Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10600
Peer-review started: March 15, 2022
First decision: April 13, 2022
Revised: April 15, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: October 16, 2022
Processing time: 197 Days and 14.6 Hours
Diagnosis of emphysematous pyelonephritis has been described around the world for some decades, frequently associated with Escherichia coli and other anaerobic, gas-forming bacteria and mostly in patients living with diabetes. We present a case report of emphysematous pyelonephritis in a non-diabetic patient caused by Serratia fonticola as well as a brief literature review to draw attention to this rare pathogen as a cause of pyelonephritis.
A 38-year-old female presented with fever, severe pain in the right flank and changes in urinary habits. She was admitted, and emphysematous pyelonephritis was confirmed by an abdominal computerized tomography and urine cultures; the latter showed Serratia fonticola as a single pathogen. After 3 d of being treated with piperacillin/tazobactam and percutaneous drainage she became afebrile, and the gas presence reduced.
Emphysematous pyelonephritis infections in non-diabetic patients are rare but can be severe and life-threatening. This case suggests that Serratia fonticola infection can occur in patients undergoing invasive or instrumented procedures.
Core Tip: Serratia fonticola may still be rare as a human pathogen, either associated to asymptomatic conditions or merely a bystander among other agents, but its incidence may relate to severe cases when patients undergo invasive, instrumented procedures or underlying conditions, as presented in this case of the female who developed emphysematous pyelonephritis.
- Citation: Villasuso-Alcocer V, Flores-Tapia JP, Perez-Garfias F, Rochel-Perez A, Mendez-Dominguez N. Serratia fonticola and its role as a single pathogen causing emphysematous pyelonephritis in a non-diabetic patient: A case report. World J Clin Cases 2022; 10(29): 10600-10605
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10600.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10600
Severe necrotizing infection caused by anaerobic, gas-forming bacteria in renal parenchyma generates a condition known as emphysematous pyelonephritis (EPN), frequently linked to Escherichia coli[1,2].
It has been stated that even when agent-related virulence factors, along with the underlying health condition of patients, may predispose them to EPN, diabetes mellitus with poor glycemic control has been independently associated with the odds of developing EPN[3]. Furthermore, evidence in Esche
EPN is an unusual condition originally associated with patients with diabetes mellitus[5,6] and commonly reported in single clinical case reports, although also more recently in at least one systematic review with meta-analysis[6]. The present communication was aimed at providing insights into a case where a non-diabetic patient developed EPN due to a Serratia fonticola (S. fonticola) infection and the clinical manifestations derived from the pyelonephritis caused by this uncommon agent.
A 38-year-old illiterate housewife from rural southeast Mexico, with bilateral urolithiasis attended a urology consultation at a highly specialized public hospital in Yucatan Mexico, as she had had a fever between 38 ℃ and 40 ℃ for the previous 2 d, accompanied by severe pain in the right flank irradiating to the ipsilateral suprapubic region and reported changes in frequency and quality of urination.
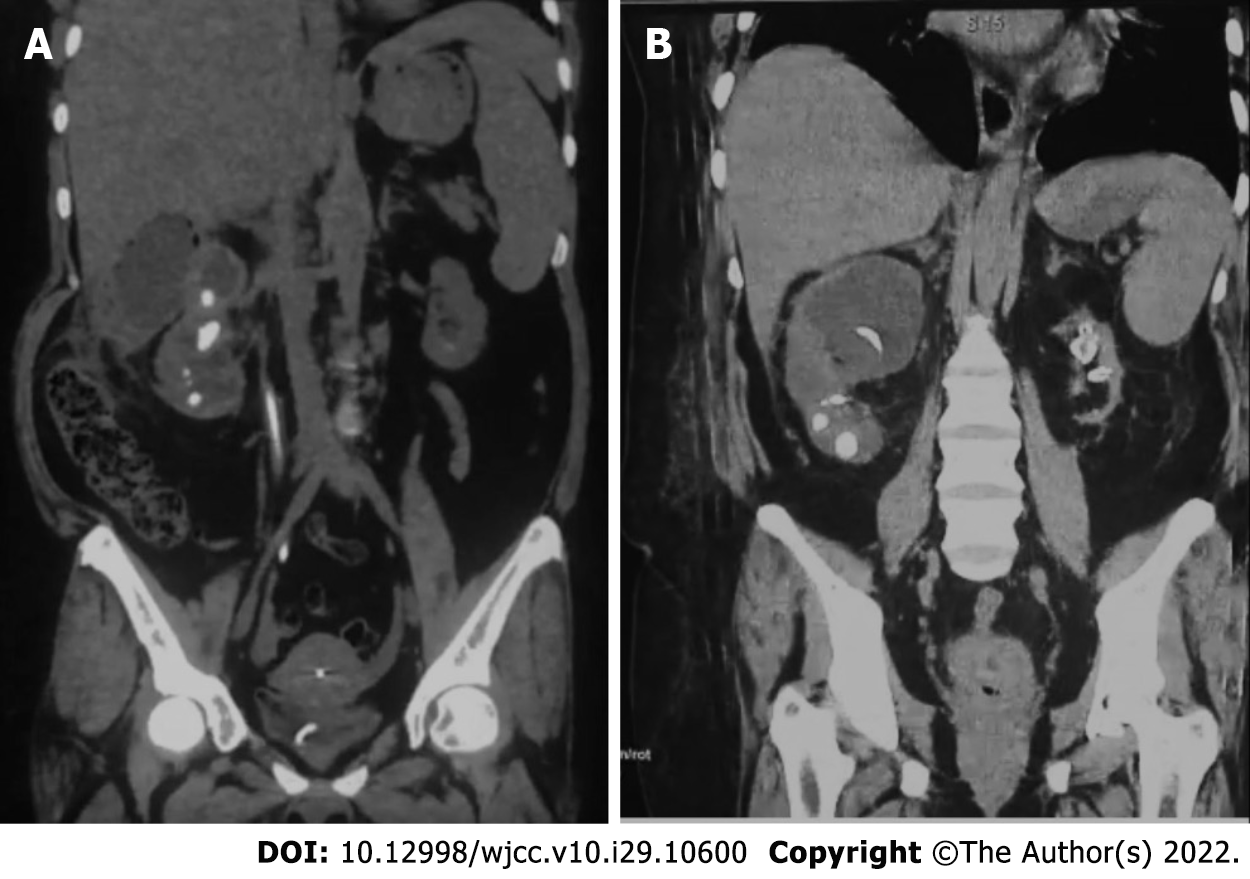
She was admitted for in-hospital care, and abdominal computerized axial tomography was performed, along with blood and urine tests, including cultures. Tomography indicated the presence of gas in the right kidney indicating EPN Huang IV and confirmed the presence of a nonfunctional left kidney (Figure 1). Blood culture was unremarkable, while urine culture reported S. fonticola as a single pathogen, sensitive to most antibiotics but resistant to trimethoprim and nitrofurantoin.
Antecedents included that she debuted with symptomatic bilateral urinary lithiasis in 2015, and she was diagnosed with chronic kidney disease Class Kidney Diseases Global Outcomes 3A that same year. Left renal exclusion was confirmed using radiotracer mercaptoacetyltriglycine gammagraphy.
In 2016 and 2017 second look percutaneous nephrolithotomy was performed, finding right ureteral stenosis due to lithiasis; JJ catheters were temporarily placed in the right kidney and removed after treatment.
Fever and right flank pain irradiating to the ipsilateral suprapubic region persisted.
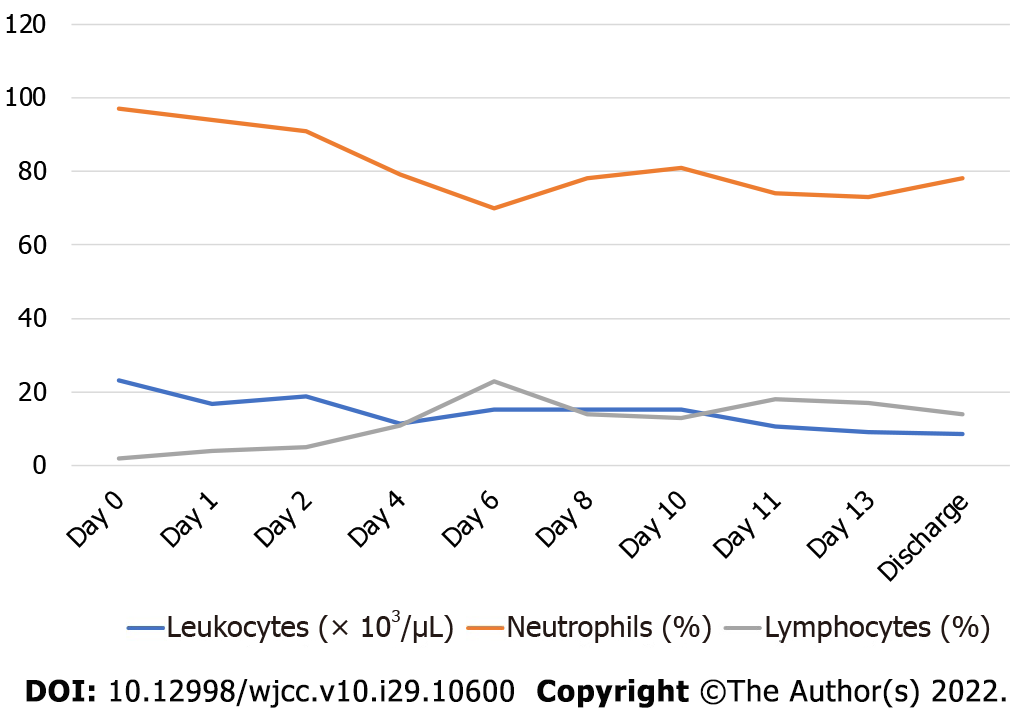
Diabetes and hypertension had been ruled out at every hospitalization, as the patient only registered normal values of fasting glucose and blood pressure. The patient was treated with piperacillin/ tazobactam; a combined endoscopic intrarenal surgery was performed to place a metallic catheter and to treat with percutaneous drainage. After 3 d of antibiotic, she became afebrile. The gas presence due to anaerobic bacteria reduced according to imaging, and a new culture was performed on day 4, which was reported negative. Her white blood cell count improved markedly. However, after 8 d her fever relapsed, and new studies were performed, finding Pseudomonas aeruginosa as a single pathogen in her urine culture along with an increase in neutrophils and white blood cell count in general. She was therefore treated with meropenem (Figure 2).
After 10 d of treatment, with tomographic and laboratory evidence for the resolution of the acute signs and symptoms, she was discharged.
EPN due to urolithiasis with S. fonticola isolation with subsequent infection due to Pseudomonas aeruginosa.
Percutaneous drainage and antibiotics
Between her discharge in 2018 and January 2021, the patient had two more hospitalizations related to urinary tract infections but none related to S. fonticola.
In previous studies, diabetes was present in 96%-98% of all patients with EPN[2,3], and renal calculi was an antecedent in approximately 7.84%[2]. In general, gas-forming bacteria are the cause of emphy
Before the 1990s, nephrectomies were considered the most effective form of intervention, as more conservative treatment had a mortality rate of 60%-80%[8]. To date, mortality has reduced significantly and even though diabetes is still recognized as a factor increasing the propensity for proliferation of gas-forming pathogens[9], it is also recognized that diabetes is not a sine qua non condition. The management of patients is somewhat controversial. While some authors suggest that the nephrectomy could provide a better prognosis in subjects with two or more risk factors, other authors suggest that medical management and drainage are currently sufficient for treating these patients[7,10].
EPN can become a recurrent condition, as reported by Ramanathan et al[11] in a three-case report communication. In the case of our patient EPN reappeared while the patient was still under clinical care, giving the opportunity to be treated promptly. Altogether, current evidence regarding EPN indicates that this condition is still potentially fatal, particularly when a timely diagnosis is not established or suboptimal treatment is provided[7]. Katib et al[12] in 2020 reported S. fonticola as a community-acquired urinary tract infection, affirming that globally this represented the third clinical case in which isolation of S. fonticola in an asymptomatic patient was reported. Therefore, the present case review may be the first reporting of a severe symptomatic infection due to S. fonticola causing EPN[13].
Community-acquired infections are susceptible to improvement with various antibiotics, but the evidence from a case in which biliary tract infection due to S. fonticola occurred in a hospital envir
Nephrectomy has been the treatment of choice when performed immediately to treat emphysematous nephritis and urosepsis[12]. For the case we have presented, nephrectomy was not an option, as the patient had a single functional kidney. Percutaneous drainage has been linked to lower mortality than medical management or emergency nephrectomy, and therefore it was the treatment of choice[7,15].
In 1979, Serratia spp. was identified as a member of the Enterobacteriae family. This study included the type, neotype and the genetic relationship of these strains by means of DNA-DNA hybridization and the relationship between them and the Serratia spp. strains known so far (S. marcescens, S. liquiefaciens, S. plymuthica and S. rubidaea)[16].
Serratia spp. belong to the Enterobacteriaceae family, which has been widely studied over the years, and there have been multiple changes to the number of species included in this family. Strains of this pathogen produce DNAase, gelatinase and lipase extracellularly, which means they can be easily differentiated from other Enterobacteriaceae genera[17].
S. fonticola is a peritrichous, motile rod-shaped gram-negative enterobacteria that was first isolated in water and soil. There are reports that show S. fonticola can also be isolated in plants, aquatic envir
In 1990, it was identified as a human pathogen in a patient wound infection abscess localized on the thigh. Since then, S. fonticola has mainly been identified in open wounds (mostly due to trauma) and in the respiratory, gastrointestinal and urinary tracts. Recently, some reports have identified S. fonticola in the biliary tract[13,18,20].
Based on clinical practice, early identification of this pathogen is important due to its structure and characteristics, which makes it resistant to a wide range of antibiotics. This can be linked to the fact that S. fonticola harbors an inducible chromosomal B-lactamase AMPC and a FOMA-type, which not only generates resistance to antibiotics (including cephalosporins up to the third generation) but can also allow factors for antimicrobial resistance to be transmitted to other bacteria, thus increasing the risk of a rapid evolution from a sectorial infection to a polymicrobial bacteremia and even septicemia[13,21,22].
Usually, Serratia spp. are nosocomial pathogens that may colonize medical devices such as urinary catheters and bronchoscopes[23]. However, in a study by Samonis et al[24] where Serratia spp. was identified as a community-acquired infection, the predominant species was S. marcescens. S. fonticola was first described as a bystander in a patient in whom it was isolated along with other agents in a urine culture. The patient was a man with neurogenic bladder paraplegia due to a combat injury and related to urinary tract catheterization[14]. Even though Serratia spp. have been linked to cases of nosocomial infections[25] and uncomplicated urinary tract infection in more susceptible patients with underlying urinary conditions and/or chronic kidney disease, S. fonticola may also be linked to severe clinical manifestations, as in this case, which was exacerbated by impaired renal function and urolithiasis, leading to EPN.
S. fonticola may still be rare as a human pathogen, either linked to asymptomatic conditions or merely a bystander among other infective agents, but its incidence may lead to severe cases when patients undergo invasive, instrumented procedures or have underlying conditions, as presented here in this case of the female who developed EPN.
To nurses and medical staff at the Urology Department.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boopathy Vijayaraghavan KM, India; Yuksel S, Turkey S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Ubee SS, McGlynn L, Fordham M. Emphysematous pyelonephritis. BJU Int. 2011;107:1474-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Tseng CC, Wu JJ, Wang MC, Hor LI, Ko YH, Huang JJ. Host and bacterial virulence factors predisposing to emphysematous pyelonephritis. Am J Kidney Dis. 2005;46:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Ho CH, Liu SP, Fan CK, Tzou KY, Wu CC, Cheng PC. Insulin Downregulated the Infection of Uropathogenic Escherichia coli (UPEC) in Bladder Cells in a High-Glucose Environment through JAK/STAT Signaling Pathway. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Rafiq N, Nabi T, Rasool S, Sheikh RY. A Prospective study of Emphysematous Pyelonephritis in Patients with Type 2 Diabetes. Indian J Nephrol. 2021;31:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Nabi T, Rafiq N, Rahman MHU, Rasool S, Wani NUD. Comparative study of emphysematous pyelonephritis and pyelonephritis in type 2 diabetes: a single-centre experience. J Diabetes Metab Disord. 2020;19:1273-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Desai R, Batura D. A systematic review and meta-analysis of risk factors and treatment choices in emphysematous pyelonephritis. Int Urol Nephrol. 2022;54:717-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Eswarappa M, Suryadevara S, John MM, Kumar M, Reddy SB, Suhail M. Emphysematous Pyelonephritis Case Series From South India. Kidney Int Rep. 2018;3:950-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Somani BK, Nabi G, Thorpe P, Hussey J, Cook J, N'Dow J; ABACUS Research Group. Is percutaneous drainage the new gold standard in the management of emphysematous pyelonephritis? J Urol. 2008;179:1844-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Falagas ME, Alexiou VG, Giannopoulou KP, Siempos II. Risk factors for mortality in patients with emphysematous pyelonephritis: a meta-analysis. J Urol. 2007;178:880-5; quiz 1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Lu YC, Chiang BJ, Pong YH, Huang KH, Hsueh PR, Huang CY, Pu YS. Predictors of failure of conservative treatment among patients with emphysematous pyelonephritis. BMC Infect Dis. 2014;14:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Ramanathan V, Nguyen PT, Van Nguyen P, Khan A, Musher D. Successful medical management of recurrent emphysematous pyelonephritis. Urology. 2006;67:623.e11-623.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Katib AA, Dajam M, Alqurashi L. Serratia fonticola microbe presented as a community-acquired urinary tract infection (UTI): a case report. J Ideas Health. 2020;3:226-227. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Das D, Pal DK. Double J stenting: A rewarding option in the management of emphysematous pyelonephritis. Urol Ann. 2016;8:261-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Aljorayid A, Viau R, Castellino L, Jump RL. Serratia fonticola, pathogen or bystander? IDCases. 2016;5:6-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Ziegelmüller BK, Szabados B, Spek A, Casuscelli J, Stief C, Staehler M. Emphysematous pyelonephritis: Case report and literature overview. Urologia. 2018;85:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Gavini F, Ferragut C, Izard D, Trinel P, Leclerc H, Lefebvre B, Mossel D. Serratia fonticola, a New Species from Water. Int J Syst Evol Microbiol. 1979;29:92-101. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Farmer JJ 3rd, Davis BR, Hickman-Brenner FW, McWhorter A, Huntley-Carter GP, Asbury MA, Riddle C, Wathen-Grady HG, Elias C, Fanning GR. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985;21:46-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 444] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Müller HE, Steigerwalt AG, Brenner DJ. Isolation of Serratia fonticola from birds. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;261:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Kunimoto D, Rennie R, Citron DM, Goldstein EJ. Bacteriology of a bear bite wound to a human: case report. J Clin Microbiol. 2004;42:3374-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Ruan J, Wang W, Zhang T, Zheng T, Zheng J, Yu S, Yu D, Huang Y. Establishment of a duplex real-time qPCR method for detection of Salmonella spp. and Serratia fonticola in fishmeal. AMB Express. 2020;10:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Bollet C, Gainnier M, Sainty JM, Orhesser P, De Micco P. Serratia fonticola isolated from a leg abscess. J Clin Microbiol. 1991;29:834-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | van Hoek AH, Veenman C, van Overbeek WM, Lynch G, de Roda Husman AM, Blaak H. Prevalence and characterization of ESBL- and AmpC-producing Enterobacteriaceae on retail vegetables. Int J Food Microbiol. 2015;204:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Hai PD, Hoa LTV, Tot NH, Phuong LL, Quang VV, Thuyet BT, Son PN. First report of biliary tract infection caused by multidrug-resistant Serratia fonticola. New Microbes New Infect. 2020;36:100692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Samonis G, Vouloumanou EK, Christofaki M, Dimopoulou D, Maraki S, Triantafyllou E, Kofteridis DP, Falagas ME. Serratia infections in a general hospital: characteristics and outcomes. Eur J Clin Microbiol Infect Dis. 2011;30:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Johnson A, Watson D, Dreyfus J, Heaton P, Lampland A, Spaulding AB. Epidemiology of Serratia Bloodstream Infections Among Hospitalized Children in the United States, 2009-2016. Pediatr Infect Dis J. 2020;39:e71-e73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |