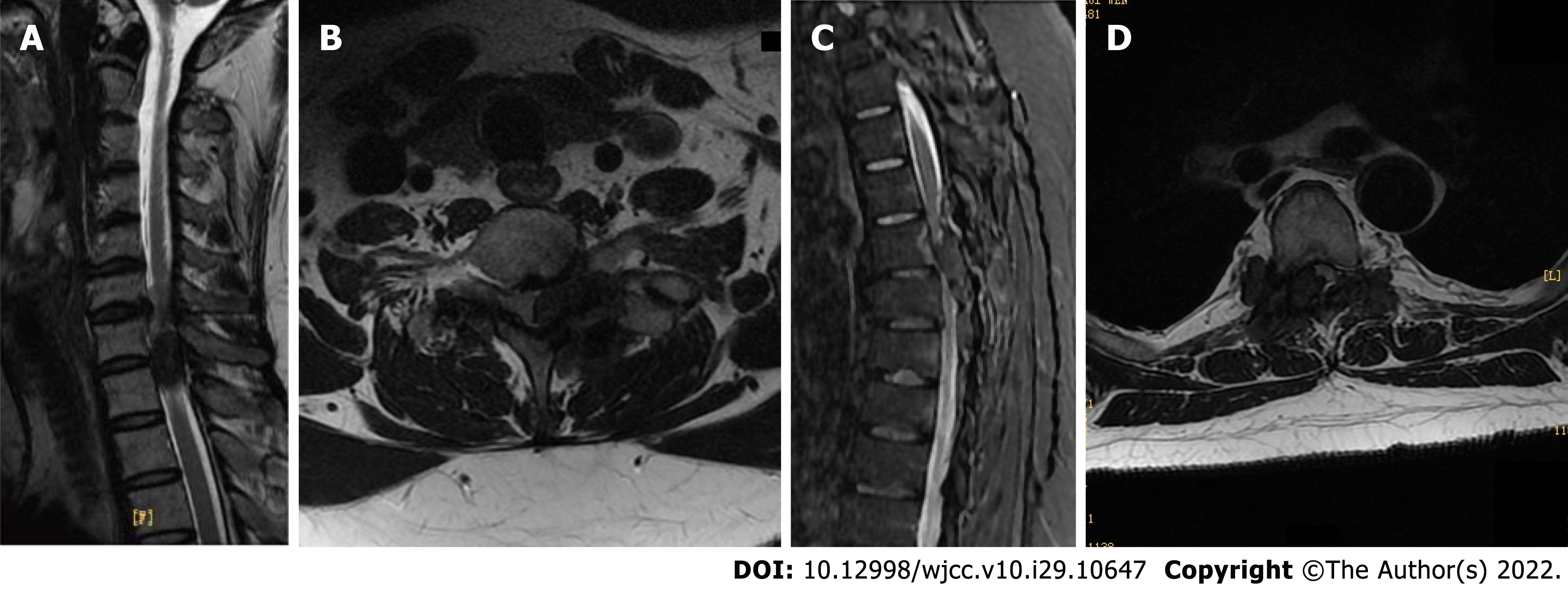
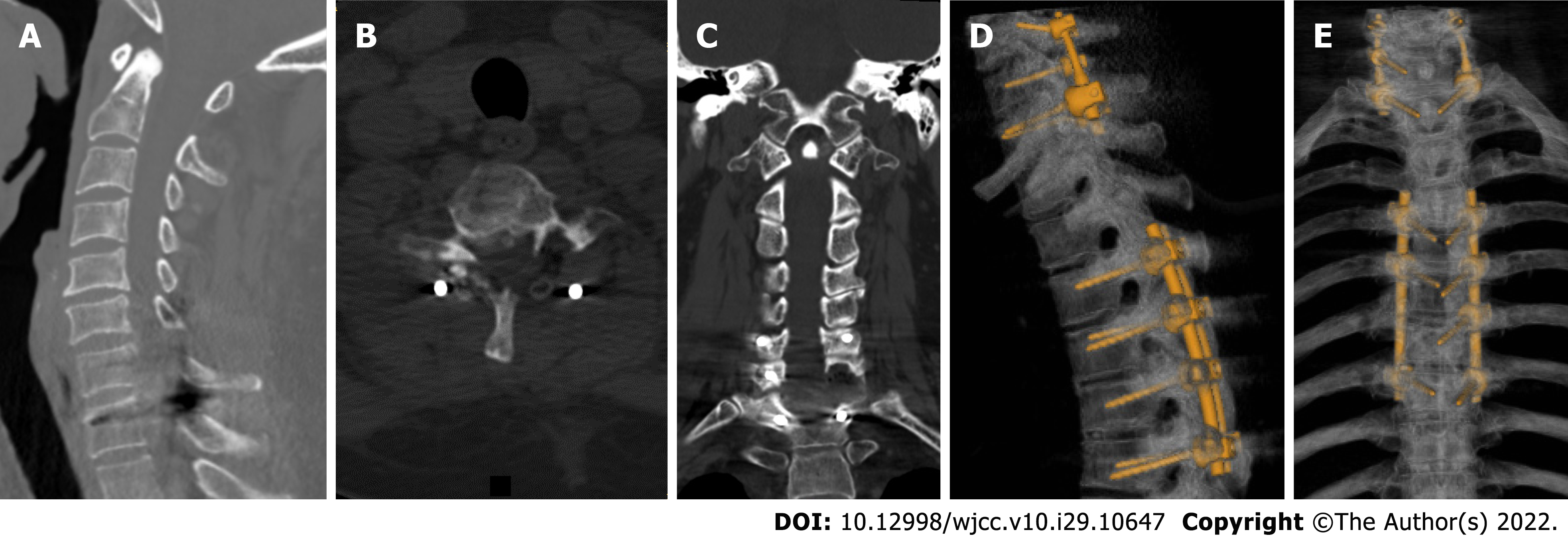
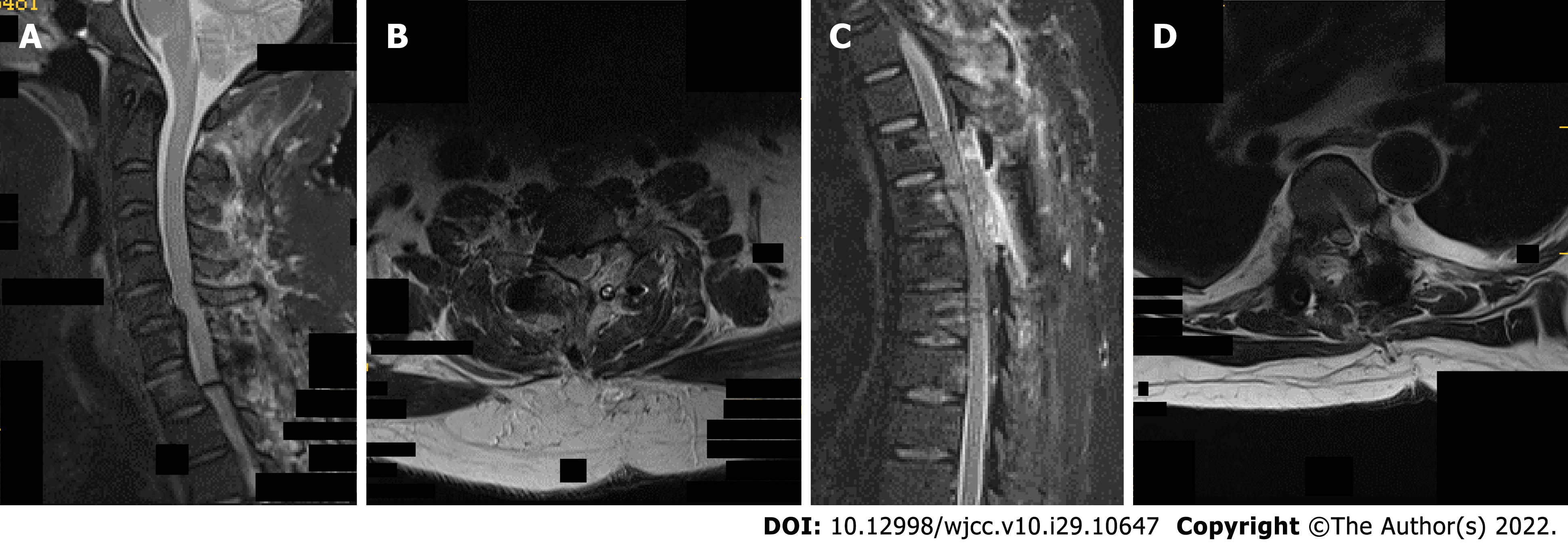
Ultrasound revealed echogenic changes in both kidneys and right kidney stones. X-ray showed bilateral elbow joints, bilateral knee joints, bilateral ankle joints, gouty arthritic changes in both hands, destruction of the left olecranon and right medial humeral condyle, and multiple metacarpals and carpal bones in both hands. Defects of the right foot 1st metatarsal with exposed stump was considered postoperative changes. Preoperative Computed tomography (CT) of the cervical, thoracic, lumbar, and sacral spine showed: (1) Multiple tophi throughout the spine, with spinal cord compression and degeneration at C7/T1, T5, and T6 Levels; and (2) multiple abnormal high signals that might indicate tophus deposition in the lumbar and sacral vertebral subtalar joints (Figure 1). Cervical and thoracic spine magnetic resonance imaging (MRI) suggested: (1) Multiple tophus deposits in and around the left vertebral subtalar joint at C7/T1, right T4/5 and bilateral subtalar joints at T5/6, and spinal cord compression and degeneration at C7/T1, T5 and T6 Levels (Figure 2).
Due to the suspicion of cervicothoracic spinal canal occupancy with spinal cord injury, decreased muscle strength of the extremities, unsatisfactory results of conservative treatment, and the presence of surgical indications, further diagnosis and treatment for this patient were necessary.
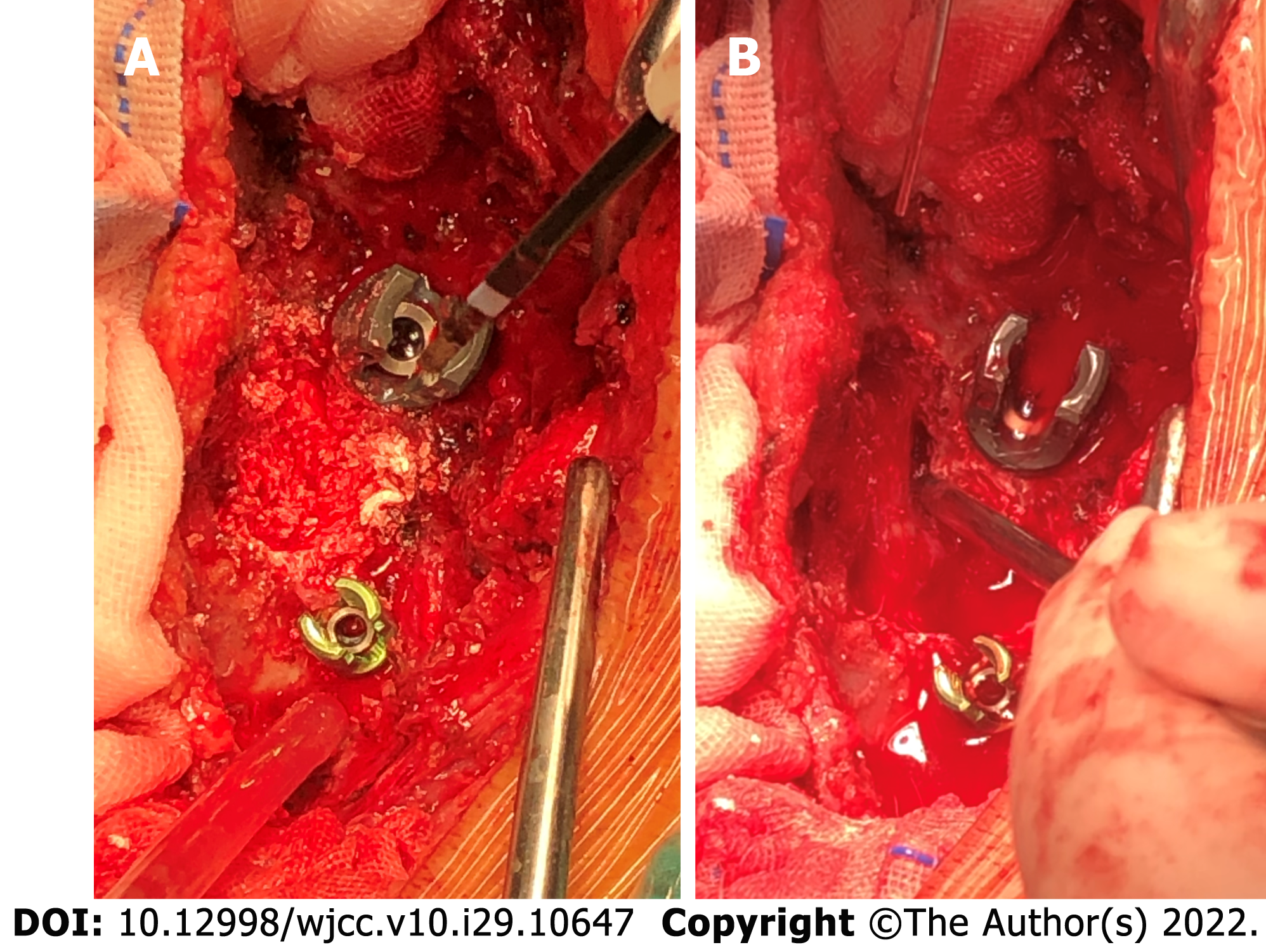
After the operation, the C6-T7 vertebral plate and upper and lower articular processes were exposed, and a large number of cocoon-like, lime-like tophi were seen (Figure 3 and 4). The tophi were removed under the protection of the cervical spinal cord, and the pressure was fully decompressed. Right unilateral laminectomy at T5 and T6 was then performed, and a large number of crystalline tophi were seen in the spinal canal, compressing the cervical and thoracic spinal cord. The tophi were removed, and the spinal cord and nerve roots were free of compression.
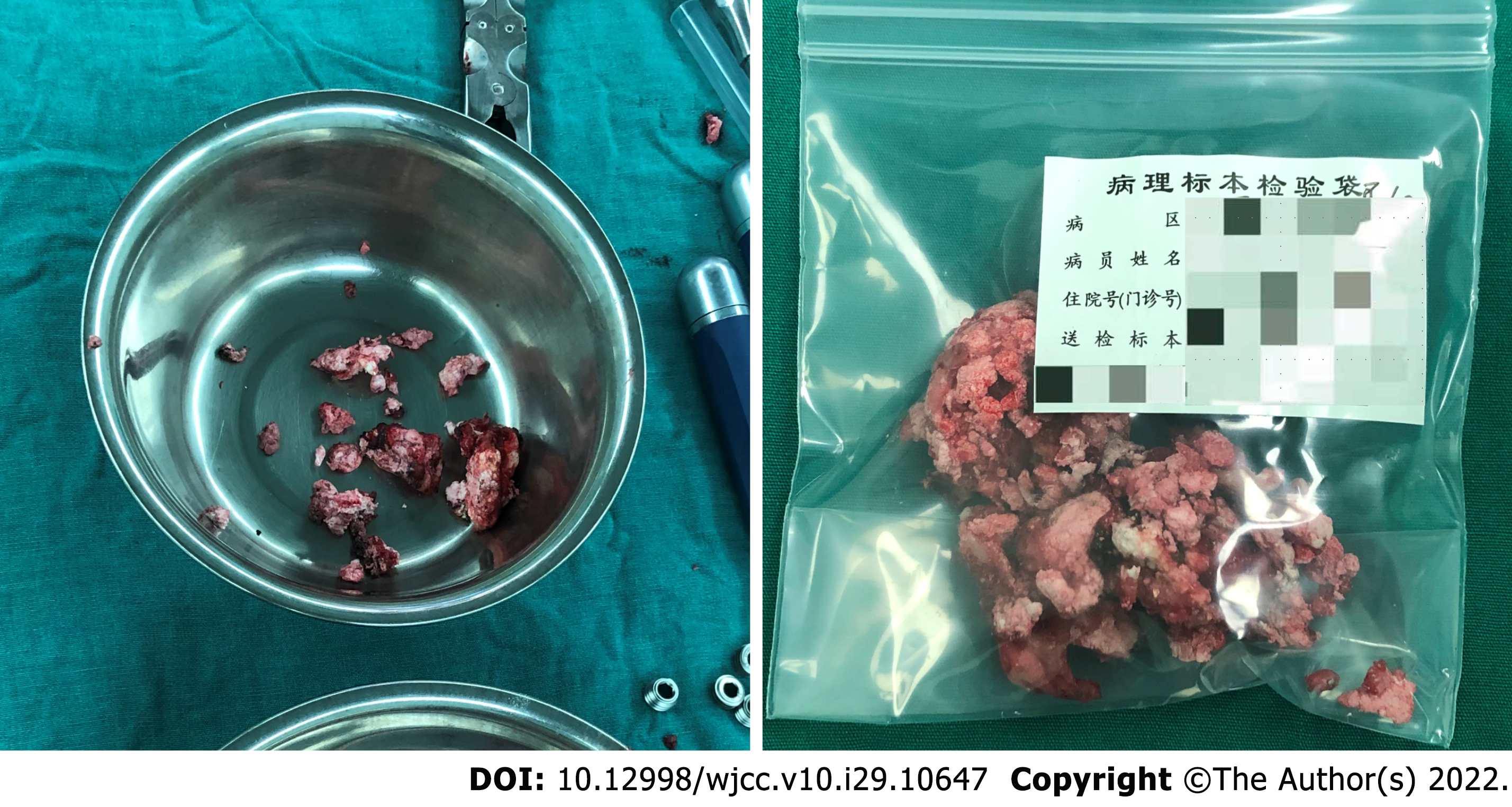
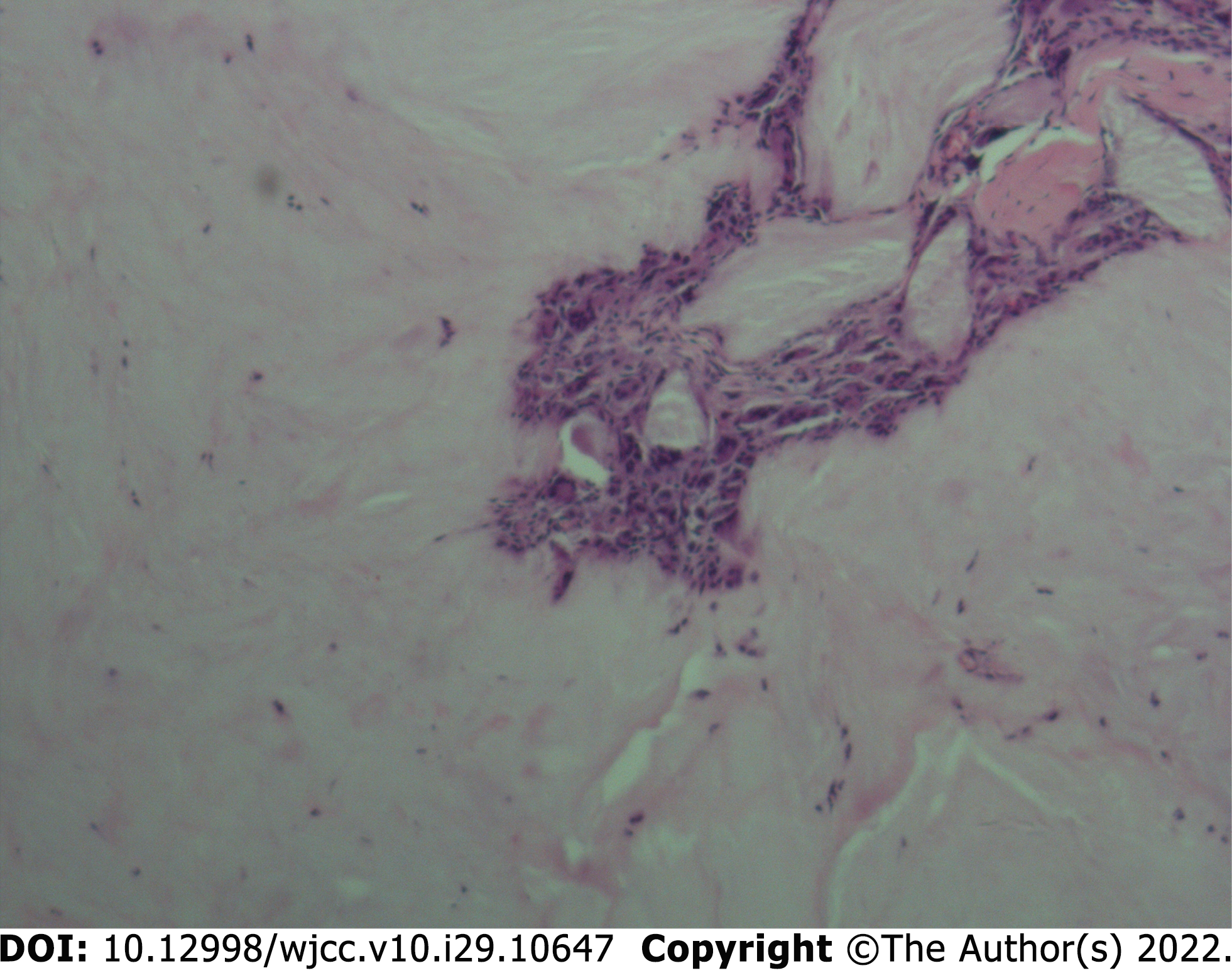
The intraoperatively collected tophi were sent for histopathological examination, and the results suggested crystal deposition (Figure 5). Considering that the patient had no radicular symptoms and nerve damage in the lumbar spine, symptomatic treatment such as uric acid control was temporarily given, and the patient was closely observed. Postoperative X-ray films showed that the C6-T1 and T4-7 vertebrae were fixed in place without loosening. Postoperative sensory numbness and weakness of the extremities were significantly improved compared with the preoperative period, and no intraoperative infection was observed (Figure 6 and 7). The patient was discharged in May 2021.
Based on these observations, common diagnoses (such as histopathological findings) were ruled out. Finally, the man was diagnosed with SG.
On April 15, 2021, a posterior cervicothoracic dissection and decompression combined with tophus excision and internal fixation using the nail-and-rod system was performed under general anesthesia.
The patient kept a low-purine diet and was recommended to perform healthy exercises. Allopurinol and febuxostat were administered for medication control to sustain urate-lowering therapy (ULT). After completing regular follow-up visits (3rd, 6th, 9th, and 12th months after surgery), the patient’s limb muscle strength was changes to grade V and sensation in the limbs returned to normal. The patient had good compliance.
Gout is a chronic disease caused by monosodium urate (MSU) crystal deposition. Elevated serum urate level (hyperuricemia) is a major risk factor for MSU crystal deposition and the development of gout[3]. Hyperuricemia often occurs in the joints of the extremities such as ankles, knees, elbows, hands, feet, and wrists, and may affect the patient's skin condition.
Diagnosis of SG is challenging, considering that most patients are asymptomatic. Konatalapalli et al[4] reported a prevalence of SG of 35% in patients with a history of gouty arthritis (in accordance with American College of Rheumatology criteria) that was poorly controlled by conservative treatment for more than 3 years. SG only affecting small spine joints and peripheral soft tissues may be easily missed during a medical checkup. Some studies have suggested that low back pain is an early symptom of SG. Yet, Tkach[5] found that when patients with long-standing gout disease presented with low back pain, they might have osteophytic spinal stenosis or small arthritic spine. Thus, low back pain is not considered a direct evidence of SG. However, when gout affects the spinal canal, compressing the nerves and spinal cord extensively, or when the patient presents with neurological symptoms, clinicians may suspect SG. Therefore, the prevalence of SG is actually to be much higher than currently believed[6]. SG should be reasonably suspected when patients suffer from long-term gout accompanied with low back pain, limited spinal mobility and neurological symptoms[7].
In a retrospective study of 131 cases in New York, only one spine region was involved in most SG cases (80.6%); in 32 (24.8%) cases, gout only affected the cervical spine; in 49 cases (38.0%), it only affected the lumbar spine; in 23 cases (17.8%), it only affected the thoracic spine; in 18 cases (14.0%), both the lumbar spine and the upper sacrum (S1) were affected; in 6 cases (4.7%), both the thoracic and lumbar spine were affected; and in one case (0.7%), both the cervical and thoracic spine were affected[8]. Therefore, it was relatively rare for the patient to present with multiple tophi throughout the spine.
Hyperuricemia is the underlying condition for the development of gout. However, most patients with hyperacidity do not develop gout because the deposition of urate crystals and inflammatory responses at the site of crystallization are key elements for the development of gout. Narang et al[9] suggested three essential steps for urate crystallization: reduction of urate solubility (leading to urate supersaturation), nucleation of urate crystals, and growth of urate crystals. The complex process of urate crystal deposition and the underlying mechanism remain a hot research topic today.
Whole-spine polygout affecting the vertebral tubercle and the epidural spinal canal was seen in our patient. MSU crystal dissolution by ULT may further weaken these areas and induce their collapse before extensive tophi damage the bone scaffold[10]. Tophi erode the central column of the spine, causing instability and the risk of collapse during ULT. With this in mind, we made an early surgical decision and performed a long-segment internal fixation (C6-T1 and T4-7).
However, the mechanism underlying gout occurrence is not conclusive. The development of gout is a complex event involving a combination of genetic, endocrine, immune, and other factors. Furthermore, most of the current trials on the factors influencing MSU crystallization are based on in vitro methods that measure MSU crystallization by changing a single factor in a single session in vitro. Therefore, we need to comprehensively consider the potential effects of multiple different environmental factors on the process of urate crystallization in vivo.
Biopsy remains the gold standard for SG diagnosis[11]. For early diagnosis, X-ray, Color ultrasound, CT, and MRI may also be used. Yet, these imaging methods allow a limited view and lack specificity for gout diagnosis. Color ultrasound is useful for examining tophi in superficial locations of the joints in extremities[12]. DECT shows considerable sensitivity and specificity for identifying urate crystal deposits in the spine and can be used for quantitative and qualitative diagnosis of gout crystals[13,14].
In terms of treatment, allopurinol, febuxostat, and other uric acid-lowering drugs are commonly used to control uric acid levels. Nonsteroidal anti-inflammatory drugs, colchicine, and glucocorticoids are preferred for anti-inflammatory treatment, regardless of whether patients opt for surgical intervention. Ribeiro et al[15] concluded that pharmacological treatment of gout is mandatory for patients with or without surgical intervention. Unfortunately, there is a lack of clinical trials on SG treatment in China, and surgeries procedures are performed only when patients present with nerve damage, which increases both the physical and psychological burden of the patient as well as the socioeconomic burden. Therefore, gout patients should be aware of SG early screening as they remain alert for spinal symptoms.
Surgery is considered the second-line treatment for patients with SG. A systematic study of previous literature has shown that indications for surgery in SG patients include: (1) SG stone deposition in the spinal canal, leading to spinal stenosis, nerve root and dural sac compression; (2) spinal instability with axial pain due to bone destruction of the vertebral bodies and spinal appendages; and (3) in acute exacerbation of SG, pharmacological treatment fails to relieve pain[16]. There are no relevant clinical randomized controlled trials reporting which treatment (surgery vs medication) is more effective. However, complications after SG surgery remain an important concern for surgeons, including skin condition, uric acid levels, other systemic complications, and prevention of postoperative infections[17]. Surgical treatment can address spinal physical compression and occupancy, but it is important to manage the uric acid levels and educate the patients throughout the treatment process. Previous studies have not discussed the surgical approaches for SG, which are usually individualized based on the patient’s own gout presentation, the degree of spinal destruction, and whether the spinal canal is occupied.
A one-stage posterior cervicothoracic unilateral laminectomy with bilateral decompression, tophus removal, and internal fixation using a nail-and-rod system was used in this case. This method was selected to preserve the patient’s unilateral pedicle and lamina and preserve some of the posterior column structures, thus re-establishing postoperative spinal stability and sagittal balance, reducing operative time, bleeding, and improving postoperative healing rates[18]. A 5-year retrospective study conducted at the New Zealand University Hospital showed no significant differences between reope
Clinicians should suspect SG in patients with long-term gout disease, especially in those with gouty arthritis of the extremities who presented with low back pain and neurospinal compression. Drug therapy should be recommended throughout the entire management of SG. Moreover, early decompression and debridement surgery is important to relieve neurological symptoms and prevent severe secondary neurological deficits.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gálvez Salazar P, Ecuador; Jatuworapruk K, Thailand; Rothschild B, United States S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Kersley GD, Mandel L, Jeffrey MR. Gout; an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly. Ann Rheum Dis. 1950;9:282-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Konatalapalli RM, Lumezanu E, Jelinek JS, Murphey MD, Wang H, Weinstein A. Correlates of axial gout: a cross-sectional study. J Rheumatol. 2012;39:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, Stamp LK. Gout. Nat Rev Dis Primers. 2019;5:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 4. | Konatalapalli RM, Demarco PJ, Jelinek JS, Murphey M, Gibson M, Jennings B, Weinstein A. Gout in the axial skeleton. J Rheumatol. 2009;36:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Tkach S. Gouty arthritis of the spine. Clin Orthop Relat Res. 1970;71:81-86. [PubMed] |
| 6. | Lumezanu E, Konatalapalli R, Weinstein A. Axial (spinal) gout. Curr Rheumatol Rep. 2012;14:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Wang G, Zhuo N, Tian F, Wen Z, Li J. Spinal gout with erector spinae muscle entrapment. Rheumatology (Oxford). 2022;61:e129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Toprover M, Krasnokutsky S, Pillinger MH. Gout in the Spine: Imaging, Diagnosis, and Outcomes. Curr Rheumatol Rep. 2015;17:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Narang RK, Dalbeth N. Pathophysiology of Gout. Semin Nephrol. 2020;40:550-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Bardin T, Nguyen QD, Hieu NL, Tran KM, Dalbeth N, Do MD, Ea HK, Richette P, Resche-Rigon M, Bousson V. The shrinking toe sign in gout. Semin Arthritis Rheum. 2022;53:151981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Hasegawa EM, de Mello FM, Goldenstein-Schainberg C, Fuller R. Gout in the spine. Rev Bras Reumatol. 2013;53:296-302. [PubMed] |
| 12. | Cao L, Zhao T, Xie C, Zheng S, Wan W, Zou H, Zhu X. Performance of Ultrasound in the Clinical Evaluation of Gout and Hyperuricemia. J Immunol Res. 2021;2021:5550626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Khanna I, Pietro R, Ali Y. What Has Dual Energy CT Taught Us About Gout? Curr Rheumatol Rep. 2021;23:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Bongartz T, Glazebrook KN, Kavros SJ, Murthy NS, Merry SP, Franz WB 3rd, Michet CJ, Veetil BM, Davis JM 3rd, Mason TG 2nd, Warrington KJ, Ytterberg SR, Matteson EL, Crowson CS, Leng S, McCollough CH. Dual-energy CT for the diagnosis of gout: an accuracy and diagnostic yield study. Ann Rheum Dis. 2015;74:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Ribeiro da Cunha P, Peliz AJ, Barbosa M. Tophaceous gout of the lumbar spine mimicking a spinal meningioma. Eur Spine J. 2018;27:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Yan Bingshan LY, Zhang Hong, Shan Duo, Bu Guoyun, Liu Peijia, Xu Hongda, Hu Yongcheng. Gout in thoracic spinal canal: A case report and systematic review. Zhonghua Guke Zazhi. 2021;41:790-799. [DOI] [Full Text] |
| 17. | Carcione J, Bodofsky S, LaMoreaux B, Schlesinger N. Beyond Medical Treatment: Surgical Treatment of Gout. Curr Rheumatol Rep. 2020;23:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Lei D, Zhou Y, Yao D, Zhang F, Wang X, Jiang X, Xiong N, Zhao H. Efficacy of unilateral hemilaminectomy for intraspinal tumor resection: a systematic review and meta-analysis. Ann Palliat Med. 2021;10:984-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Bouknaitir JB, Carreon LY, Brorson S, Pedersen CF, Andersen MØ. Wide Laminectomy, Segmental Bilateral Laminotomies, or Unilateral Hemi-Laminectomy for Lumbar Spinal Stenosis: Five-year Patient-reported Outcomes in Propensity-matched Cohorts. Spine (Phila Pa 1976). 2021;46:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |