Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10629
Peer-review started: April 19, 2022
First decision: June 2, 2022
Revised: June 21, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 163 Days and 6.7 Hours
Mesenteric ischemia represents an uncommon complication of splanchnic vein thrombosis, and it is less infrequently seen in young women using oral contraceptives. Diagnosis is often delayed in the emergency room; thus, surgical inter
We report a 28-year-old female patient on oral contraceptives who presented with acute abdominal pain. Her physical examination findings were not consistent with her symptoms of severe pain and abdominal distention. These findings and her abnormal blood tests raised suspicion of acute mesenteric ischemia (AMI) induced by splanchnic vein thrombosis. Contrast-enhanced abdominal computed tomography revealed ischemia of the small intestine with portomesenteric and splenic vein thrombosis (PMSVT). We treated the case promptly by anticoagulation after diagnosis. We then performed delayed segmental bowel resection after thrombus regression and established collateral circulation guided by collaboration with a multidisciplinary team. The patient had an uneventful post
AMI induced by PMSVT should be considered in young women who are taking oral contraceptives and have acute abdominal pain. Prompt anticoagulation followed by surgery is an effective treatment strategy.
Core Tip: Mesenteric ischaemia is an uncommon complication of portomesenteric and splenic vein thrombosis (PMSVT) due to oral contraceptive. We report here a case of mesenteric ischaemia secondary to PMSVT, in which contrast-enhanced abdominal computed tomography played a key role in confirming the diagnosis. This report aims to contribute more information concerning the clinical characteristics as well as demonstrate that prompt anticoagulation followed by surgical invention is an effective strategy and importance of collaboration of multi-disciplinary teamwork for management of acute mensenteric ischemia caused by PMSVT.
- Citation: Zhao JW, Cui XH, Zhao WY, Wang L, Xing L, Jiang XY, Gong X, Yu L. Acute mesenteric ischemia secondary to oral contraceptive-induced portomesenteric and splenic vein thrombosis: A case report. World J Clin Cases 2022; 10(29): 10629-10637
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10629.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10629
Acute mesenteric venous thrombosis (AMVT) is an uncommon cause of acute abdominal disease and accounts for 1/1000 of emergency department admissions[1]. Contrast-enhanced computed tomography (CECT) is a highly sensitive technique for diagnosing MV thrombus (MVT), and can accurately visualize the extent of thrombosis in the splanchnic venous system[2]. Although thrombosis of the splanchnic venous system is uncommon, the disease is increasingly reported from better investigation facilities such as CECT[2]. Common causes of splanchnic vein thrombosis include underlying malignancy, cirrhosis, pancreatitis, and postoperative complications[3,4]. However, there are also reports that prothrombotic disease and oral contraceptives can cause portomesenteric venous thrombosis[5,6]. The etiology of MVT in 75% of patients can be identified, with MVT induced by oral contraceptives accounting for 4%–5% of all MVTs and for 9%–18% in young women[7]. The main treatment for MVT includes anticoagulation therapy to prevent thrombotic expansion, intestinal necrosis due to increased intestinal ischemia, and recurrent thrombosis. Multidisciplinary teamwork (MDT) is essential for the treatment of refractory acute mesenteric ischemia (AMI) secondary to portomesenteric and splenic vein thrombosis (PMSVT).
We here describe a young female patient with acute PMSVT and bowel ischemia due to oral contraceptives. She was treated with prompt anticoagulation therapy and subsequent segmental bowel resection guided by consultation with MDT.
A 28-year-old woman was admitted to the emergency department because of continuous abdominal pain around the umbilicus and epigastrium accompanied by abdominal distension, nausea, and vomiting for 11 d. She denied fever, diarrhea, constipation, hematochezia, and melena. She had been hospitalized in a local hospital due to these symptoms, where she underwent laboratory tests, abdominal ultrasonography, and abdominal/pelvic X-rays, and was diagnosed with ileus, and treatment included fasting and water deprivation, gastrointestinal decompression, intravenous antibiotics, and fluid replacement. None of these treatments alleviated her abdominal pain and distention but did reduce nausea and vomiting. However, the etiology of ileus could not be clearly defined and the patient’s symptoms did not improve significantly during hospitalization. She was then transferred to our hospital for further diagnosis and treatment.
The patient had been hospitalized for 11 d in a local hospital due to continuous abdominal pain accompanied by abdominal distension, nausea, and vomiting prior to admission to our hospital. The symptoms started 11 d ago and the patient complained of persistent abdominal pain and distention.
The patient had been taking oral contraceptives (ethinyl estradiol 0.03 mg and drospirenone 3 mg/d) to treat abnormal uterine bleeding, prescribed at the gynecology department of a local hospital for 13 mo prior to presentation.
The patient denied smoking or alcohol consumption. She had no personal or family history of thrombosis, thrombophilia, cancer, or pregnancy loss.
The patient’s initial vital signs were stable, including blood pressure of 108/84 mmHg, heart rate of 90 bpm, respiratory rate of 16 breaths/min, and body temperature of 36.4 °C. Her body mass index was 20.5 kg/m2, weight was 50 kg, and height was 156 cm. Physical examination revealed tenderness in the epigastrium and periumbilical region, and no significant peritoneal signs, and rectal examination was normal. These findings were not consistent with her symptoms of severe pain and distension.
Laboratory tests performed on the day of admission showed an elevated white blood cell count of 25.5 × 109/L (normal range, 3.5-9.5 × 109/L), C-reactive protein (CRP) of 206 mg/mL (normal range, 0.0-6.0 mg/mL), procalcitonin (PCT) of 0.6236 ng/mL (normal range, 0.0000-0.5000 ng/mL), and D-dimer of 15.88 μg/mL (normal range, 0.00-1.00 μg/mL). Hematocrit, platelet count, and kidney and liver function tests were normal. Prothrombin time was 13.6 s (normal range, 9.4-12.5 s), activated partial thromboplastin time (aPTT) was 30.2 s (normal range, 25.4-32.4 s), and international normalized ratio (INR) was 1.16 (normal range, 0.80-1.20). Arterial blood gas analysis showed the following: pH, 7.40; partial pressure of oxygen, 89 mmHg; partial pressure of carbon dioxide, 35 mmHg; bicarbonate concentration, 23.1 mmol/L; and lactate concentration, 1.1 mg/dL. Prothrombotic testing revealed the following: Protein S, 67% (normal range 55%-145%); protein C, 81% (normal range 60%-140%); antithrombin-III, 90% (normal range 75%-125%); anticardiolipin antibody IgG, 7.5 RU/mL (normal range 0-12 RU/mL); anti-β2-glycoprotein I antibodies, 14 RU/mL (normal range 0-20 RU/mL); and lupus anticoagulant ratio, 1.0 (normal range 0.8-1.2). None of the following were detected by polymerase chain reaction assay: Factor V leiden mutation, prothrombin G20210A mutation, and JAK2V617F mutation. These results were obtained 10 d after the specimens were sent to the laboratory.
Subsequent laboratory tests on day 7 after admission showed elevated CRP of 20.4 mg/mL and D-dimer of 7.24 μg/mL; other blood tests, for example, PCT, PT, aPTT, INR, white blood cell count, and plasma lactate concentration, returned to normal. Routine blood tests performed on days 15 and 31 after admission were normal.
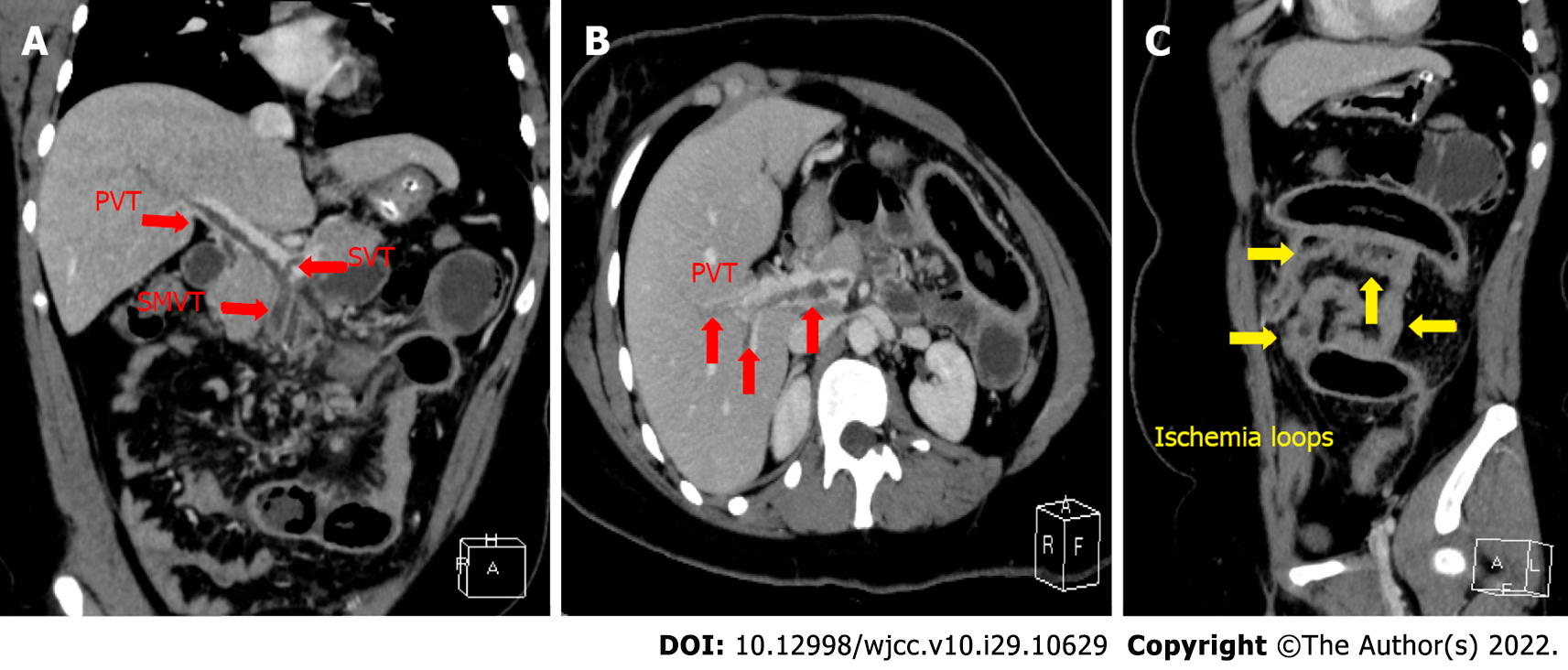
Imaging examinations performed on the day of admission showed that chest radiography was normal, and abdominal radiography revealed scattered air fluid levels with no free air. Abdominal ultrasound showed no fluid collection. CECT of the abdomen and pelvis revealed a large thrombus in the superior mesenteric vein (SMV), right branch of the portal vein (PV), and main vessels of the PV and splenic vein (SV). CECT showed segmented small bowel with increased fat concentration in the intestinal mesentery, intestinal wall thickening, and luminal stenosis. The segmented small bowel was dilated proximal to the intestinal stenosis, and there were no signs of intestinal pneumatosis or vein gas. There were no signs of intra-abdominal cancer or inflammatory conditions (Figure 1).
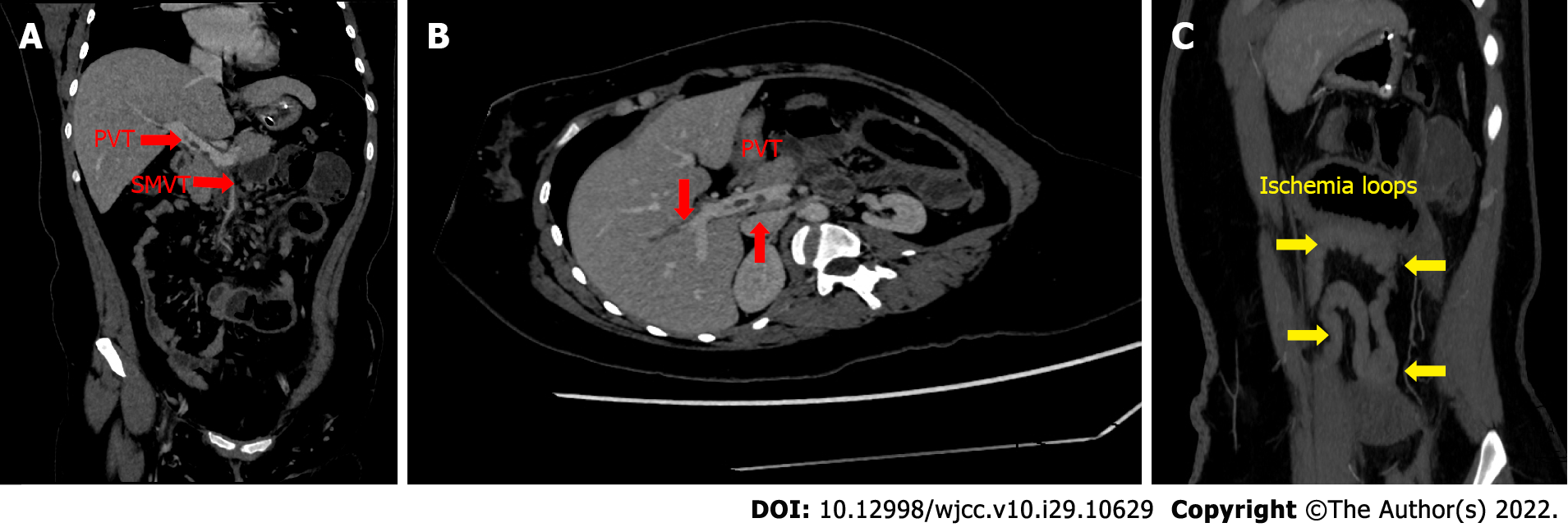
Follow-up CECT on day 7 after admission showed that the large thrombus in the right branches and main vessels of the PV and SV had slightly regressed (Figure 2).
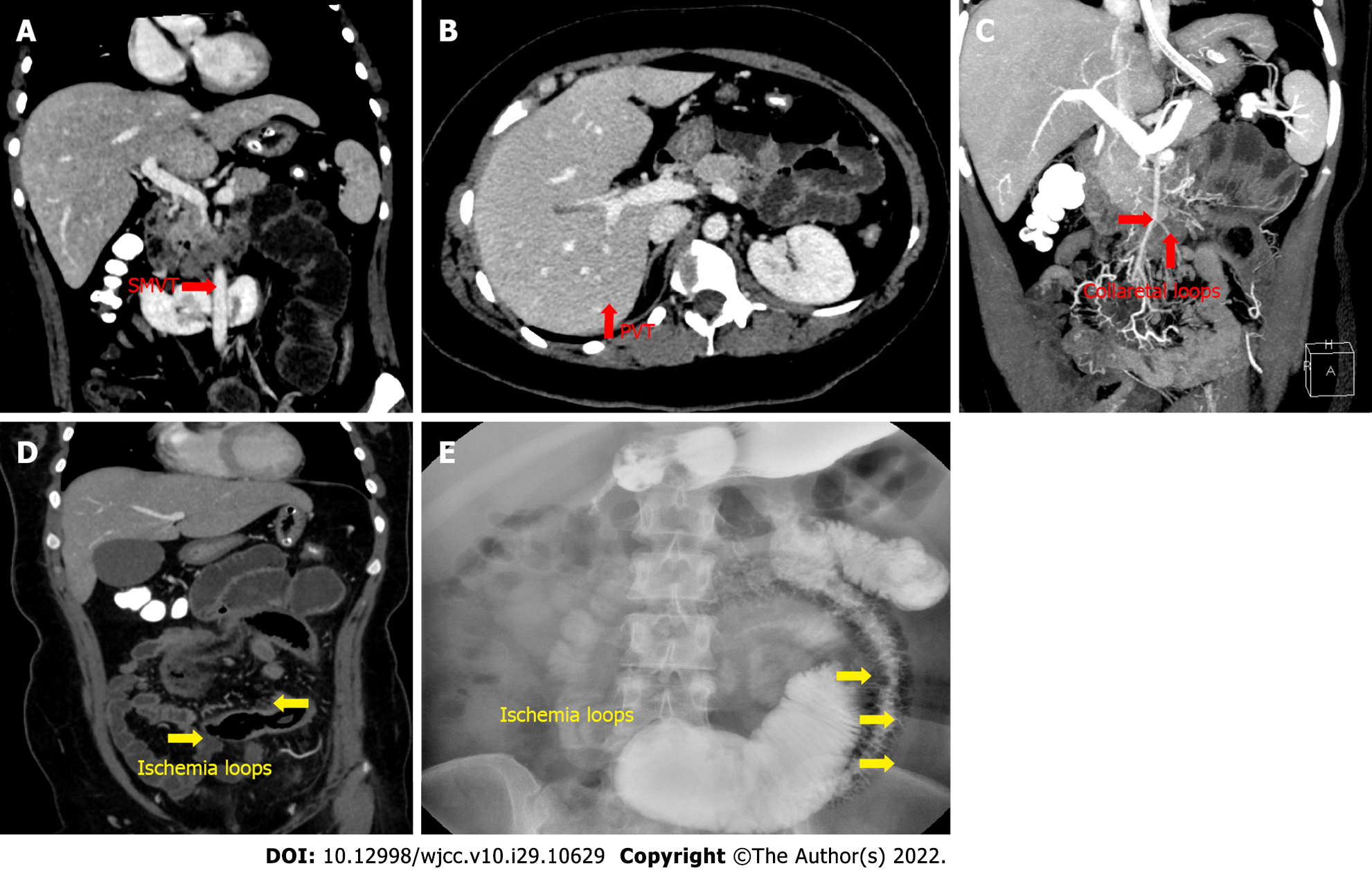
Follow-up CECT on day 15 after admission showed that the thrombus in the right branches of the PV had significantly regressed. There were no signs of thrombus in the main PV and SV, and the MVT had slightly subsided and collateral circulation was established (Figure 3). Bowel dilation was improved, and total gastroenterography revealed intestinal segmentation and luminal stenosis (Figure 3).
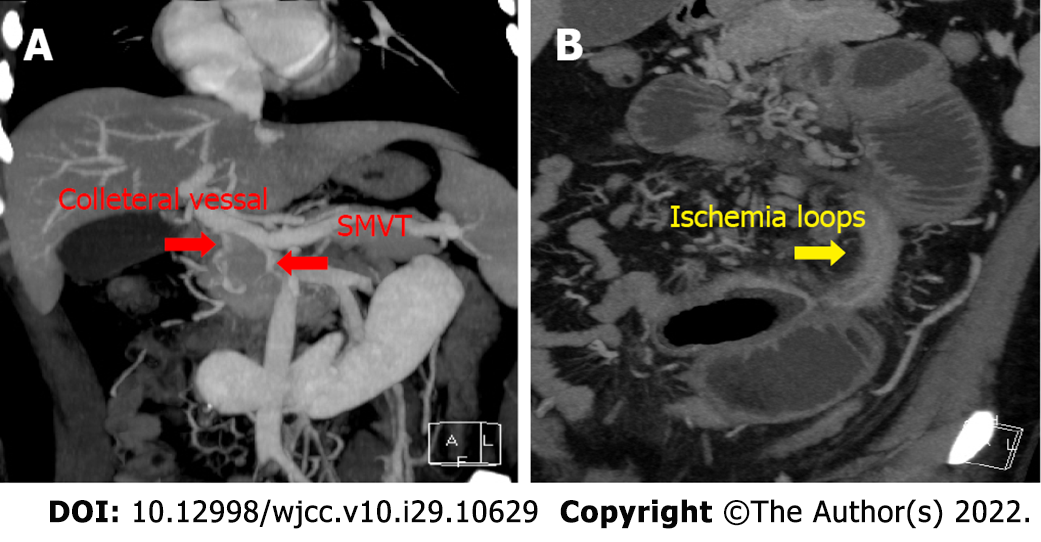
Follow-up CECT on day 31 after admission revealed complete resolution of PV and SV thrombi, obliteration of the remnant thrombus in the SMV, and a well-developed collateral circulation. CECT also showed segmental stricture of the distal jejunum with proximal dilatated jejunum (Figure 4).
The final diagnosis was AMI secondary to PMSVT caused by oral contraceptives.
After admission, we suspected that incomplete intestinal obstruction caused by AMI may be related to splanchnic vein thrombosis as CECT showed no other potential cause of bowel obstruction. It was difficult to decide whether to use anticoagulation or exploratory laparotomy as a therapeutic strategy. Hence, multidisciplinary consultations were conducted with emergency surgeons, vascular surgeons, anesthetists, anticoagulation specialists, nutrition specialists, and gynecologists, and treatment plans were: Gastrointestinal decompression; total parenteral nutrition; intravenous antibiotic cefminox 1.0 g, twice daily; and anticoagulation with low molecular weight heparin (LMWH) sodium 5000 U (100 U/kg), subcutaneous injection twice daily, which was commenced as the initial conventional therapy. Thrombolytic therapy with urokinase was added if this treatment was ineffective. If necessary, endovascular treatment by transcatheter thrombolysis was planned. The treatment endpoint was to delay the removal of diseased bowel after the thrombus had completely resolved or a collateral circulation was established. Further urgent surgical intervention should be performed when intestinal necrosis with impending perforation or peritonitis is suspected. The gynecologists recommended that oral contraceptive be discontinued immediately, and a contraceptive ring for birth control should be used when the patient recovered.
During the treatment, the patient’s vital signs, along with routine investigations and blood tests, were monitored regularly. Clinical features and plasma lactate level were used as markers of progression to extensive bowel ischemia and bowel infarction, and PT was maintained within the normal range. Her symptoms gradually improved after 1 wk of conservative management. Follow-up CECT showed that the large thrombus had slightly regressed, and blood tests showed elevated CRP of 20.4 mg/mL and D-dimer of 7.24 μg/mL, and other measures had returned to normal. With continuing parenteral nutrition and anticoagulation, her clinical condition stabilized 15 d after initiation of treatment; her routine blood tests were normal, and follow-up CECT showed that the thrombus in the right branches of the PV had regressed. There were no signs of thrombus in the main PV and SV, and the MVT had subsided and collateral circulation was established. Additionally, bowel dilatation was improved, and total gastroenterography revealed segmental intestinal luminal stenosis.
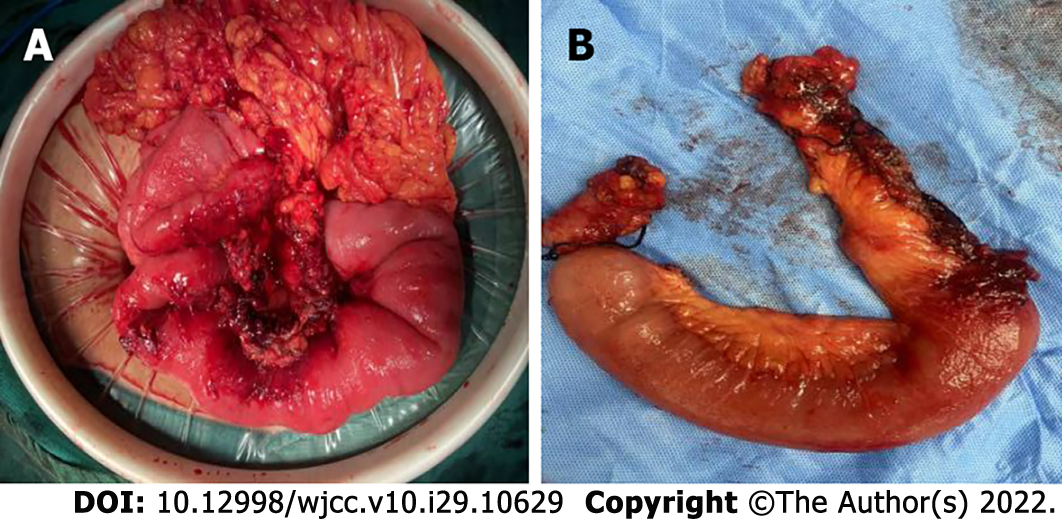
To improve her treatment, multidisciplinary consultation was conducted again. Total enteral nutrition was performed with nasointestinal tubes according to the nutrition specialist’s advice, and the anticoagulation specialists recommended that 20 mg rivaroxaban should be given orally for anticoagulation instead of LMWH injection, and the PT level was monitored according to her prothrombotic test results. However, intermittent abdominal discomfort and fullness occurred when she tried to increase nutrient intake. We were concerned about bowel stricture and discussed the necessity of surgical resection of the narrow bowel with the patient. On day 31 after admission, follow-up CECT revealed that the thrombus in the PV and SV had completely resolved, remnant thrombus in the SMV was obliterated, and collateral circulation was well developed. She underwent scheduled laparotomy, in which 25 cm of stenotic small bowel was resected and a functional end-to-end anastomosis was created using staplers. Intraoperative inspection of the stenosed jejunum showed significant narrowing of the intestinal lumen, severely fibrotic bowel wall, and swollen mesentery (Figure 5). As planned, the nutrition team advised starting total parenteral nutrition after surgery, and simultaneously, she initiated subcutaneous LMWH 5000 U/d twice a day as advised by the anticoagulation specialist. On postope
The patient was instructed to take one tablet of 20 mg rivaroxaban orally once daily for at least 6 mo. During the subsequent follow-up period, the patient has not complained of any other discomfort.
We administered successful treatment for oral contraceptive-induced PMSVT with bowel ischemia, under multidisciplinary collaboration. Our treatment was predominantly conservative with systemic anticoagulation and supportive treatment that was initiated as soon as the diagnosis was confirmed by CECT. Surgical exploration is limited to patients with persistent or worsening symptoms and the development of frank perforation or signs of peritonitis, following comprehensive examination including physical findings, laboratory data, and imaging results. Planned delayed bowel resection was performed after the thrombi were completely resolved or collateral circulation was established. Our case suggests that emergency surgeons should consider intestinal ischemia induced by MVT in young women who are taking oral contraceptives and present with severe acute abdominal pain. Although MVT only accounts for 6%–9% of cases of AMI[1], oral contraceptive-related MVT accounts for 4%–5% of all MVTs, and is a potentially life-threatening condition[7]. Although splanchnic thrombosis is rare, the widespread use of CECT in patients with abdominal pain can advance diagnosis from 1 wk to 1 d[8]. A filling defect in the MV is the most common finding on CT imaging in patients with MVT. Characteristic CT findings of intestinal wall ischemia include bowel wall thickening and persistent enhan
Bowel ischemia caused by PMSVT is an uncommon complication in young women on oral contraceptives. Clinicians should consider AMI in young women on oral contraceptives who develop sudden severe abdominal pain not consistent with physical examination findings. Our case demonstrated that prompt anticoagulation followed by surgical intervention is an effective strategy for the management of AMI induced by splanchnic thrombosis, and avoidance of thrombotic expansion and excessive bowel resection. We also highlight the importance of multidisciplinary collaboration in the management of acute ischemia due to PMSVT.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dubois-Silva Á, Spain; Pappachan JM, United Kingdom; Tripathi D, United Kingdom S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Singal AK, Kamath PS, Tefferi A. Mesenteric venous thrombosis. Mayo Clin Proc. 2013;88:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Rajesh S, Mukund A, Arora A. Imaging Diagnosis of Splanchnic Venous Thrombosis. Gastroenterol Res Pract. 2015;2015:101029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Thatipelli MR, McBane RD, Hodge DO, Wysokinski WE. Survival and recurrence in patients with splanchnic vein thromboses. Clin Gastroenterol Hepatol. 2010;8:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | De Stefano V, Martinelli I. Splanchnic vein thrombosis: clinical presentation, risk factors and treatment. Intern Emerg Med. 2010;5:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Osti NP, Sah DN, Bhandari RS. Successful medical management of acute mesenteric ischemia due to superior mesenteric and portal vein thrombosis in a 27-year-old man with protein S deficiency: a case report. J Med Case Rep. 2017;11:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Hunninghake J, Murray BP, Ferraro D, Gancayco J. Acute intestinal ischaemia from a portal vein thrombosis in a young female smoker on an oral contraceptive. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Zhang J, Duan ZQ, Song QB, Luo YW, Xin SJ, Zhang Q. Acute mesenteric venous thrombosis: a better outcome achieved through improved imaging techniques and a changed policy of clinical management. Eur J Vasc Endovasc Surg. 2004;28:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Haddad MC, Clark DC, Sharif HS, al Shahed M, Aideyan O, Sammak BM. MR, CT, and ultrasonography of splanchnic venous thrombosis. Gastrointest Radiol. 1992;17:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Milone M, Di Minno MN, Musella M, Maietta P, Iaccarino V, Barone G, Milone F. Computed tomography findings of pneumatosis and portomesenteric venous gas in acute bowel ischemia. World J Gastroenterol. 2013;19:6579-6584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 524] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 12. | DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 648] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 13. | Sturm L, Bettinger D, Klinger C, Krauss T, Engel H, Huber JP, Schmidt A, Caca K, Thimme R, Schultheiss M. Validation of color Doppler ultrasound and computed tomography in the radiologic assessment of non-malignant acute splanchnic vein thrombosis. PLoS One. 2021;16:e0261499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Tessler FN, Gehring BJ, Gomes AS, Perrella RR, Ragavendra N, Busuttil RW, Grant EG. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. 1991;157:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Oliva IB, Davarpanah AH, Rybicki FJ, Desjardins B, Flamm SD, Francois CJ, Gerhard-Herman MD, Kalva SP, Ashraf Mansour M, Mohler ER 3rd, Schenker MP, Weiss C, Dill KE. ACR Appropriateness Criteria ® imaging of mesenteric ischemia. Abdom Imaging. 2013;38:714-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Reginelli A, Genovese E, Cappabianca S, Iacobellis F, Berritto D, Fonio P, Coppolino F, Grassi R. Intestinal Ischemia: US-CT findings correlations. Crit Ultrasound J. 2013;5 Suppl 1:S7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Meaney JF. Non-invasive evaluation of the visceral arteries with magnetic resonance angiography. Eur Radiol. 1999;9:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Yang S, Fan X, Ding W, Liu B, Meng J, Wang K, Wu X, Li J. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93:e270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019;94:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 20. | Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15:407-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Salim S, Ekberg O, Elf J, Zarrouk M, Gottsäter A, Acosta S. Clinical implications of CT findings in mesenteric venous thrombosis at admission. Emerg Radiol. 2018;25:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1435] [Article Influence: 478.3] [Reference Citation Analysis (2)] |
| 23. | Janczak DT, Mimier MK, McBane RD, Kamath PS, Simmons BS, Bott-Kitslaar DM, Lenz CJ, Vargas ER, Hodge DO, Wysokinski WE. Rivaroxaban and Apixaban for Initial Treatment of Acute Venous Thromboembolism of Atypical Location. Mayo Clin Proc. 2018;93:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban vs warfarin in the management of acute non-neoplastic portal vein thrombosis. Vascul Pharmacol. 2019;113:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 25. | Paraskeva P, Akoh JA. Small bowel stricture as a late sequela of superior mesenteric vein thrombosis. Int J Surg Case Rep. 2015;6C:118-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kim HK, Chun JM, Huh S. Anticoagulation and delayed bowel resection in the management of mesenteric venous thrombosis. World J Gastroenterol. 2013;19:5025-5028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Yang J, Shen L, Zheng X, Zhu Y, Liu Z. Small bowel stricture complicating superior mesenteric vein thrombosis. J Huazhong Univ Sci Technolog Med Sci. 2012;32:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Kim JY, Ha HK, Byun JY, Lee JM, Yong BK, Kim IC, Lee JY, Park WS, Shinn KS. Intestinal infarction secondary to mesenteric venous thrombosis: CT-pathologic correlation. J Comput Assist Tomogr. 1993;17:382-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |