Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10559
Peer-review started: May 12, 2022
First decision: July 14, 2022
Revised: July 17, 2022
Accepted: September 7, 2022
Article in press: September 7, 2022
Published online: October 16, 2022
Processing time: 140 Days and 5.3 Hours
Patent foramen ovale (PFO) is the most common congenital heart disease and is associated with several diseases, including stroke and migraine. PFO diagnosis involves transoesophageal echocardiography, transthoracic echocardiography, and transcranial Doppler. Recent studies have shown that intracardiac echocardiography (ICE) can be used to diagnose and guide percutaneous transcatheter clo
A 70-year-old male presented with paroxysmal dizziness and limb weakness for the past 3 mo. Magnetic resonance imaging revealed a history of stroke, and a bubble test revealed the presence of PFO. The patient was then transferred to our hospital for PFO closure. Under ICE guidance, the separation of the septum pr
ICE can guide PFO closure in patients with a history of stroke. When PFO is not evident under ICE, a Swartz catheter can be used.
Core Tip: Patent foramen ovale (PFO) is the most common congenital heart disease and is associated with several diseases, including stroke and migraine. PFO diagnosis involves transoesophageal echocardiography, transthoracic echocardiography, and transcranial Doppler. Recent studies have shown that intracardiac echocardiography (ICE) can be used to diagnose and guide percutaneous transcatheter closure. Here, we represent the use of ICE in PFO detection and closure for PFO patient with a history of stroke. Importantly, when PFO is unobvious, Swartz catheter can be used to physically separate the septum primum and septum secundum.
- Citation: Han KN, Yang SW, Zhou YJ. Novel way of patent foramen ovale detection and percutaneous closure by intracardiac echocardiography: A case report. World J Clin Cases 2022; 10(29): 10559-10564
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10559.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10559
Patent foramen ovale (PFO) caused by failed fusion of the septum primum and septum secundum is the most common congenital heart disease and is present in approximately 25% of the general population[1]. Although most patients with PFO are asymptomatic, many studies have shown that PFO is implicated in diseases including migraine and decompression illness[2]. PFO may cause cryptogenic stroke, especially when accompanied by deep venous thrombosis and other structural abnormalities, which could be explained by the hypothesis that thrombi from the right heart system can directly pass the PFO to the left atrium when the pressure of the right heart increases[3]. However, the association between PFO and cryptogenic stroke remains unclear because the only one prospective longitudinal study has shown a negative result[1]. The Food and Drug Administration approved the use of percutaneous transcatheter closure (PTC) for PFO because closure can significantly decrease the rate of recurrent stroke. Furthermore, the Society for Cardiovascular Angiography and Interventions (SCAI) guidelines recommend PFO closure in patients with prior PFO-associated stroke[4]. PFO diagnosis involves transoesophageal echocardiography (TEE), transthoracic echocardiography (TTE), transcranial Doppler (TCD), and intracardiac echocardiography (ICE)[5]. TEE, with its relatively high resolution, was once considered the gold standard. Recently, a study has demonstrated the advantages of ICE in diagnosing and guiding PTC, including having higher resolution, the flexibility of obtaining multi-angle images, continuous guidance during the procedure, avoidance of general anaesthesia, and the capability of detecting other abnormalities[6]. Here, we shared one case of ICE use in diagnosis and PTC in a patient who had previously undergone cryptogenic stroke. We showed that if the PFO is not obvious, a Swartz catheter can be used.
A 70-year-old male presented with paroxysmal dizziness and limb weakness for the past 3 mo.
Three months ago, the patient got a sudden paroxysmal dizziness and limb weakness. Two months prior, the patient experienced more severe dizziness, weakness, vomiting, and nausea. Then, the patient was admitted to our hospital.
The patient has no history of hypertension, diabetes, hyperlipidaemia, coronary artery disease, or atrial fibrillation.
The patient had a 50-year smoking history (30 cigarettes per day). No family history.
The patient has a muscular weakness of limbs.
Laboratory tests showed that the hemoglobin A1c level was 6.2% and fasting plasma glucose was 7.47 mmol/L.
Three months prior, magnetic resonance imaging (MRI) of the local hospital showed remote cerebral infarction. Two months ago, MRI showed right cerebellar hemisphere infarction with local hemosiderin deposition. Echocardiography revealed ascending aortic enlargement, and the left ventricular ejection fraction was 59%. Bubble test of the right atrium indicated PFO.
Final diagnosis is PFO.
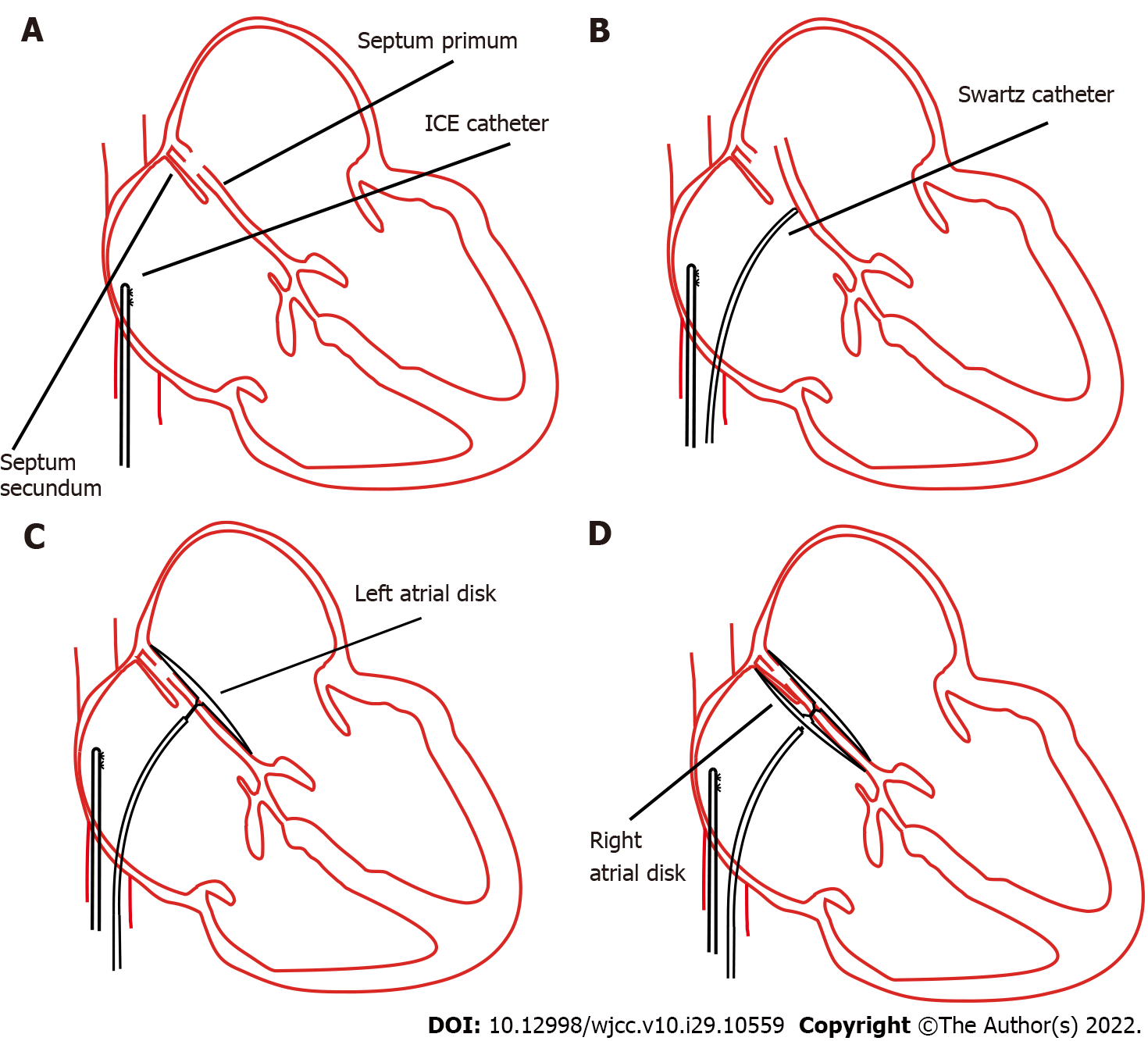
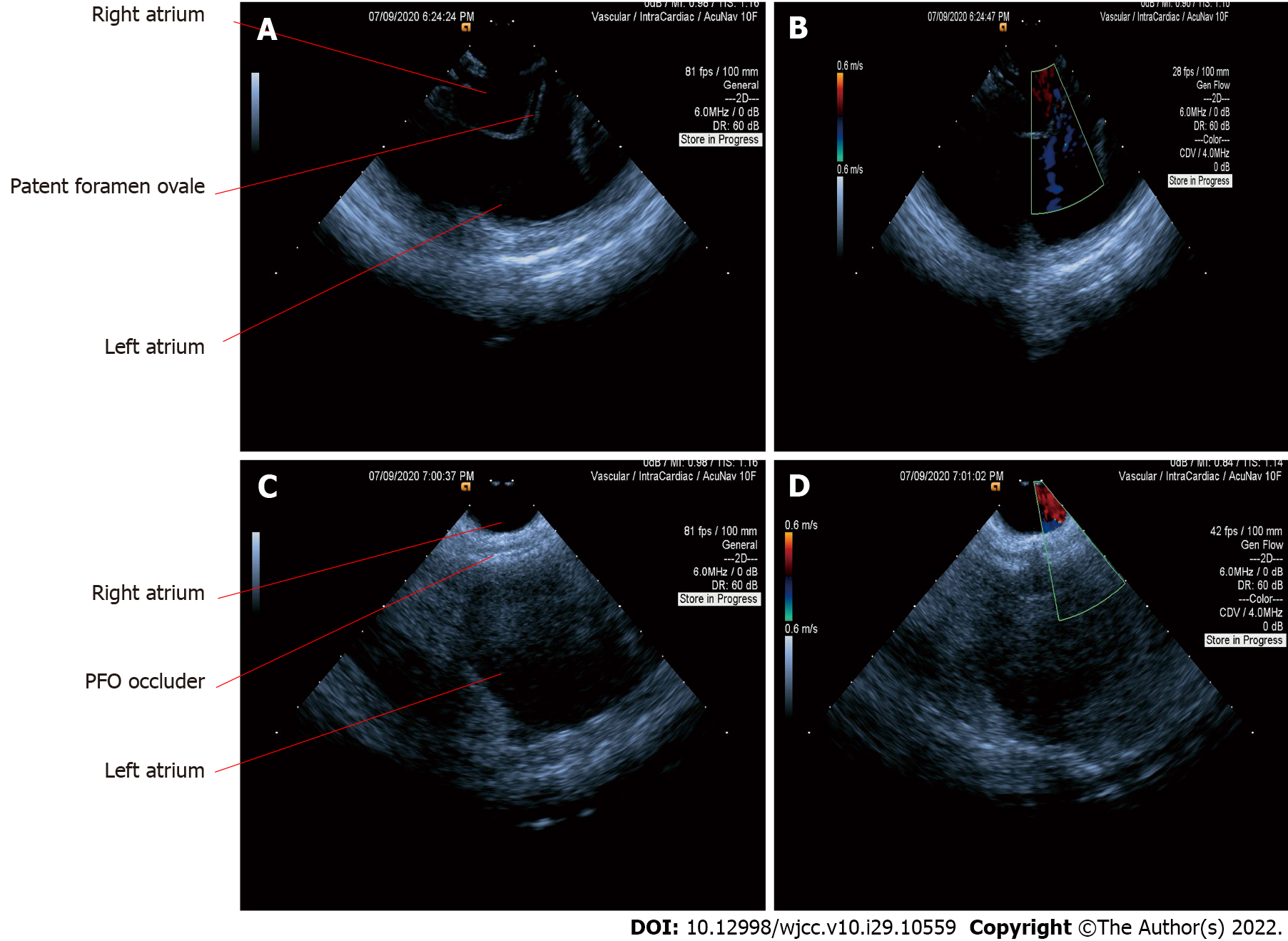
PTC was then indicated according to the symptoms and examination findings[7]. Afterwards, we proceeded with the PFO confirmation using ICE. Firstly, the ICE catheter (Johnson & Johnson Medical, United States) was advanced into the right atrium from the left femoral vein, under fluoroscopic guidance. In the “home view”, the right atrium, right ventricle, tricuspid valve, and long axis of the right ventricular outflow tract can be observed (Figure 1A). Subsequently, by flexing the ICE catheter posteriorly and slightly tilting rightward, the transducer directly faced the interatrial septum, and the entire length of the interatrial septum became visible. However, we could not find the separation of the septum primum and septum secundum, even with the assistance of the colour Doppler test. Fortunately, PFO was confirmed using an 8.5 F Swartz sheath (Abbott, United States). The Swartz sheath was advanced into the inferior vena cava through the right femoral vein, and then to the right side of the interatrial septum. Then, by applying slight physical pressure on the right side of the interatrial septum, the septum primum was manually separated from septum secundum, which can avoid the use of aerated saline contrast and Valsalva maneuver (Figures 1B and 2A). Colour Doppler imaging confirmed right-to-left shunting (Figure 2B).
After careful measurement of the PFO and surrounding rims, the PTC procedure was performed and an Amplatzer PFO occluder (St. Jude Medical, United States) was implanted. The distal disk of the occluding device was first deployed in the left atrium through the withdrawal of the sheath (Figure 1C). The device and sheath were then pulled back against the atrial septum. When the left disk was in close contact with the septum, the right atrial disk of the occluding device was deployed via further sheath withdrawal (Figure 1D). After deployment, the position of the device was confirmed by ICE (Figure 2C), and the colour Doppler was used to ensure that there were no residual leaks (Figure 2D).
The patient experienced no complications during the procedure. Three months later, the frequency and degree of dizziness significantly decreased. Furthermore, the patient did not have recurrent stroke, and his subjective quality of life improved.
The association between PFO and stroke has not yet been consolidated. Our patient had a history of cryptogenic stroke, and according to the SCAI guidelines, we recommended performing PFO closure to prevent stroke. However, closure is fraught with possible complications during follow-up. Patients may develop complications, including arrhythmia, thrombus formation on the surface of the device, and even chest pain. Therefore, it is important to fully evaluate whether patients should undergo PFO closure, and long-term follow-up is needed.
As shown in previous studies, even though TEE was once considered the gold standard for detecting PFO, it has some limitations for diagnosis and PTC when compared to ICE. In our case, we demonstrated the detailed application of ICE to confirm PFO and guide PTC. Previous studies have shown that compared to TEE, ICE can find more abnormalities, including the Chiari network and atrial myxoma[8]. ICE can be used to easily visualise the posterior and inferior rims of the interatrial septum. Additionally, ICE can help visualise clinical problems such as lead endocarditis masses and post-lead extraction floating masses inside the right atrial chamber[9,10]. Moreover, TEE reportedly misses the diagnosis in approximately 10% of patients[11]. Furthermore, compared to TEE, ICE does not require general anaesthesia, an oesophageal probe, or assistance from an additional sonographer. Therefore, complications related to general anaesthesia and intubation can be avoided, and interventional cardiologists can perform both the imaging and catheterisation procedures. In addition, it is difficult for the patients to do the Valsalva manoeuvre using TEE. When using ICE, a cardiologist can directly and efficiently obtain ideal images, which can decrease the operation and fluoroscopy time[12]. ICE can also be used to detect residual shunting immediately after PTC[13]. It can directly monitor procedure-related complications such as thrombus formation and pericardial effusion.
Although ICE can directly visualise the PFO and separation of the septum primum from the septum secundum or can be supported by a colour Doppler (Figures 2A and 2B), most PFO is asymptomatic and shunting may only occur when the right heart pressure increases. Therefore, it is necessary to manually create shunting. In our case, we used a Swartz sheath to further confirm PFO by manually applying physical pressure on the interatrial septum. To the best of our knowledge, the present study is the first to use a Swartz sheath. Previous studies used aerated saline bubbles assisted by the Valsalva manoeuvre or by coughing to increase right heart pressure and create shunting of the contrast. However, if the PFO is not apparent, the efficacy of the Valsalva manoeuvre or coughing becomes low; moreover, these approaches cannot be performed under general sedation. Furthermore, a study showed that saline contrast may cause cerebral ischaemic events[14]; therefore, our method could be safer. In patients who require atrial septal puncture, the Swartz sheath can be used for both detection and puncture without additional expense.
ICE can fully evaluate the surrounding structures, confirm that there are no other abnormalities, and measure the rims of PFO. It can also provide continuous guidance for septal puncture and closure deployment. Other diagnostic methods for PFO include TTE and TCD. TTE is the most commonly used method for the initial diagnosis, but this approach has relatively lower sensitivity. TCD can indirectly detect PFO by detecting shunting between the left and right atria, and its sensitivity is between those of TTE and TEE[15]. However, because TCD cannot directly observe shunting in the atrium, it can be difficult to distinguish between PFO, atrial septal defects, and intrapulmonary shunts. Although the sensitivities of TTE and TCD are lower than those of TEE and ICE, they are convenient and non-invasive. Therefore, we hypothesised that TTE and TCD can be used to screen for PFO. If the screening result is positive and the patient has a history of stroke, PFO closure and ICE will be the first choice. If the screening result is positive and the patient has no history of stroke, the patient needs to be followed up regularly because some PFO could automatically close with ageing.
Detection and closure of PFO are of utmost significance. Here, we presented the use of ICE for PFO detection and closure in a patient with a history of stroke. More importantly, we demonstrated that when the PFO is not obvious, a Swartz catheter can be used to physically separate the septum primum and septum secundum.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Beijing Municipal Administration of Hospitals’ Mission plan, SML20180601.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caiati C, Italy; Gupta P, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, Whisnant JP, Wiebers DO, Covalt JL, Petterson TM, Christianson TJ, Agmon Y. Patent foramen ovale: innocent or guilty? J Am Coll Cardiol. 2006;47:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Johansson MC, Eriksson P, Dellborg M. The significance of patent foramen ovale: a current review of associated conditions and treatment. Int J Cardiol. 2009;134:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Zietz A, Sutter R, De Marchis GM. Deep Vein Thrombosis and Pulmonary Embolism Among Patients With a Cryptogenic Stroke Linked to Patent Foramen Ovale-A Review of the Literature. Front Neurol. 2020;11:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Kavinsky CJ, Szerlip M, Goldsweig AM, Falck-Ytter Y, Babatunde I, Morgan RL, Amin Z, Boudoulas KD, Carroll JD. SCAI Guidelines for the Management of Patent Foramen Ovale. J Soc Cardiovasc Angiogr Interv. 2022;1:100039. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Homma S, Messé SR, Rundek T, Sun YP, Franke J, Davidson K, Sievert H, Sacco RL, Di Tullio MR. Patent foramen ovale. Nat Rev Dis Primers. 2016;2:15086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Kavvouras C, Vavuranakis M, Vaina S, Lampropoulos K, Bazoukis G, Tse G, Tousoulis D. Intracardiac echocardiography for percutaneous patent foramen ovale and atrial septal defect occlusion. Herz. 2019;44:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Farb A, Ibrahim NG, Zuckerman BD. Patent Foramen Ovale after Cryptogenic Stroke - Assessing the Evidence for Closure. N Engl J Med. 2017;377:1006-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Bartel T, Konorza T, Arjumand J, Ebradlidze T, Eggebrecht H, Caspari G, Neudorf U, Erbel R. Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation. 2003;107:795-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Caiati C, Luzzi G, Pollice P, Favale S, Lepera ME. A Novel Clinical Perspective on New Masses after Lead Extraction (Ghosts) by Means of Intracardiac Echocardiography. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Caiati C, Pollice P, Lepera ME, Favale S. Pacemaker Lead Endocarditis Investigated with Intracardiac Echocardiography: Factors Modulating the Size of Vegetations and Larger Vegetation Embolic Risk during Lead Extraction. Antibiotics (Basel). 2019;8:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Augoustides JG, Weiss SJ, Weiner J, Mancini J, Savino JS, Cheung AT. Diagnosis of patent foramen ovale with multiplane transesophageal echocardiography in adult cardiac surgical patients. J Cardiothorac Vasc Anesth. 2004;18:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Boccalandro F, Baptista E, Muench A, Carter C, Smalling RW. Comparison of intracardiac echocardiography versus transesophageal echocardiography guidance for percutaneous transcatheter closure of atrial septal defect. Am J Cardiol. 2004;93:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Mahmoud AN, Elgendy IY, Agarwal N, Tobis JM, Mojadidi MK. Identification and Quantification of Patent Foramen Ovale-Mediated Shunts: Echocardiography and Transcranial Doppler. Interv Cardiol Clin. 2017;6:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Romero JR, Frey JL, Schwamm LH, Demaerschalk BM, Chaliki HP, Parikh G, Burke RF, Babikian VL. Cerebral ischemic events associated with 'bubble study' for identification of right to left shunts. Stroke. 2009;40:2343-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke. 1993;24:1020-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 196] [Article Influence: 6.1] [Reference Citation Analysis (0)] |