Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10606
Peer-review started: April 13, 2022
First decision: June 27, 2022
Revised: July 7, 2022
Accepted: September 7, 2022
Article in press: September 7, 2022
Published online: October 16, 2022
Processing time: 168 Days and 19 Hours
Left cardiac myxoma (CM) is the most common benign tumor of primary cardiac tumors, but because of its special position caused by pathological physiology cha
A 67-year-old male patient suddenly appeared numbness and weakness of the left lower limb and could not walk without obvious incentive. The patient was finally diagnosed as left CM complicated with acute lower limb arterial embolism after completing cardiac ultrasound, computer tomography angiography, and histopathological analysis, such as hematoxylin-eosin stain staining, immunohistochemistry and special staining including alcian blue staining and periodic acid schiff staining. Arterial thrombosis was removed successfully by femoral artery thrombectomy, postoperative numbness and weakness of the patient's left lower limb disappeared, skin temperature became warm, and dorsal foot artery puls
Although CM is rare, it may be considered as the source of embolism in patients with acute limb ischemia. Repeated loss of thrombus on the tumor and its surface may lead to repeated embolism of peripheral vessels. Cardiac ultrasound is helpful for early diagnosis. Here, we use this case re
Core Tip: Cardiac myxoma (CM), although rare, is a unique cause of acute arterial embolism, we report a rare case of this type and provide insights into the diagnosis and treatment of acute lower extremity arterial embolism caused by shedding of left CM. We hope that by improving the recognition of the disease, we can reduce intervention time and thus avoid delays in diagnosis and treatment.
- Citation: Meng XH, Xie LS, Xie XP, Liu YC, Huang CP, Wang LJ, Zhang GH, Xu D, Cai XC, Fang X. Cardiac myxoma shedding leads to lower extremity arterial embolism: A case report. World J Clin Cases 2022; 10(29): 10606-10613
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10606.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10606
The most common primary tumor of the heart is left cardiac myxoma (CM). CM is rare and benign and has an incidence of approximately 0.5 per 1 million people per year[1]. There are thrombi of different sizes, and once they fall off, they can easily cause embolisms in the peripheral blood vessels, thus causing serious complications. The clinical manifestations of left atrial myxoma vary from asym
A 67-year-old male patient suddenly developed numbness and weakness of the left lower extremity without an obvious origin 6 h ago, and he could not walk.
Six hours prior to admission, the patient suddenly developed numbness and weakness of the left lower extremity without an obvious origin, so he went to the emergency department of our hospital.
Although he had a history of hypertension and was treated with oral antihypertensive drugs, his blood pressure was well controlled. He denied a history of atrial fibrillation. In 2016, he had an arterial embolism in both lower extremities; he also had a history of bilateral femoral artery thrombectomy, and long-term treatment with oral Bayer aspirin.
The patient was not a descendant of a consanguineous marriage, and there was no family history of a similar disease.
During physical examination, the bilateral femoral artery and the right popliteal, posterior tibial, and dorsal pedis arteries were palpable, but the left popliteal, posterior tibial, and dorsal pedis arteries were not palpable. The skin temperature of the right lower extremity was normal, but the left lower extremity was cool and pale.
The laboratory results at admission were normal, including routine blood tests, coagulation function, liver and kidney function, tumor markers, and urine and stool tests, but the d-dimer results (670 µg/L) were not normal (0-500 µg/L).
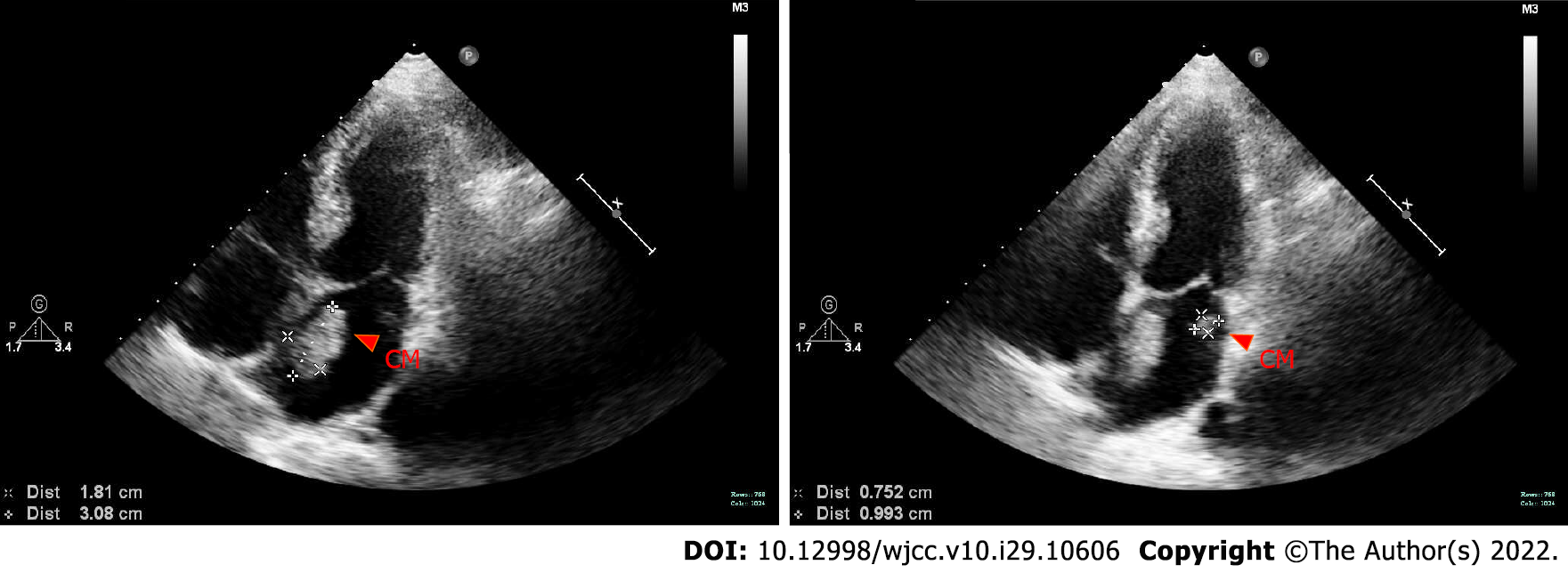
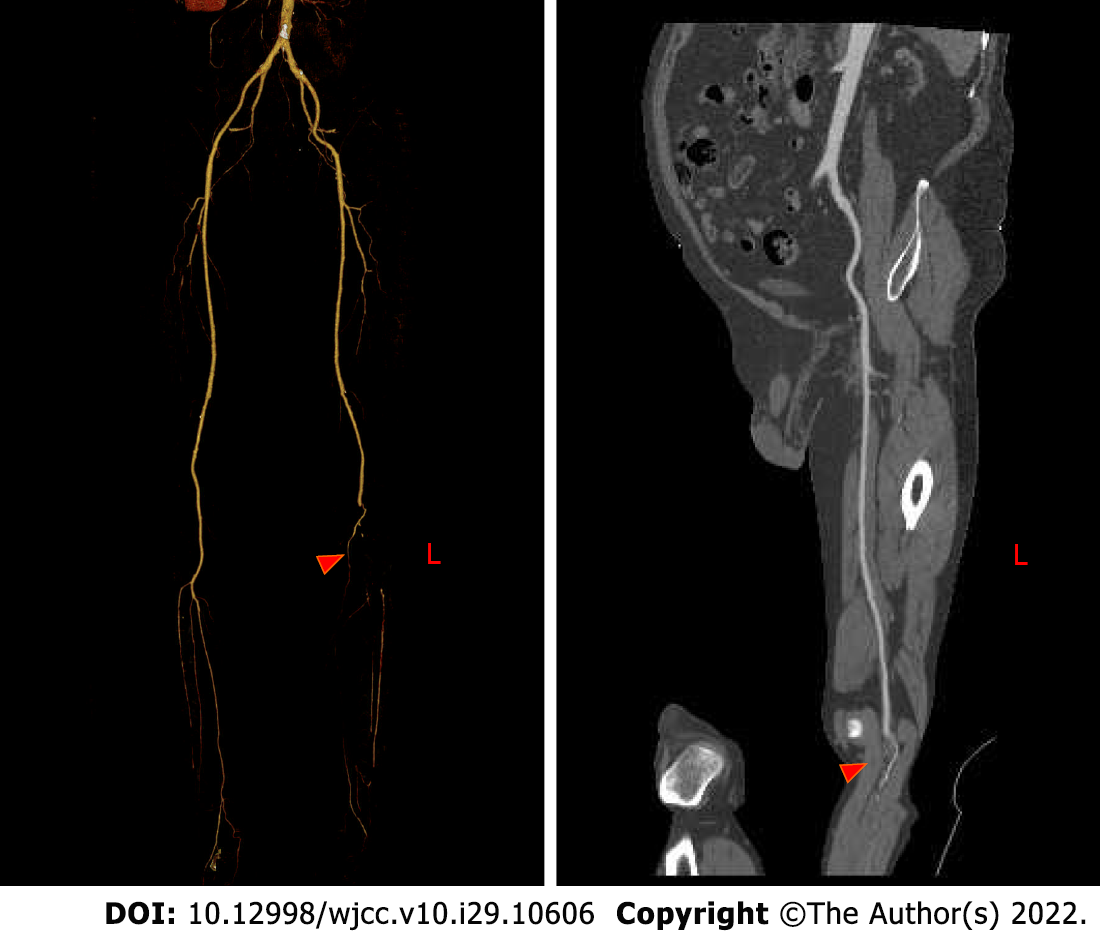
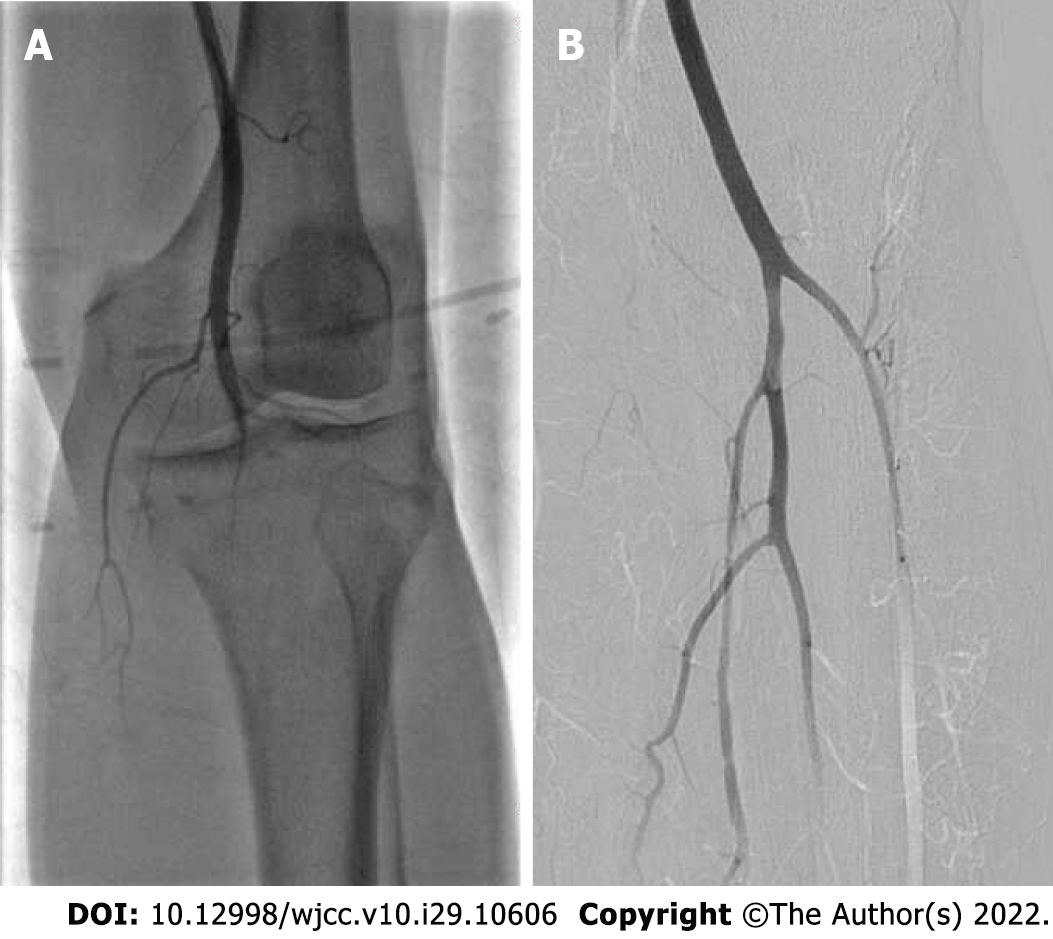
Bedside echocardiography showed two abnormal echo groups in the left atrium (shown by the red arrow), which were 3.1 cm × 1.8 cm and 1.0 cm × 0.8 cm in size, and there was an acceptable range of motion (Figure 1). Computer tomography angiography (CTA) of the lower extremity vessels showed that the left popliteal artery was not visualized shown by the red arrow, so a lumen embolism was considered (Figure 2). The patient’s left lower extremity ischemic symptoms were severe, and there was a risk of lower extremity necrosis. The surgical indications were clear. After completing the preoperative examination and excluding surgical contraindications, an emergency left femoral artery incision was made, and a thrombectomy was performed.
The patient recovered well after the operation. The numbness and weakness of the left lower extremity disappeared, the skin temperature became warmer, and the left popliteal, posterior tibial and dorsal foot arteries were palpable.
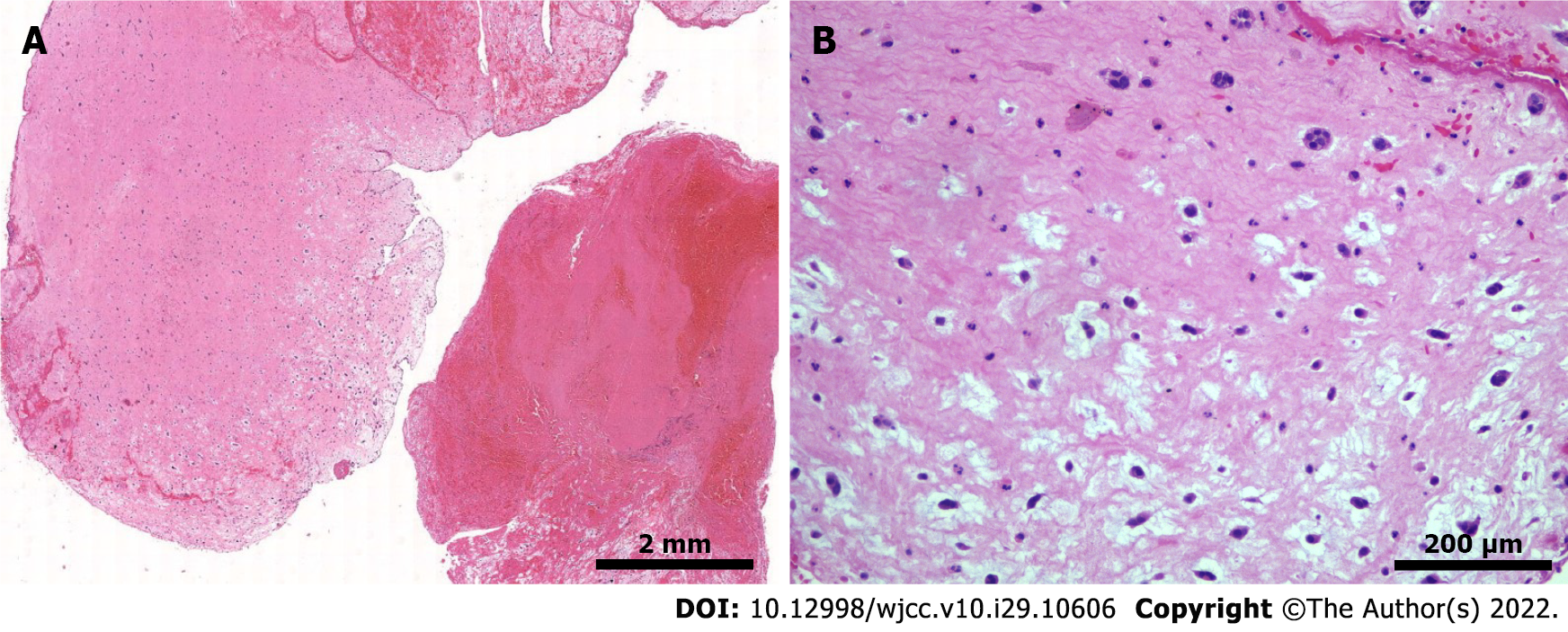
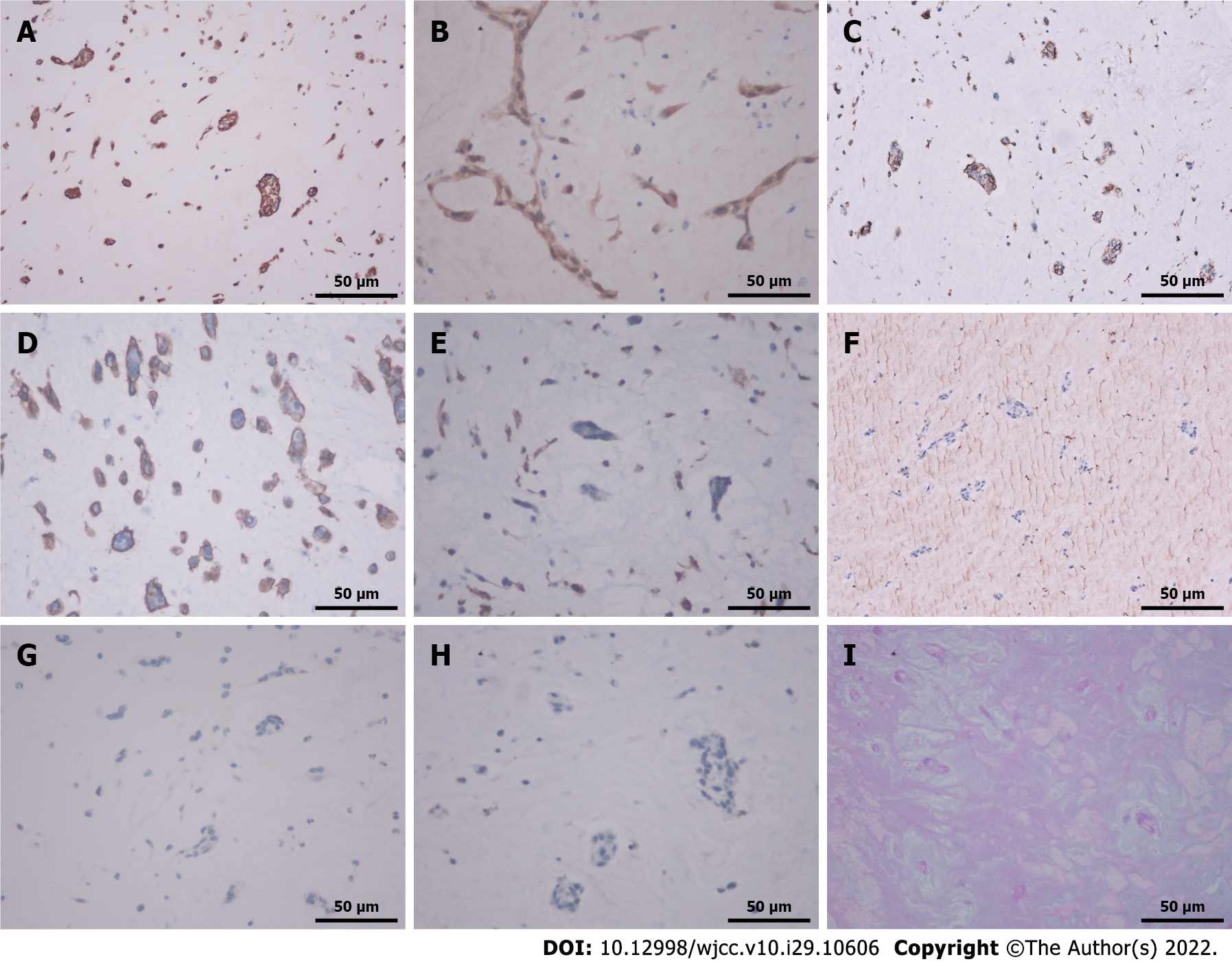
Assessment of the pathological specimen was based on microscopic examination. Hematoxylin-eosin staining showed thrombi and many myxoid materials, and free clusters of round and polygonal cells were seen inside, which was consistent with the pathological characteristics of myxoma (Figure 3). Immunohistochemical studies showed that vimentin, calretinin, CD31, and CD34 were positive, while CD68, cytokeratin (CK), CK5/6, and factor 8-related antigen (F8) were negative. Alcian blue staining (AB) and periodic acid Schiff staining (PAS) were also positive (Figure 4). The above pathological findings were consistent with the pathological features of myxoma[5-7]. Combined with the results of preoperative echocardiography and postoperative pathological examination, it can be inferred that the acute lower extremity arterial embolism was caused by shedding of a left CM.
Acute lower limb arterial embolism secondary to CM.
A 10-cm incision was made along the surface of the left common femoral artery under the inguinal ligament, and the common femoral artery, deep femoral artery and superficial femoral artery were dissociated and exposed. After an intravenous injection of 5000 units of heparin, the common femoral artery and deep femoral artery were blocked. The superficial femoral artery and the common femoral artery were longitudinally incised to the origin of the deep femoral artery; the incision was approximately 1 cm long, and the left superficial femoral artery had smooth, unrestricted blood flow and mild stenosis. The lateral popliteal artery was not visualized; thus, a lumen embolism was considered, and the peroneal artery, posterior tibial artery, and proximal tibial artery occlusion were observed (Figure 5A). The guide wire catheter passed through the diseased section and continued to the distal ends of the anterior tibial artery and posterior tibial artery. The guide wire passed through without resistance, and the 4F Fogarty thrombectomy catheter reached the popliteal artery. After multiple thrombectomies, the thrombus had a total length of approximately 10 cm and was dark red and transparent at the head end. The final angiography showed that the blood flow of the superficial femoral artery, popliteal artery, tibiofibular trunk, anterior tibial artery, peroneal artery and dorsal pedis artery was smooth (Figure 5B), and the blood supply to the foot was significantly improved compared with the previous angiography. Blood vessels and various layers of tissue were sutured, and the operation ended. After the operation, the left dorsal artery pulse was increased, the skin temperature of the foot was significantly higher, and the thrombus was examined by histopathology. After the operation, 4100 units of low molecular weight heparin were administered, and anticoagulants were subcutaneously injected every 12 h.
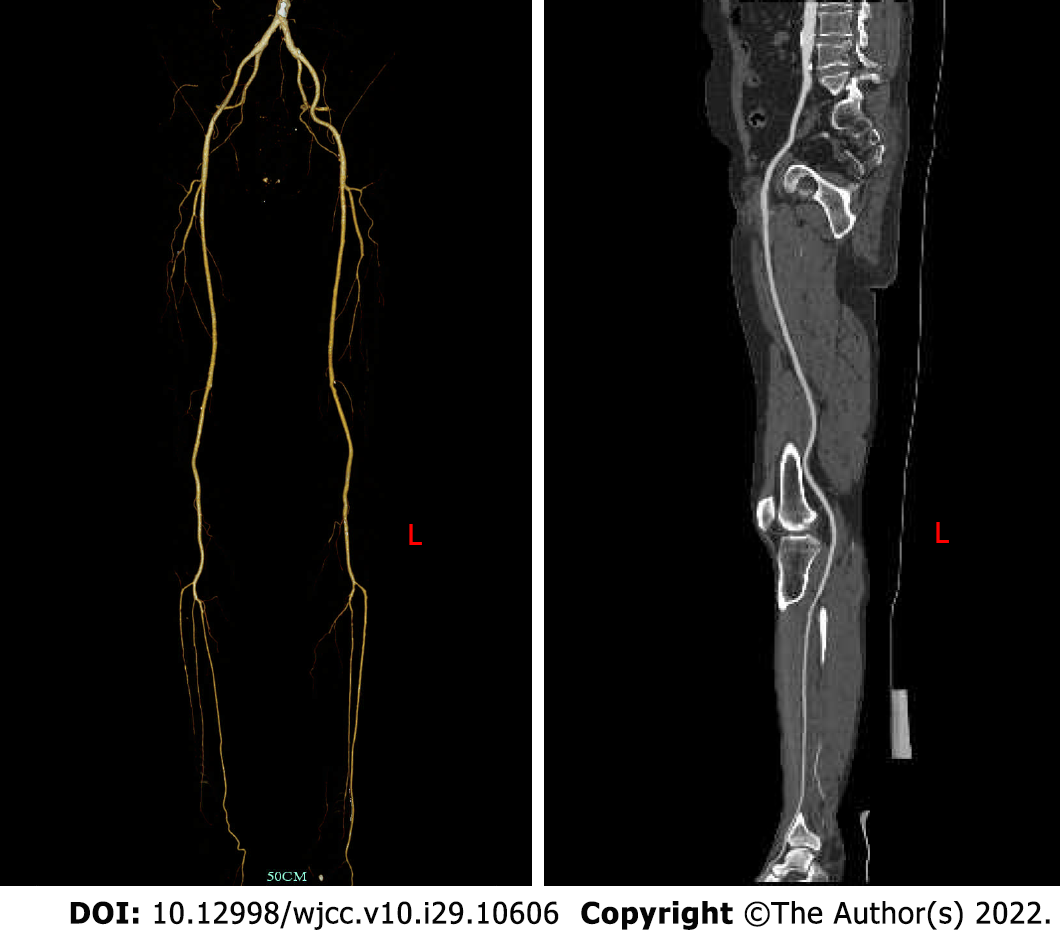
The patient had no postoperative complications, such as incision infection, hemorrhage, hematoma, pseudoaneurysm or lower extremity ischemia–reperfusion injury, and was discharged 6 d after the operation. One month after discharge, there were no obvious ischemic symptoms in the left lower extremity, and the left popliteal artery and dorsal pedis artery pulse were accessible; at the 6th months after discharge, CTA of the lower extremity blood vessels showed that the blood flow of the left popliteal artery, anterior tibial artery and peroneal artery was still unobstructed (Figure 6). We recom
Most CMs occur at the edge of the fossa ovalis, and approximately 10% occur at other sites[8]. The clinical manifestations and signs of CM depend on the location and activity of the tumor. Most left CMs have atypical symptoms, including hemodynamic changes, systemic symptoms, and the triad of peripheral vascular embolism[9]. However, approximately 3.2%-46.4% of patients with CM are asymptomatic[1,10]. CM occurs in all age groups, with an average age of 42-66 years[11]. Familial CM is less frequently reported in younger patients than in older patients. CM is most commonly found in the left atrium; however, unusual locations of CM include the right atrium (0.7% to 7.5%), right ventricle (0.7% to 2.5%), left ventricle (0.7% to 3.6%), and heart valves (less than 1%) [12]. Approximately 72% to 92% of patients have been reported that CM is located in the left atrium by most studies [5].
The diagnosis of CM is based on clinical examination, electrocardiogram, transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), chest computed tomography, and chest or cardiac magnetic resonance imaging, and there is no evidence that a specific blood test is useful in diagnosis. The clinical examination of the patient includes auscultation for possible systolic or diastolic murmurs, which may be heard if the myxoma prolapses or blocks the valve orifice of the heart. Heart murmurs are not specific to CM. Furthermore, concomitant organic heart disease may be confused with CM. There is no standard electrocardiograph diagnosis for CM because arrhythmias are common in other heart diseases [13]. Nevertheless, the accuracy of TTE and TEE in diagnosing CM is as high as 90% to 96%. TTE is the most convenient and useful examination for patients with heart disease, especially cardiac tumors[2]. Echocardiography is the most reliable method for diagnosing left atrial myxoma in every hospital. In conventional clinical practice, it can quickly and safely analyze the structure and location of cardiac tumors. If TTE is unable to identify the nature, structure, and activity of CM, TEE can accurately assess tumor characteristics. The diagnostic test for CM has a high sensitivity of more than 90%[14]. Systemic embolic events have been reported in 30% to 50% of patients with left atrial myxoma, half of which have cerebrovascular involvement. In addition, left atrial myxoma can be repeatedly shed, resulting in recurrent acute lower extremity arterial embolism[4]. Acute limb ischemia is caused by a sudden decrease in limb perfusion that threatens limb vitality, which can lead to loss of sensation, movement, or even a limb, and requires urgent intervention. Of peripheral arterial emboli, 80% have a cardiac origin (rheumatic valve disease, atrial fibrillation, appendicitis, wall thrombus, infective endocarditis, prosthetic valve thrombus, or tumor), and only 1.5% are cardiac tumors that require surgery to save a limb[4,15]. Therefore, accurate and timely diagnosis and treatment are essential to restore normal blood flow. Lower extremity arterial ultrasound and CTA are crucial for the timely diagnosis and assessment of acute lower extremity arterial embolism. Lower extremity arteriotomy, thrombectomy with a Fogarty catheter, and catheter thrombolysis are the first-line interventions for acute lower extremity ischemia[16]. The lower extremity vascular CTA showed mild stenosis of the left superficial femoral artery; the left popliteal artery was not visualized; and the peroneal artery, posterior tibial artery, and proximal end of the anterior tibial artery were occluded. Considering the patient's medical history, it was determined that it was caused by shedding of the emboli of the left atrial myxoma. Echocardiography showed multiple abnormal echo groups in the left atrium, and the intraoperative findings and postoperative pathology were essentially consistent with the preoperative examination. Pathological immunohistochemical results showed that the emboli stained positive for vimentin, calretinin, CD31 and CD34 but were negative for CD68, CK, CK5/6 and F8. In addition, AB and PAS were positive, and the above results are consistent with the pathological characteristics of CM[5-7].
Although rare, myxoma is a unique cause of acute arterial embolism that needs to be fully recognized. Systemic anticoagulation and immediate surgical thrombectomy are first-choice treatments for acute lower extremity arterial embolism. Early surgical tumor resection is the main treatment method to prevent further cardiovascular complications[4]. CM is an identifying indicator in patients with acute limb ischemia presenting to the emergency department, and the timely use of bedside echocardiography can aid in diagnosis and reduce intervention time, which is crucial for a good prognosis in such cases.
CM, although rare, is a unique cause of acute arterial embolism, and echocardiography and arterial CTA are critical for the timely diagnosis of this disease. We report a rare case of this type and provide insights into the diagnosis and treatment of acute lower extremity arterial embolism caused by shedding of the left CM. We hope that by improving the recognition of the disease, we can reduce intervention time and thus avoid delays in diagnosis and treatment.
We thank all the histology technologists at the Department of Pathology, Hangzhou First People’s Hospital for their technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashimoto K, Japan; Ito S, Japan S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Samanidis G, Khoury M, Balanika M, Perrea DN. Current challenges in the diagnosis and treatment of cardiac myxoma. Kardiol Pol. 2020;78:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Mahavar RK, Arora D, Singh A, Mishra M. Recurrent cardiac myxoma: A case report. Ann Card Anaesth. 2021;24:490-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Ho KKF, Barsoum R, Shepherd B, McGahan T. Bilateral acute lower limb ischemia secondary to complete embolization of cardiac myxoma. J Vasc Surg. 2020;71:1759-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Mathew R, Agrawal N, Aggarwal P, Jamshed N. Atrial Myxoma Presenting as Acute Bilateral Limb Ischemia. J Emerg Med. 2019;57:710-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Jantuan E, Chiu B, Shen F, Oudit GY, Sergi C. The tumor microenvironment may trigger lymphoproliferation in cardiac myxoma. Transl Oncol. 2021;14:100911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Sussman MA. Atrial myxoma: the cardiac chameleon. Eur Heart J. 2020;41:4346-4348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Ma H, Niu Y, Tian M, Liu L, Gong W, Zheng M. A study of 399 cardiac tumors: Characteristics of echocardiography and pathological features. Echocardiography. 2022;39:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Chang WS, Li N, Liu H, Yin JJ, Zhang HQ. Thrombolysis and embolectomy in treatment of acute stroke as a bridge to open-heart resection of giant cardiac myxoma: A case report. World J Clin Cases. 2021;9:7572-7578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Liu SZ, Hong Y, Huang KL, Li XP. Multiple left ventricular myxomas combined with severe rheumatic valvular lesions: A case report. World J Clin Cases. 2021;9:5535-5539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Cianciulli TF, Cozzarin A, Soumoulou JB, Saccheri MC, Méndez RJ, Beck MA, Gagliardi JA, Lax JA. Twenty Years of Clinical Experience with Cardiac Myxomas: Diagnosis, Treatment, and Follow Up. J Cardiovasc Imaging. 2019;27:37-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Bianchi G, Margaryan R, Kallushi E, Cerillo AG, Farneti PA, Pucci A, Solinas M. Outcomes of Video-assisted Minimally Invasive Cardiac Myxoma Resection. Heart Lung Circ. 2019;28:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Karabinis A, Samanidis G, Khoury M, Stavridis G, Perreas K. Clinical presentation and treatment of cardiac myxoma in 153 patients. Medicine (Baltimore). 2018;97:e12397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Nehaj F, Sokol J, Mokan M, Jankovicova V, Kovar F, Kubaskova M, Mizera S. Outcomes of Patients with Newly Diagnosed Cardiac Myxoma: A Retrospective Multicentric Study. Biomed Res Int. 2018;2018:8320793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Jiang CX, Wang JG, Qi RD, Wang W, Gao LJ, Zhao JH, Zhang CX, Zhou MC, Tu X, Shang MS, Yao Y. Long-term outcome of patients with atrial myxoma after surgical intervention: analysis of 403 cases. J Geriatr Cardiol. 2019;16:338-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Liu L, Zhang X, Huang M, Li J, Zhao Z, Huang J. The effectiveness of percutaneous mechanical thrombectomy in the treatment of acute thromboembolic occlusions of lower extremity. Vascular. 2021;29:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Teodoro C, Bertanha M, Girard FPCM, Sobreira ML, Yoshida RA, Moura R, Jaldin RG, Yoshida WB. Results of treatment of acute occlusions of limb arteries at a university hospital - retrospective study. J Vasc Bras. 2020;19:e20200031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |