Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10575
Peer-review started: December 28, 2021
First decision: March 13, 2022
Revised: March 26, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: October 16, 2022
Processing time: 274 Days and 23.8 Hours
Primary hepatic neuroendocrine carcinoma (NEC) is rare, and a combination with hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) is extremely rare. To date, only four combination cases have been reported. The present paper describes the fifth patient.
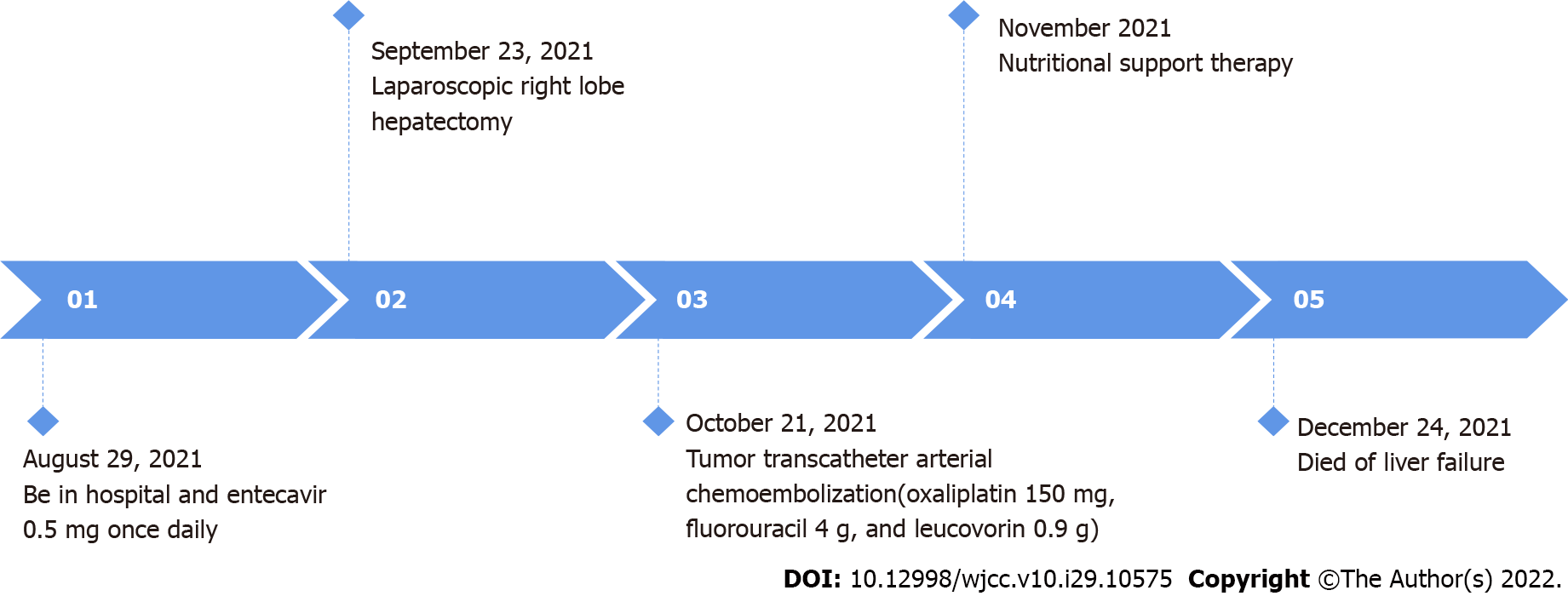
A 32-year-old Chinese man with chronic hepatitis B was hospitalized for persistent upper abdominal pain. Abdominal computed tomography (CT) examination revealed a liver mass. The tumor was located in the 7th and 8th segments of the liver, and CT and magnetic resonance imaging findings were consistent with the diagnosis of HCC. Laboratory examinations revealed the following: Alanine aminotransferase, 243 U/L; aspartate aminotransferase, 167 U/L; alpha-fetoprotein, 4519 μg/L. Laparoscopic right lobe hepatectomy was performed on the liver mass. Postoperative pathology showed low differentiation HCC plus medium and low differentiation CCA combined with NEC. One month after the surgery, the patient suffered from epigastric pain again. Liver metastasis was detected by CT, and tumor transcatheter arterial chemoembolization was performed. Unfortunately, the liver tumor was progressively increased and enlarged, and after 1 mo, the patient died of liver failure.
This is a rare case, wherein the tumor is highly aggressive, grows rapidly, and metastasizes in a short period. Imaging and laboratory tests can easily misdiagnose or miss such cases; thus, the final diagnosis relies on pathology.
Core Tip: Hepatocellular carcinoma (HCC) is the most common subtype of primary liver cancer. However, the combination of HCC, cholangiocarcinoma, and neuroendocrine carcinoma exhibiting three differentiation pathways is extremely rare. This has been described previously only in four patients. We report a case of a similar tumor in a 32-year-old man. It was diagnosed according to the computed tomography and magnetic resonance imaging findings and histopathology. This report aims to raise awareness and improve the treatment of the disease.
- Citation: Wu Y, Xie CB, He YH, Ke D, Huang Q, Zhao KF, Shi RS. Three-in-one incidence of hepatocellular carcinoma, cholangiocellular carcinoma, and neuroendocrine carcinoma: A case report. World J Clin Cases 2022; 10(29): 10575-10582
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10575.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10575
Hepatocellular carcinoma (HCC) is the most common subtype of primary liver cancer (PLC)[1], followed by cholangiocarcinoma (CCA). Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a tumor with both hepatocytic and biliary components. The incidence of cHCC-CCA among PLCs is 0.4%-14.2%[2]. Also, other unusual PLCs with combined components have been recorded. Tumors with an HCC and neuroendocrine carcinoma (NEC) differentiation have been published[3]. However, hepatocellular tumors showing three differentiation pathways are sporadic. The combination of HCC, CCA, and NEC differentiation has been described previously only in four patients. Herein, we report another case of a similar tumor in a 32-year-old man, with an aim to increase cognition and improve the treatment of the disease.
A 32-year-old male patient was hospitalized at the Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou Province, China on August 29, 2021, due to pain in the right upper abdomen for 30 d.
The patient’s symptoms started 30 d ago, with repetitive right upper abdominal pain.
The patient presented hepatitis B 5 years ago and did not receive regular treatment.
The patient had a smoking and drinking history of > 10 years: 15 cigarettes/d and about 100 mL wine/d. His parents were healthy and had no family history of cancer.
The abdomen was soft with tenderness in the right upper abdomen but no rebound pain and muscle tension. A mass of about 80 m × 80 m could be felt under the right costal margin of the liver; it was tough and tender, with a clear boundary, and did not move when touched.
Laboratory examinations revealed the following: Alanine aminotransferase, 243 (normal range 9-50) U/L and aspartate aminotransferase, 167 (normal range 15-50) U/L; abnormal alpha-fetoprotein (AFP), 4519 (normal range < 9) μg/L; normal carbohydrate antigen, carcinoembryonic antigen (CEA), and neuron-specific enolase (NSE) levels; hepatitis B virus (HBV) DNA, 2.327 × 102 (normal range < 1000) IU/mL, hepatitis B surface antigen, 250 (normal range < 0.05) IU/mL, and hepatitis B core antibody 8.35 (normal range < 1) cutoff index.
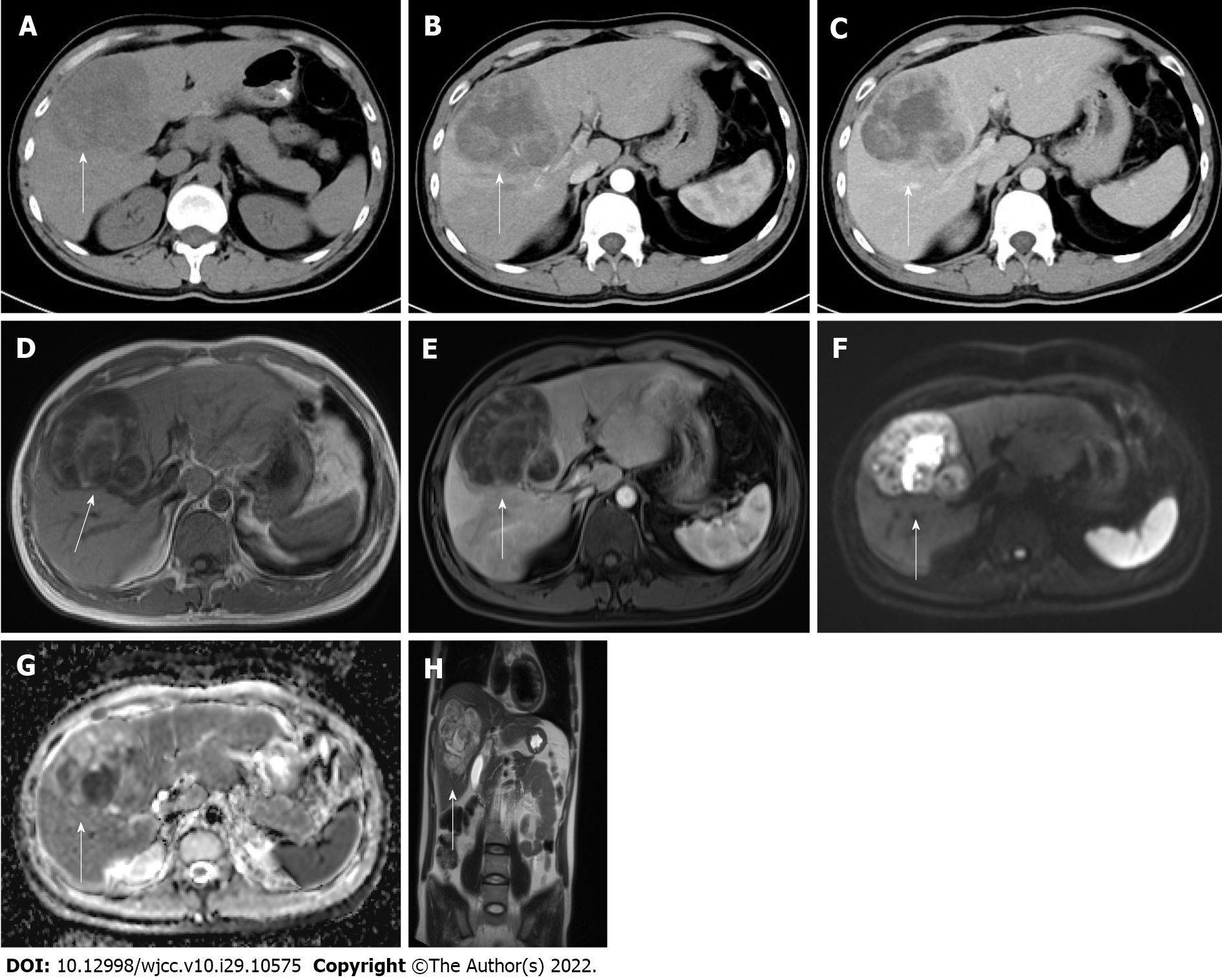
Abdomen computed tomography (CT) displayed a mass of 81 mm × 83 mm in the 7th and 8th hepatic segments as a mild external protrusion. It showed inhomogeneous enhancement compared to the surrounding liver parenchyma in the arterial phase and low density with a clear portal border. Magnetic resonance imaging (MRI) with gadoxetate disodium (Eovist®) revealed significant enhancement. Intriguingly, massive mixed signals were detected from the mass: A low signal on T1-weighted image (T1WI), a high signal on T2WI, multiple equal and low signals, and segmentation. Diffusion-weighted imaging (DWI) showed an uneven high signal, apparent diffusion coefficient (ADC) images showed an uneven low signal, and no signal reduction area was observed in the out-phase. In the enhanced arterial phase, the edge was slightly uneven, and the enhancement was weaker than that of the normal liver. In the delayed phase, the lesion center and edge were significantly uneven, and the enhancement degree was higher than that of the normal liver, indicating gradually delayed enhancement (Figure 1). Chest CT, gastrofibroscopy, and colonoscopy did not find any evidence of another origin site.
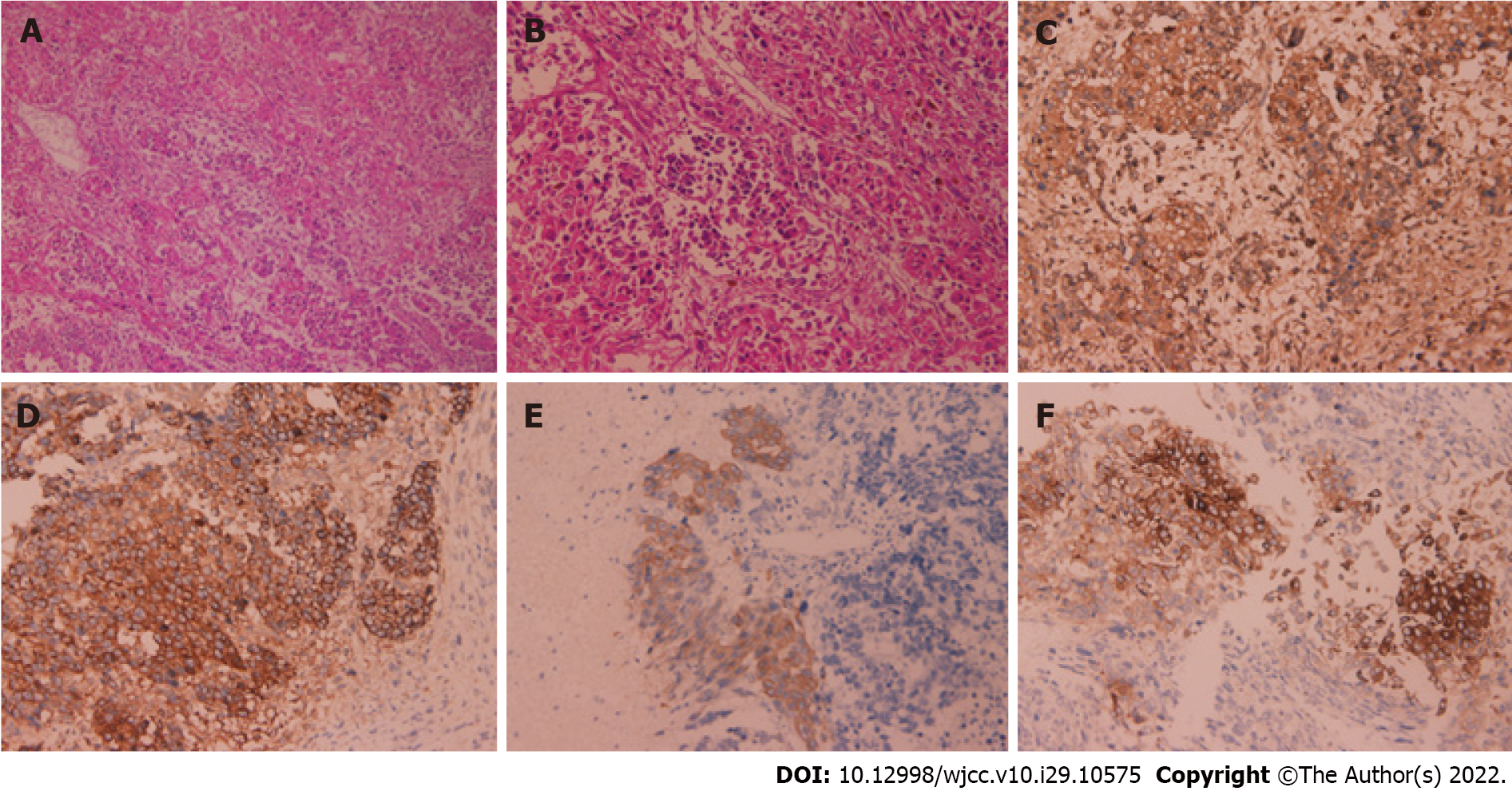
Pathological and immunohistochemical examination results on the excised liver tissues after surgery (Figure 2) were as follows: CK (++), AFP (++), hepatocyte (++), CK19 (+), synaptophysin (Syn) (+), CD56 (+), catenin (+), vimentin (+), chromogranin A (CgA) (-), Glypican-3 (+), Ki-67 (60%+), vascular invasion (+), nerve invasion (-), no tumor involvement at the cutting edge of the liver, and the broken end of the gallbladder neck. According to the histopathological results and immunohistochemical features, this tumor included HCC, intrahepatic CCA (iCCA), and NEC.
The final diagnosis was low differentiation HCC plus medium and low differentiation CCA combined with NEC.
With the consent of the patient and his family, he was treated by laparoscopic partial hepatic lobectomy and cholecystectomy by an experienced hepatobiliary surgeon. The intraoperative findings were as follows: A mass with a diameter of about 10 cm was located in the right lobe of the liver, protruding from the liver capsule; it was intact and had a clear boundary. The whole mass and the surrounding liver tissue, about 2 cm, and major blood vessels were resected.
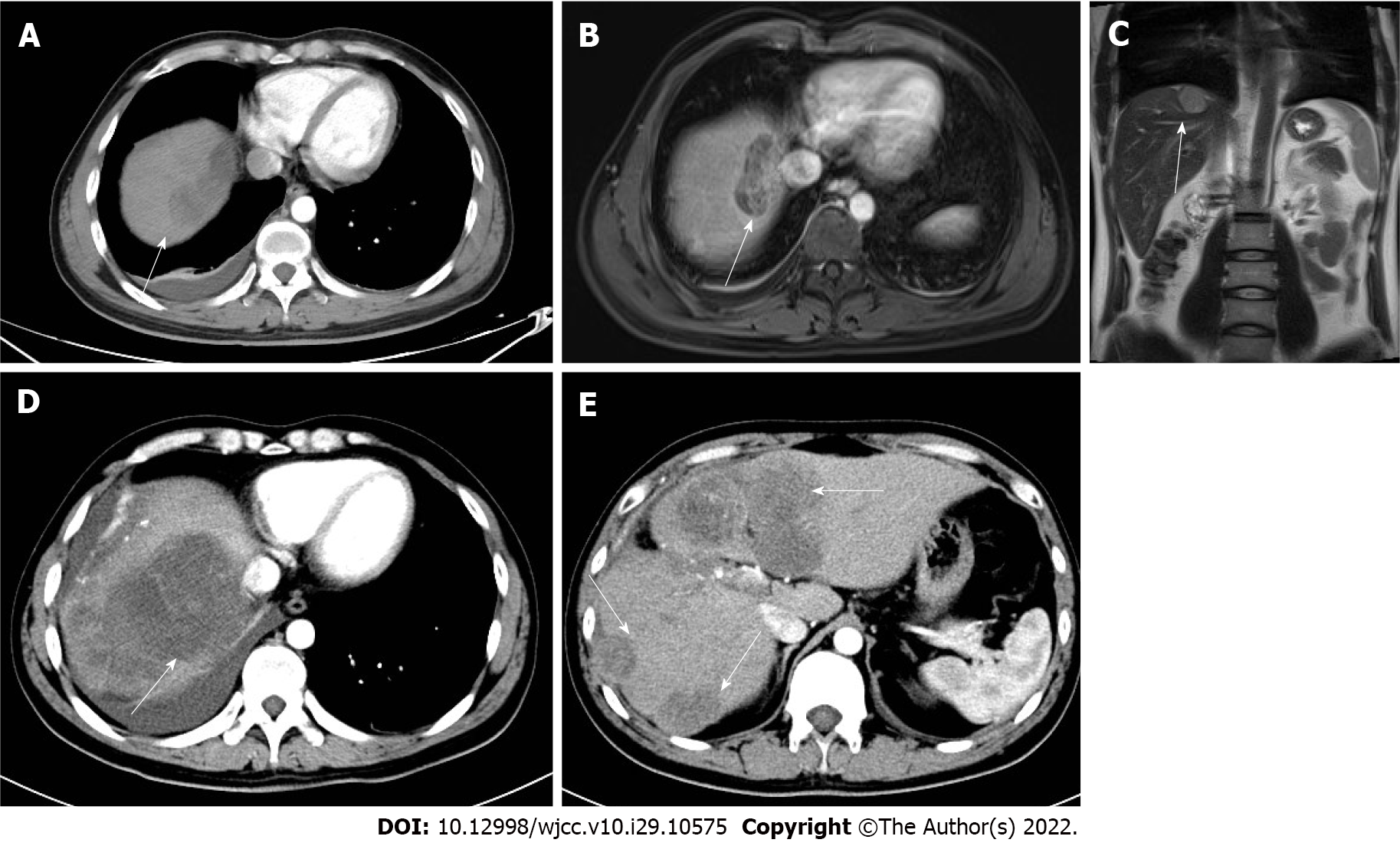
One month post-surgery, the patient was hospitalized on October 20, 2021 due to epigastric pain. Abdominal enhanced CT and MRI suggested multiple metastases in the liver (Figure 3). In order to control tumor growth, the patient was treated by transarterial chemoembolization (TACE) to embolize the blood vessels supplying the tumor; also, oxaliplatin (150 mg), fluorouracil (1 g), and leucovorin (0.4 g) were administered. HBV infection was treated with entecavir (0.5 mg, once daily). Subsequently, the abdominal pain was relieved, and the patient was discharged from the hospital. However, he was treated again at our hospital on November 24, 2021 due to the aggravation of epigastric pain. Abdominal CT showed that liver metastases had increased and enlarged (Figure 3), with a maximum diameter of 62 m × 65 m.
The patient eventually died of liver failure in December 2021 (Figure 4).
PLC is the fourth driving cause of cancer-related deaths worldwide, and the incidence is persistently rising in Western countries[4]. It is a heterogeneous tumor related to various hazard factors, clinical results, and histological and molecular features. Among these malignancies, HCC and iCCA are the most common cancers that represent the two extremes of primary malignancies. cHCC-CCA is a subset of liver neoplasms that might display hepatocytic and biliary differentiation. Compared to HCC and iCCA, these biphenotypic tumors are rarer, accounting for < 5% of all liver cancers[3]. Conversely, the concurrent occurrence of HCC and NEC is rarer than the HCC plus CCA type in the liver because the rate of primary hepatic NEC is very low as opposed to incidental intrahepatic metastasis of NEC. Interestingly, HCC, iCCA, and NEC are extremely rare, and only four cases have yet been reported (Table 1).
| Cases of liver tumors with triple differentiation described in the literature | Ref. |
| Two Chinese male patients (average age: 57.5 yr) were positive for hepatitis B and exhibited hepatic tumors with triple differentiation | He et al[18], 2013 |
| A healthy 19-year-old Caucasian man developed a large hepatic tumor showing triple differentiation | Beard et al[16], 2017 |
| A 65-year-old woman with a history of hepatitis C and rectal “carcinoid” developed a hepatic tumor with triple differentiation | Dimopoulos et al[17], 2021 |
| A 32-year-old Chinese man with a history of hepatitis B and wine-drinking exhibited hepatic tumors with triple differentiation | Current case report |
Neuroendocrine tumors are mainly localized in the gastrointestinal system and frequently metastasize to the liver[5]. Primary neuroendocrine tumors are exceptionally uncommon in the liver. The morphology of primary hepatic neuroendocrine tumors is hazy. Currently, there are two hypotheses explaining this phenomenon. One is that the stem cell forebody of malignant cells from another pernicious hepatic tumor differentiate into a neuroendocrine tumor. Another is that such tumors come from neuroendocrine cells in the intrahepatic bile conduit epithelium[6,7]. Primary neuroendocrine tumors with HCC in the liver are rare. HCC with carcinoid tumors was first docu
Serum markers are often utilized to evaluate liver tumors. Donadon et al[11] showed that the indicators of primary HCC, such as AFP, CEA, and carbohydrate antigen 19-9, have little value for the diagnosis of liver NEC. Specific immunohistochemical markers of NEC include NSE, CgA, Syn, CD57, and bombesin; those of HCC are HEPPAR-1 and AFP, and for CCA they are CK-7 and CK-19[3]. Park et al[12] demonstrated that serum CgA was an indicator for NEC diagnosis, with an 87%-100% sensitivity and 92% specificity. In addition, serum 5-hydroxytryptamine and 24-h urine 5-hydoxyindoleacetic acid also had a high sensitivity and specificity for the diagnosis of the disease. In this patient, the immunohistochemistry staining for CK, AFP, hepatocyte, CK19, Syn, and CD56 was positive, which was consistent with the diagnosis of HCC, iCCA, and NEC.
Imaging examination is a crucial method to judge the quality of mass. Contrast-enhanced CT showed rich blood supply tumors for hepatic NEC, while on the plain scan, slightly low-density lesions with clear boundaries, uniformly enhanced smaller lesions, and irregular necrotic areas in larger lesions were observed. Moreover, the enhancement was “fast in and slow out”. The lesions in the arterial phase showed rosette and patchy enhancement, while those in the portal vein phase showed centripetal enhancement, and those in the delayed phase showed equal or slightly high-density and no enhancement in the necrotic area[13,14]. This was different from the rich blood supply and “fast in and fast out” enhancement of typical HCC. MRI is a valuable diagnostic method for HCC. HCC exhibited a low-intensity signal on T1WI and slightly high-intensity signal on T2WI, and dynamic enhancement scanning showed uneven images in the arterial phase, further enhancement in the portal vein phase, decreased enhancement in the delayed phase, and low-intensity signal and annular capsule enhancement in the later stage[15]. The characteristics of this patient were consistent with the previous description. DWI showed a high signal, and ADC image showed a low signal, indicating limited diffusion and suggesting a malignant tumor.
Mixed tumors with three differentiation pathways are extremely rare, and therefore no meaningful conclusions could be derived with respect to the risk factors, the origin of the cells, prognosis, and treatment options. Previously, a case of blended tumor with three separation pathways was reported in a young Caucasian man with no known identifiable hazard variables[16]. Another study described a patient with a history of hepatitis C[17]. Also, two Chinese male patients were positive for hepatitis B[18]. In this case, the Chinese patient had a history of hepatitis B with long-term smoking and drinking, and 3/5 patients had hepatitis B, indicating that hepatitis B is a major risk factor for this disease (Table 2).
| Gender | Age (yr) | Country | Site of primary lesion | Hepatitis | AFP (μg/L) | Ki-67 | Therapy | Lifetime |
| Male | 61 | China | Liver | B | 1210 | 5% | Surgical resection + TACE | Short |
| Male | 54 | China | Liver | B | 1400 | 80% | Surgical resection + TACE | 2 mo |
| Male | 19 | United States | Liver | / | Normal | High | Surgical resection + Cisplatin and gemcitabine chemotherapy | > 8 mo |
| Female | 65 | United States | Rectum | C | 2198 | High | Surgical resection + Cisplatin and gemcitabine chemotherapy | 5 mo |
| Male | 32 | United States | Liver | B | 4519 | 60% | Surgical resection + TACE | 4 mo |
HCCs with NEC components are related to invasive behavior and unfavorable results. However, whether the HCC or the NEC component determines the prognosis of patients is yet to be clarified. Interestingly, the Ki-67 proliferation index of NEC is significantly higher than that of HCC. Additionally, lymph node or distant metastases are often present in NEC; thus, the prognosis of primary HCC combined with primary NEC might be positively correlated with the NEC component. The more the NEC component, the worse the prognosis and the higher the probability of recurrence and metastasis. Although the leading treatment for mixed liver tumors is surgery, the other treatments include TACE, radioembolization, chemotherapy, or liver transplantation. The decisions to treat patients with adjuvant therapy and other alternatives are based on the assessment of the tumor. For example, Ki-67 proliferation index is a satisfactory indicator; a high value indicates a high risk of tumor invasion and recurrence. Our patient had Ki-67 > 60%, vascular invasion (+), and multiple metastases in the liver 1 mo after surgery. Despite aggressive treatment, the patient had a very rapid disease progression that could be attributed to three differentiation pathways.
Herein, we report a rare and easily misdiagnosed case, and several key points deserve close attention. First, clinical examination suggested HCC or other malignant tumors[19]. The patient was infected with HBV and had an alcohol history and abnormal AFP levels. The contrast-enhanced CT showed heterogeneous enhancement in the arterial and delayed phases. MRI showed a predominantly long T1 signal. T1WI showed mild enhancement in some lesions, while DWI showed a high signal, and ADC image showed a low signal in the center of the lesion. Postoperative pathology revealed a blended neuroendocrine-non-neuroendocrine neoplasm that was a crucial pathological determinant. Strikingly, a few cells were positive for NEC, iCCA, and HCC markers, indicating that a few “undifferentiated cells” were plastic amid the differentiation period, regardless of whether they were pernicious hepatic tumor cells or ancestral cells. Surgical resection is the preferred treatment for mixed liver tumors. The postoperative use of a combination of chemotherapy-based measures with other modalities might improve the prognosis of patients. In conclusion, the co-occurrence of HCC, iCCA, and NEC with high invasiveness, rapid growth, easy recurrence, and metastasis in a short duration is very rare. Imaging and laboratory tests could easily miss or misdiagnose the cancer, and thus, the final diagnosis relies on pathology. Therefore, additional case studies are required to elucidate the characteristics, diagnosis, and optimal therapy for HCC, iCCA, and NEC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Cerwenka H, Austria S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000-4013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 2. | Adány R, Szegedi A, Ablin RJ, Muszbek L. Fibrinolysis resistant fibrin deposits in lymph nodes with Hodgkin's disease. Thromb Haemost. 1988;60:293-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 258] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 4. | Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 5. | Nishino H, Hatano E, Seo S, Shibuya S, Anazawa T, Iida T, Masui T, Taura K, Haga H, Uemoto S. Histological features of mixed neuroendocrine carcinoma and hepatocellular carcinoma in the liver: a case report and literature review. Clin J Gastroenterol. 2016;9:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Pilichowska M, Kimura N, Ouchi A, Lin H, Mizuno Y, Nagura H. Primary hepatic carcinoid and neuroendocrine carcinoma: clinicopathological and immunohistochemical study of five cases. Pathol Int. 1999;49:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Gould VE, Banner BF, Baerwaldt M. Neuroendocrine neoplasms in unusual primary sites. Diagn Histopathol. 1981;4:263-277. [PubMed] |
| 8. | Barsky SH, Linnoila I, Triche TJ, Costa J. Hepatocellular carcinoma with carcinoid features. Hum Pathol. 1984;15:892-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Yang CS, Wen MC, Jan YJ, Wang J, Wu CC. Combined primary neuroendocrine carcinoma and hepatocellular carcinoma of the liver. J Chin Med Assoc. 2009;72:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Nomura Y, Nakashima O, Akiba J, Ogasawara S, Fukutomi S, Yamaguchi R, Kusano H, Kage M, Okuda K, Yano H. Clinicopathological features of neoplasms with neuroendocrine differentiation occurring in the liver. J Clin Pathol. 2017;70:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Donadon M, Torzilli G, Palmisano A, Del Fabbro D, Panizzo V, Maggioni M, Santambrogio R, Montorsi M. Liver resection for primary hepatic neuroendocrine tumours: report of three cases and review of the literature. Eur J Surg Oncol. 2006;32:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Park CH, Chung JW, Jang SJ, Chung MJ, Bang S, Park SW, Song SY, Chung JB, Park JY. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol. 2012;27:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Yang K, Cheng YS, Yang JJ, Jiang X, Guo JX. Primary hepatic neuroendocrine tumors: multi-modal imaging features with pathological correlations. Cancer Imaging. 2017;17:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Huang J, Yu JQ, Sun JY. Computer tomography and magnetic resonance image manifestations of primary hepatic neuroendocrine cell carcinomas. Asian Pac J Cancer Prev. 2014;15:2759-2764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Cha DI, Kang TW, Jang KM, Kim YK, Kim SH, Ha SY, Sohn I. Hepatic neuroendocrine tumors: gadoxetic acid-enhanced magnetic resonance imaging findings with an emphasis on differentiation between primary and secondary tumors. Abdom Radiol (NY). 2018;43:3331-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Beard RE, Finkelstein SD, Borhani AA, Minervini MI, Marsh JW. A massive hepatic tumor demonstrating hepatocellular, cholangiocarcinoma and neuroendocrine lineages: A case report and review of the literature. Int J Surg Case Rep. 2017;37:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Dimopoulos YP, Winslow ER, He AR, Ozdemirli M. Hepatocellular carcinoma with biliary and neuroendocrine differentiation: A case report. World J Clin Oncol. 2021;12:262-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | He C, Yin HF, Liu P, Zhang Y, Zhang JB. [Clinicopathologic features of combined hepatic carcinoma]. Zhonghua Bing Li Xue Za Zhi. 2013;42:824-828. [PubMed] |
| 19. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 401] [Article Influence: 57.3] [Reference Citation Analysis (1)] |