Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10638
Peer-review started: April 14, 2022
First decision: June 16, 2022
Revised: July 13, 2022
Accepted: September 7, 2022
Article in press: September 7, 2022
Published online: October 16, 2022
Processing time: 168 Days and 2.2 Hours
Preoperative conditions in pediatric liver transplant recipients are understandably complex. Compared with adults, children have lesser compensatory abilities and demand greater precision during procedural executions. In the setting of end-stage liver disease, the heightened perioperative risk of coexistent cardiovascular pathology may impact graft survival as well. Requirements for anesthesia and perioperative management are thus more rigorous, calling for individualized treatments that reflect specific cardiovascular constraints and proposed surgical plans.
Reports of perioperative anesthesia management and liver transplant prognostication in pediatric patients with concurrent atrial septal defects are scarce. Her
Children with atrial septal defects bear substantially more than customary perioperative risk during orthotopic liver transplants, given their compromised cardiopulmonary reserves and functional states. Comprehensive preoperative cardi
Core Tip: Children with atrial septal defects bear substantially more than customary perioperative risk during orthotopic liver transplants. Although perioperative anesthesia management must reconcile individual cardiovascular constraints with proposed surgical plans, published reports offering necessary guidance are scarce. Herein, we address this complex scenario, focusing on issues of perioperative anesthesia management. Comprehensive preoperative cardiovascular assessments, with multidisciplinary input, may offer insights into structural cardiac pathophysiologic effects and transplant-related hemodynamic changes that impact new grafts. Active, effective hemodynamic monitoring and other measures are also essential during the perioperative period to ensure transplantation success and graft survival.
- Citation: Liu L, Chen P, Fang LL, Yu LN. Perioperative anesthesia management in pediatric liver transplant recipient with atrial septal defect: A case report. World J Clin Cases 2022; 10(29): 10638-10646
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10638.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10638
Biliary atresia (BA) affects 1-2 of every 15000 Live births and is the primary indication for liver transplantation (LT) in the pediatric population[1,2]. Congenital heart disease (CHD) is the most common form of birth defect, atrial septal defect (ASD) being the leading type of CHD (approximately 10%-15%)[3]. However, even among those born with multiple congenital disorders, BA and CHD coexist in just 2%-3%[4]. Preoperative conditions of children requiring LT are complex. Compared with adults, their compensatory abilities are less adept, and they demand greater surgical precision. The combined burden of end-stage liver disease (ESLD) and existing cardiac pathology stands to increase perioperative cardiac risk and affect graft survival. Anesthesiologists must therefore individualize treatment, based on cardiovascular status and specific surgical requirements. Unfortunately, reports of perioperative anesthesia management in pediatric living-donor liver transplantation (LDLT) candidates with concurrent ASD are scarce. The objective at present was to share our related experience and assess the actual impact of ASD on LDLT outcome. We have thus detailed the applicable therapeutic approach and discussed key aspects of perioperative anesthesia management.
A female child, 2 years and 5 mo old, was admitted to our hospital for subsequent LDLT.
The child had been seen at our hospital 1 year prior for pre-LT evaluation. She was then placed on a waiting list for LT while being followed as an outpatient.
ASD and extrahepatic BA had been diagnosed shortly after birth. Biliary tract exploration was done at 3 mo of age, performing a Kasai procedure (i.e., portoenterostomy) to establish drainage. Stool color returned to normal postoperatively, although liver dysfunction persisted in subsequent visits.
The child’s parents denied any family history of other illnesses, such as respiratory and nervous system disorders or malignant tumors.
At the time of transplantation (weight, 12.7 kg; height, 89 cm), the patient was conscious and had stable vital signs. On room air, O2 saturation was 99% (without cyanosis). However, there were several distinctive clinical features of advanced liver disease, namely slightly jaundiced skin, a swollen but soft abdomen (liver palpable below umbilicus), and inferior splenic border protrusion 7.2 cm below costal margin (although soft, non-tender). A surgical scar (9 cm) of right abdomen was also visible.
Various liver function tests were abnormal, including alanine aminotransferase [67 U/L (normal, 6-45 U/L)], aspartate aminotransferase [71 U/L (normal, 20-60 U/L)], alkaline phosphatase [427 U/L (normal, 145-320 U/L)], total bile acids [41.3 µmol/L (normal, < 10.0 µmol/L)], total bilirubin [22.5 µmol/L (normal, 5.0-21.0 µmol/L)], total protein [64.7 g/L (normal, 66.0-83.0 g/L)], free fatty acid [1516.7 µmol/L (normal, 129-769 µmol/L)], and fasting plasma glucose [2.87 mmol/L (normal, 3.9-6.1 mmol/L)]. Routine blood and urine analytes otherwise tested normal, as did coagulation indices.
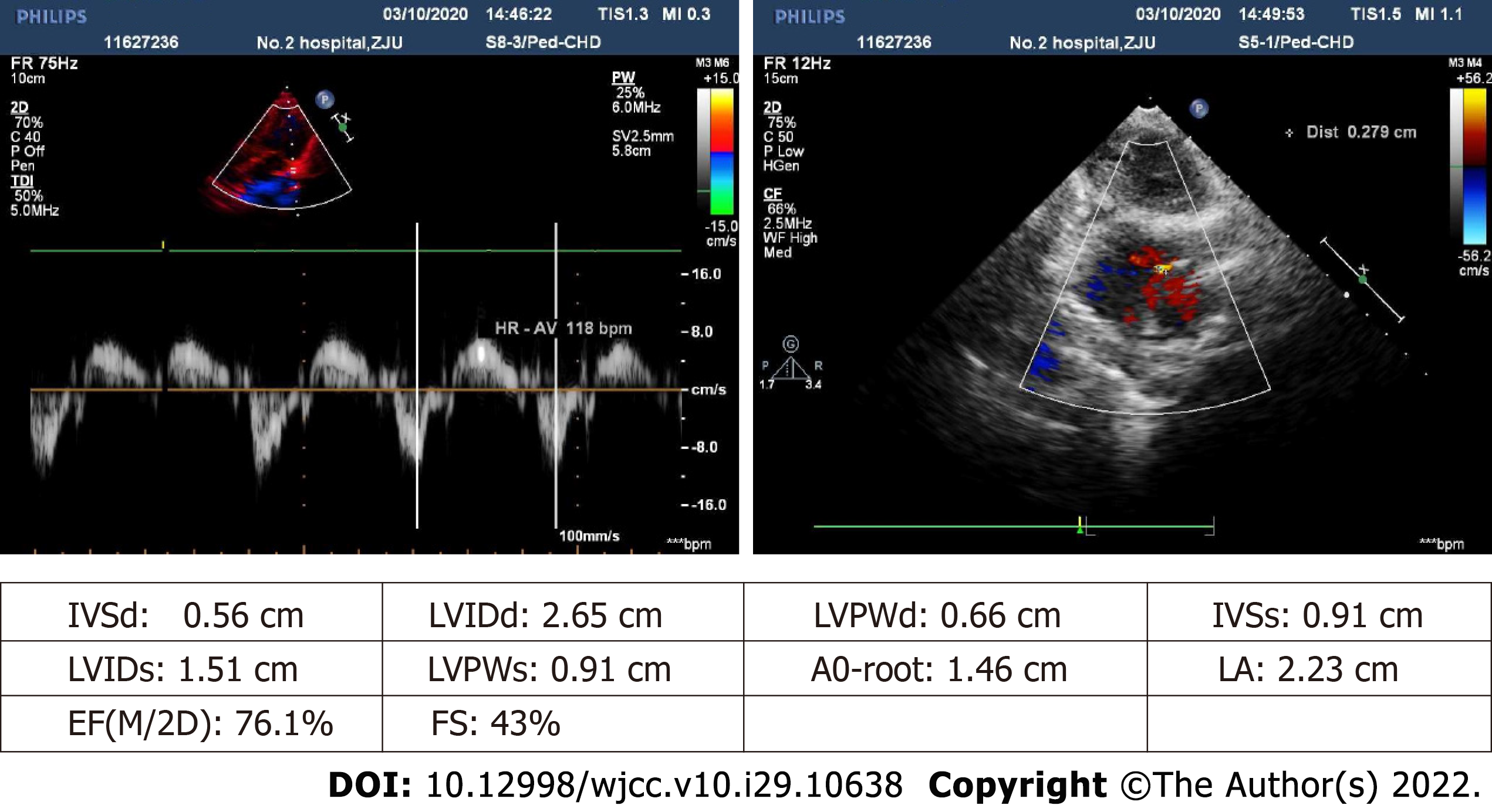
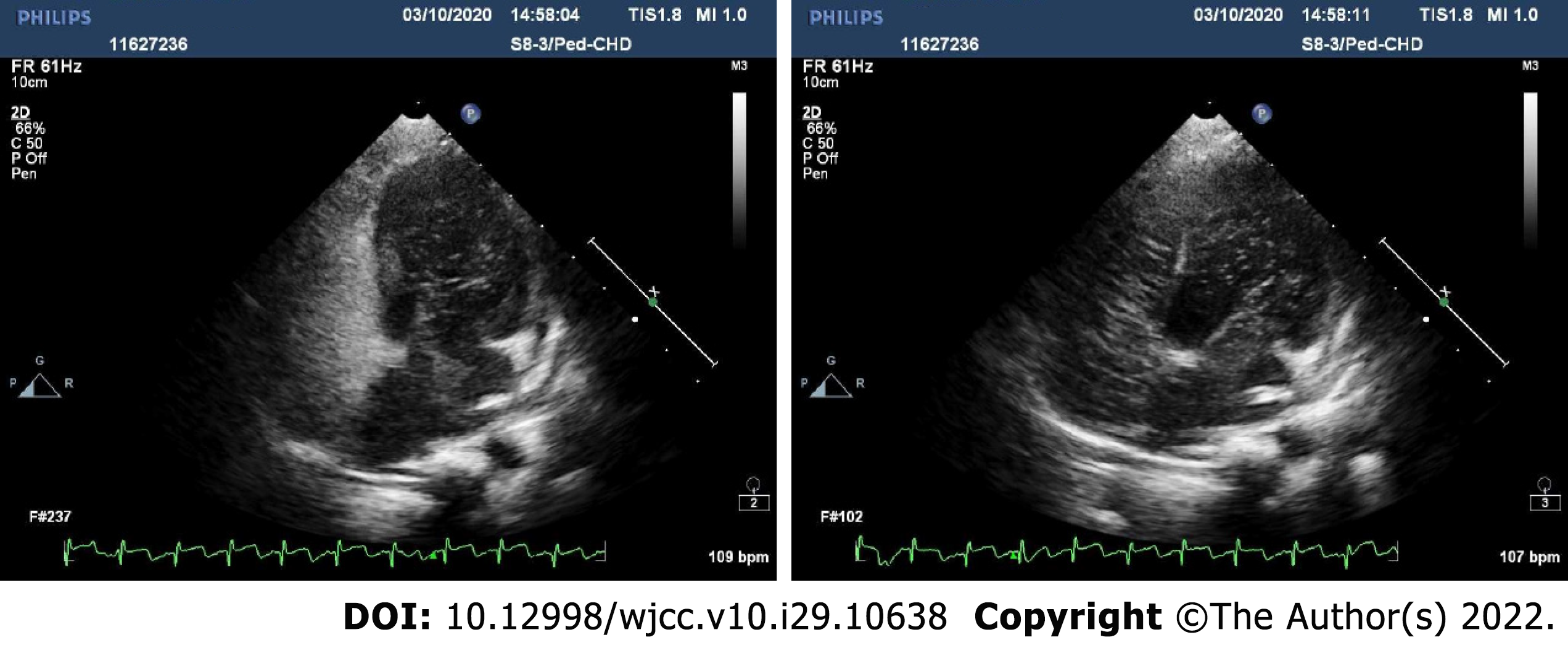
Color doppler ultrasound images of the abdomen revealed considerable cirrhosis, a widened main portal vein, localized post-cholecystectomy dilatation of left intrahepatic bile duct, and splenomegaly, in the absence of ascites. Preoperative transthoracic echocardiography indicated an echoic interruption of middle and lower atrial septum, 0.3 cm across (slightly less than in prior year), with left-to-right red-colored streamers detected by color Doppler flow imaging (Figure 1). Shunt velocity was not measured. Agitated-saline contrast echocardiography (ASCE) was performed to further clarify the nature of intracardial shunting. After injecting vibrated normal saline (3 mL) into cubital vein, the right heart filled well. In resting state, few left heart air bubbles formed during the third cardiac cycle; but many more (> 35 per frame) arose during the fifth cycle, ostensibly from left and right upper pulmonary veins. Valsalva maneuver during the third cardiac cycle also produced a flurry of bubbles (> 35/frame) within left heart. These findings suggest atrial-level right-left shunting, pulmonary arteriovenous fistula not excluded (Figure 2). No other determinative testing was attempted.
The Model for Pediatric End-stage Liver Disease score was 14[5,6]. Concurrent ASD was also investigated in detail. Remarkably, cyanosis and digital clubbing were absent, and lung auscultation was clear. We then assembled a multidisciplinary team (MDT) of specialists in LT surgery, pediatrics, cardiology, cardiovascular surgery, ultrasound, radiology, anesthesiology, and intensive care to discuss this child’s cardiovascular profile, the feasibility of LDLT, and any need for specific cardiac inter
Our final diagnoses, including historical information provided, were congenital biliary ASD.
Upon admission, the child received compound glycyrrhizin tablets (for hepatic protection) and ursodeoxycholic acid capsules (to relieve cholestasis) as oral symptomatic treatments. Once in the operating room, standard monitoring practices of the American Society of Anesthesiologists were implemented and initial vital signs recorded [blood pressure (BP), 96/48 mmHg; heart rate (HR), 99 bpm; pulse oxygen saturation, 100%]. Continuous invasive BP readings were obtained by radial artery catheterization, using the femoral artery for pulse-induced contour cardiac output (PiCCO) analysis. Given the potential for intracardiac shunting, it was mandatory that all veins and monitoring lines be free of air bubbles (regardless of their direction), preventing entry of gas into the circulation for the duration of the procedure. The LT procedure took place under general anesthesia, induced by midazolam, propofol, and sufentanil after 100% preoxygenation. Following cisatracurium delivery, orotracheal intubation (4.0 mm tube) was performed. Anesthesia was then maintained via sevoflurane, oxygen/air mixture, sufentanil, and propofol, adding cisatracurium for muscle relaxation. We kept intraoperative mean arterial pressure (MAP) at 60-70 mmHg, HR at 90-120 bpm, and hematocrit (Hct) at just under 30%. Central venous blood gas analyses (i.e., electrolytes, Hct, and lactate levels) were run after the operation began, 5 min before anhepatic phase, 5 min before and 1 h after perfusing the new liver, and prior to completing the operation (Table 1).
| Analyte | After start of operation | Five min before anhepatic phase | Five min before perfusing new liver | One hour after perfusing new liver | Before completing operation |
| HCT (%) | 29.1 | 29.1 | 29.5 | 29.2 | 28.7 |
| Hb (g/dl) | 10.12 | 10.04 | 10.41 | 10.24 | 10.45 |
| pH | 7.341 | 7.372 | 7.371 | 7.341 | 7.37 |
| PvCO2 (mmHg) | 41.7 | 35.1 | 33.8 | 40.4 | 37.8 |
| PvO2 (mmHg) | 115.8 | 75.7 | 129.6 | 84.7 | 77.3 |
| SpO2 (%) | 100 | 97 | 98 | 98 | 98 |
| BE (mmol/L) | -3.8 | -5.4 | -6.1 | -4.4 | -3.9 |
| K+ (mmol/L) | 4.03 | 3.9 | 3.72 | 3.54 | 3.83 |
| Ca++ (mmol/L) | 1.12 | 1.11 | 1.3 | 1.19 | 1.14 |
| Glu (mmol/L) | 5.2 | 6.6 | 5.16 | 12 | 6.2 |
| Lactate (mmol/L) | 1.1 | 1.6 | 1.9 | 1.8 | 1.6 |
Perioperative infusions of isosmotic solution (compensating for loss to third space), glucose (for normoglycemic maintenance), and albumin (as volume expander) were sufficient to maintain hemoglobin at approximately 8-10 g/dL. Fluid input and output totals recorded during surgery are listed in Table 2. The entire operation lasted 6 h and 40 min, including a pre-anhepatic stage of 3 h and 36 min and an anhepatic stage of 35 min. No significant procedure-related pulmonary or hemodynamic derangements occurred. Using a right-liver split graft, the surgical team chose a piggyback technique that proved successful. The still-intubated patient was taken to the intensive care unit (ICU) under sedation. Once extubated (Day 1 after surgery), inhaled O2 was supplied by nasal catheter. No posto
| Total input /output | Volume |
| Normal saline | 100 mL |
| 5% glucose injection | 120 mL |
| Multiple electrolyte injections | 970 mL |
| 20% human albumin | 50 mL |
| Urine | 480 mL |
| Blood loss | 200 mL |
During the 1-year postoperative follow-up period, the graft functioned consistently well. Liver ultrasound and enhanced abdominal computed tomography (CT) scans acquired periodically indicated no obvious abnormalities of the grafted liver, and hepatic blood flow velocity was normal. The child also displayed good growth and development, showing greater capacity for exercise endurance and no symptoms of poor heart function (i.e., shortness of breath, skin cyanosis, or frequent colds). Conse
LT is now the standard of care for pediatric patients with ESLD and metabolic disorders involving the liver. However, children with CHD who undergo LT procedures may experience severe hemodynamic fluctuations during the perioperative period due to their cardiac defects and limited myocardial reserves[7,8]. In our patient, we were obliged to consider whether her heart might withstand the LT procedure, and whether the graft would survive well postoperatively. Perioperative risks, such as sudden right ventricular failure, possible volume overload, and severe congestion of the new graft, may heighten the risk of postoperative transplant failure. A series of 14 pediatric patients with ASD and ESLD, all candidates for orthotopic LT (OLT), has been reported previously by Concejero et al[9]. The authors concluded that in pediatric patients with variably sized ASDs but stable preoperative hemodynamics, there is apparently no impact on outcomes of LT. Tam et al[10] have also examined a small and similar case series, with comparable perioperative and long-term outcomes. Nevertheless, an editorial by Pigula et al[11] still contends that treatment of comorbidities must be a priority if disease combinations are complex. The risk of performing OLT in pediatric patients with unrepaired CHD is therefore believed prohibitive, but no criteria are yet defined to prioritize care. Although a number of studies have addressed either single cases or limited numbers of children with CHD who undergo LT, few articles offer detailed perioperative anesthesia management and prognostication of LT in the setting of ASD. The present report describes a pediatric candidate for LT with ASD, focusing on perioperative anesthesia management. It also highlights the important contribution of anesthesiologists to positive therapeutic outcomes in this complex clinical situation through their preoperative evaluations and perioperative diligence.
In pediatric LT candidates, especially those with CHD, preoperative evaluations and preparatory activities are key measures for surgical success and postoperative graft survival. Detailed medical histories and physical exams performed upfront may expose relevant ongoing patient issues. Preoperative assessments for pediatric LT procedures are aimed at nutrition, growth and development, cardio hepatic and renal function, respiratory and central nervous system compromise, coagulation status, and internal environment/electrolyte standing. A comprehensive preoperative assessment of the cardiovascular system is especially important[12]. In patients with CHD, the anesthesiologist must fully appreciate in advance of surgical proceedings the nature and pathophysiology of an existing disorder, the current level of cardiopulmonary function, and a patient’s exercise capacity. For ASD specifically, this includes an awareness of defect type and size, cardiac reserve, shunt conditions, and extent of cyanosis.
A complete plan for anesthesia must be formulated beforehand, based on thorough understanding of the patient's pathophysiologic state and severity of clinical symptoms, making cardiac preparations as needed. Communicating with both child and parents and educating them on the process of anesthesia are essential parts of the patient visit that serve to ease anxiety levels[13]. ASCE is a simple and easy method for examining various aspects of the right ventricular system, aiding in recognition of subtle morphologic anomalies or characterization of blood flow (patterns/timing) through suspected cardiac shunts or lesions[14]. One may inspect compartmental anatomic structures and flow dynamics to help differentiate intracardiac (i.e., patent foramen ovale, ASD) from extracardiac (i.e., pulmonary arteriovenous fistula) right-to-left shunting. If performed before surgery, ASCE affords better definition of a child’s cardiovascular status and existing shunt, suggesting possible preemptive repairs and bolstering intraoperative hemodynamic stabilization efforts.
Preoperative planning in such complex and difficult cases also calls for MDT input to optimize the decision-making process[15,16]. Unlike a single-source diagnostic and treatment model, MDT management may actually improve diagnostic accuracy and broaden therapeutic options or rationales[17]. The various experts work in concert to formulate scientific, reasonable, and standardized plans that minimize misdiagnosis and treatment errors, inspiring the best possible choices and greatly enhancing patient prognoses. We convened a MDT to discuss the condition of this pediatric patient. Given her cardiovascular status and hepatorenal function on admission, we debated whether major surgery of this sort would be tolerated and questioned the appropriateness of prior medical treatment, defect correction, or concurrent heart-liver transplantation. This discourse guided perioperative anesthesia management as well. Pertinent narcotics and rescue agents should be prepared before anesthesia induction. All drugs and required items must be within easy reach, and all equipment should be in standby mode. Crying upon entry to the operating room should be avoided to mitigate its hemodynamic impact. All invasive procedures should be conducted as late after sedation or anesthesia induction as possible.
In pediatric patients with ASD, substantial hemodynamic instability may occur during and after LT procedures (depending on defect extent and available cardiac reserve)[18] and potentially increase chances of new graft failure. Chronic cholestatic disease may also culminate in cirrhotic cardiomyopathy, marked by reduced cardiovascular responsiveness to stress, hypotension during anesthesia, and diminished sensitivity to catecholamines or vasopressors, clearly elevating perioperative cardiac risk. Various monitoring and treatment measures are therefore essential to maintain intraoperative hemodynamic stability. Intracardiac shunts may be bidirectional, causing hypoxemia, exaggerated pulmonary blood flow, and greater risk of intraoperative embolic events (i.e., air embolism). However, anesthesia-related maneuvers, such as positive-pressure ventilation, may help by redirecting abnormal flow. Given the particular manner of shunting associated with ASDs, any actions taken during anesthesia and surgery must not allow air into the circulatory system to permit distal organ embolization. Reperfusion is another phase during which embolism may occur[19]. Surgeons are reminded to flush donor organs very carefully, ensuring that no air enters during vascular anastomoses. Reducing congestion in a new graft will improve its viability and promote functional liver recovery.
It is best to individualize intraoperative anesthesia management in these patients, based on the primary underlying disease, type/features of existing CHD, present hemodynamic status, internal environmental conditions, and observed electrolyte disturbances. Adopted plans should include optimal drug dosing, appropriately regulated ventilatory settings, and customized patient monitoring, regularly analyzing blood gases and testing coagulation function. If conditions permit, muscle relaxation, intraoperative transesophageal echocardiography (TEE), and Doppler imaging of hepatic blood flow may be pursued. PiCCO monitoring is recommended to track hemodynamic changes and manage circulatory volume more effectively. Post-reperfusion syndrome (PRS)[20,21] is a serious transplant-related problem, especially in children with CHD. The fundamental objectives are to actively manage pathophysiologic changes upon portal vein flow restoration (to new liver), maintain stability of vital signs and the internal environment, and promote functional hepatic recovery. Once hepatic flow is re-established, the CVP level should not exceed 10 mmHg under stable BP conditions. The new liver should also be checked for unwanted hyperemic swelling.
Volume management is a difficult aspect of LT anesthesia in children with ASD. Goal-directed fluid therapy is one means of accurately guiding intraoperative fluid delivery[22,23]. The various stages of LT procedures call for different strategies and vasoactive drug rationales to accommodate a range of surgical maneuvers and prevent potential fluid imbalance, whether overload or deficiency. Circulatory data monitored by PiCCO may help guide volume management and vasoactive drug delivery to improve the perfusion of important organs. Parameters such as cardiac output (CO), cardiac index (CI), and others are tracked accordingly to assess the whole-heart index (reflecting cardiac function overall), thus largely avoiding pulmonary arterial catheter placement[24]. PiCCO monitoring is especially suited for patients plagued by hemodynamic instability or volume uncertainty[25]. TEE is similarly used to ascertain volume and contractility, thus guiding fluid management and inotropic therapy, and may be applied in complex cases as conditions permit.
Overall, human serum albumin is still the best available colloidal solution. Albumin is primarily given for preoperative volume expansion, increasing its concentration in children with severely low protein levels and voluminous ascites. Compound sodium acetate (without lactic acid) is also a suitable crystalloid for LT[26]. The incidence of hypoglycemia during pediatric LT is relatively high, so we routinely use 100 mL of 5% glucose and adjust the rate to blood glucose levels. Normal saline is not recommended for pediatric LT, given the potential for hyperchloric acidosis[27]. Red blood cell suspensions are infused at hemoglobin (Hb) levels < 7 g/dL to maintain Hb within a specified range (> 8 g/dL and < 10 g/dL). Plasma is infused in children with severe coagulation insufficiency (prothrombin times > 16 s).
Arterial or venous blood gases should be analyzed at all major perioperative stages of pediatric LT for dynamic monitoring of internal environmental fluctuations. Metabolic acidosis is the most common type of acid-base disturbance during LT. Children generally tolerate mild or moderate metabolic acidosis, but extreme levels (beta-hydroxybutyrate < -6 mmol/L) warrant 5% sodium bicarbonate solution. Perioperative electrolyte derangements are quite common, so appropriate electrolyte preparations may be used to supplement levels, based on blood gas analytics.
Because preoperative coagulation is abnormal in most of these children, thromboelastography (TEG) is advocated as a means of dynamically monitoring coagulation function during the perioperative period[28]. TEG enables comprehensive assessment of the entire coagulation process, including fibrinolysis and platelet function. Clotting substances should be carefully administered during the reperfusion phase to avoid intensifying the risk of portal vein or hepatic artery thrombosis. Anesthesiologists should assess surgical field bleeding and dynamic coagulation monitoring in tandem to maintain states of mild hypocoagulability in children. High blood viscosity and hematocrit levels may also reflect a predisposition to intraoperative thrombosis, so mild perioperative anemia should be maintained.
Intraoperative temperature monitoring should be done routinely. Blood temperatures measured by PiCCO catheter may detect core changes quicker and more accurately than nasopharyngeal and esophageal probes[28]. Heat preservation measures should be strengthened during operations, increasing ambient temperature of the operating room if needed and using HOTLINE fluid warmers (Smiths Medical Inc, Minneapolis, MN, United States) or variable temperature blankets.
A favorable postsurgical prognosis and careful postoperative management are inseparably linked. Once a child is transported to the ICU, continuous assessments of heart, lung, kidney, and other critical organs are imperative. Similarly, volume resuscitation and needed blood product infusion, correction of coagulation dysfunction, hemodynamic and internal environmental stability, positive trends in new liver function, and timely detection/treatment of complications are of utmost importance. Appropriate sedation and proper pain management may improve respiratory function and help shorten durations of ventilatory assistance[29]. Timely tracheal tube removal reduces the risk of lung infections as well. Through active and effective postoperative management, the majority of pediatric patients with CHD do well after LT procedures, experiencing no prolonged ICU stays or high morbidity. Their long-term prospects are excellent, some achieving resolution of cardiac issues[9].
Each pediatric patient demonstrates unique physical and pathophysiologic profiles, so a fixed therapeutic plan is usually ill-advised. One must individualize treatment strategies in a team effort, forging surgical success through good communication. As important members of a successful team, anesthesiologists must prove more flexible and creative under certain conditions. Children with ASD are at substantially greater than usual perioperative risk during OLT, given their limited cardiopulmonary reserves and functional states. Comprehensive preoperative cardiovascular assessments, rooted in ASCE use (to delineate intracardiac shunting) and MDT treatment planning, enable fuller appreciation of structural cardiac pathophysiologic effects and LT-related hemodynamic changes that impact new grafts. Active, effective monitoring and other measures must also be implemented to maintain hemodynamic stability during perioperative periods, avoid entry of bubbles into the circulation, and ease congestion in new grafts, all crucial for LT success and graft survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Teragawa H, Japan S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Fouquet V, Alves A, Branchereau S, Grabar S, Debray D, Jacquemin E, Devictor D, Durand P, Baujard C, Fabre M, Pariente D, Chardot C, Dousset B, Massault PP, Bernard D, Houssin D, Bernard O, Gauthier F, Soubrane O. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transpl. 2005;11:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Bates MD, Bucuvalas JC, Alonso MH, Ryckman FC. Biliary atresia: pathogenesis and treatment. Semin Liver Dis. 1998;18:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3680] [Cited by in RCA: 3703] [Article Influence: 161.0] [Reference Citation Analysis (1)] |
| 4. | Czeizel A. Biliary dysgenesis and congenital cardiovascular malformation association. Acta Paediatr Hung. 1987;28:63-80. [PubMed] |
| 5. | McDiarmid SV, Anand R, Lindblad AS; Principal Investigators and Institutions of the Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 273] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Bourdeaux C, Tri TT, Gras J, Sokal E, Otte JB, de Ville de Goyet J, Reding R. PELD score and posttransplant outcome in pediatric liver transplantation: a retrospective study of 100 recipients. Transplantation. 2005;79:1273-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, Fix OK, Kay N, Abecassis MI, Gheorghiade M, Flaherty JD. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Murray KF, Carithers RL Jr; AASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 9. | Concejero A, Chen CL, Liang CD, Wang CC, Wang SH, Lin CC, Liu YW, Yong CC, Yang CH, Jawan B, Cheng YF. Atrial septal defect in end-stage liver disease children before and after liver transplantation. Surgery. 2008;143:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Tam NL, Concejero AM, Liang CD. Fate of atrial septal defect in children with end-stage liver disease undergoing living donor liver transplantation. Transplant Proc. 2008;40:2510-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Pigula FA. Liver transplantation in children with congenital heart disease. Pediatr Transplant. 2007;11:461-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Png K, Veyckemans F, De Kock M, Carlier M, Sluysmans T, Otte JB, Reding R, Clement de Clety S, Sokal E, Van Obbergh L. Hemodynamic changes in patients with Alagille's syndrome during orthotopic liver transplantation. Anesth Analg. 1999;89:1137-1142. [PubMed] |
| 13. | Motoyama EK, Davis P. Smith's anesthesia for infants and children. 7th ed. Philadelphia, Pa: Mosby. 2006; 1256. |
| 14. | Arndt JW, Oyama MA. Agitated saline contrast echocardiography to diagnose a congenital heart defect in a dog. J Vet Cardiol. 2008;10:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Regunath H, Vasudevan A, Vyas K, Li-Chien C, Patil S, Terhune J, Whitt SP. A Quality Improvement Initiative: Developing a Multi-Disciplinary Team for Infective Endocarditis. Mo Med. 2019;116:291-296. [PubMed] |
| 16. | Mestres CA, Paré JC, Miró JM; Working Group on Infective Endocarditis of the Hospital Clínic de Barcelona. Organization and Functioning of a Multidisciplinary Team for the Diagnosis and Treatment of Infective Endocarditis: A 30-year Perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | He H, Yang L, Peng Y, Liu L, Xue Q, Gao S. The value of multidisciplinary team (MDT) management in the diagnosis and treatment of primary intrathoracic synovial sarcomas: a single-center experience. J Thorac Dis. 2021;13:600-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Feltracco P, Serra E, Milevoj M, Carollo C, Barbieri S, Vitale A, Gringeri E, Cillo U, Milanesi O, Ori C. Liver transplantation in children with congenital cardiac defects: a case report and a short literature review. Transplant Proc. 2013;45:2769-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Suriani RJ, Cutrone A, Feierman D, Konstadt S. Intraoperative transesophageal echocardiography during liver transplantation. J Cardiothorac Vasc Anesth. 1996;10:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Umbro I, Tinti F, Scalera I, Evison F, Gunson B, Sharif A, Ferguson J, Muiesan P, Mitterhofer AP. Acute kidney injury and post-reperfusion syndrome in liver transplantation. World J Gastroenterol. 2016;22:9314-9323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Fukazawa K, Yamada Y, Gologorsky E, Arheart KL, Pretto EA Jr. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J Cardiothorac Vasc Anesth. 2014;28:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Reydellet L, Blasco V, Mercier MF, Antonini F, Nafati C, Harti-Souab K, Leone M, Albanese J. Impact of a goal-directed therapy protocol on postoperative fluid balance in patients undergoing liver transplantation: a retrospective study. Ann Fr Anesth Reanim. 2014;33:e47-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Can J Anaesth. 2015;62:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Oren-Grinberg A. The PiCCO Monitor. Int Anesthesiol Clin. 2010;48:57-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Tibby S. Transpulmonary thermodilution: finally, a gold standard for pediatric cardiac output measurement. Pediatr Crit Care Med. 2008;9:341-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Shin WJ, Kim YK, Bang JY, Cho SK, Han SM, Hwang GS. Lactate and liver function tests after living donor right hepatectomy: a comparison of solutions with and without lactate. Acta Anaesthesiol Scand. 2011;55:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Boniatti MM, Filho EM, Cardoso PR, Vieira SR. Physicochemical evaluation of acid-base disorders after liver transplantation and the contribution from administered fluids. Transplant Proc. 2013;45:2283-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Wagener G. Liver anesthesiology and critical care medicine. New York: Springer. 2012; 473. |
| 29. | Feltracco P, Carollo C, Barbieri S, Milevoj M, Pettenuzzo T, Gringeri E, Boetto R, Ori C. Pain control after liver transplantation surgery. Transplant Proc. 2014;46:2300-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |