Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10550
Peer-review started: September 17, 2021
First decision: December 9, 2021
Revised: December 22, 2021
Accepted: September 7, 2022
Article in press: September 7, 2022
Published online: October 16, 2022
Processing time: 376 Days and 19 Hours
Immune check-point inhibitors-induced colitis (ICPIs-induced colitis) is one of the immune-related side effects. Steroids and Infliximab are commonly used to treat it. The patients of our report were treated by Vedolizumab.
The two patients went to the doctor with bloody stools and were treated by Sin
For the symptoms of bloody diarrhea after the ICPIs treatment of cancer, the pos
Core Tip: With its good efficacy, immunotherapy is increasingly being used in treating malignant tumors and attention shall be paid to the immune-related side effects caused by this therapy. This is a report on Sintilimab and Carrelizumab induced colitis which has rarely been reported in the past. Besides, a positive effect was achieved by adopting Vedolizumab combined with short-term corticosteroids therapy, which was different from the previous treatment.
- Citation: Zhang Z, Zheng CQ. Vedolizumab in the treatment of immune checkpoint inhibitor-induced colitis: Two case reports. World J Clin Cases 2022; 10(29): 10550-10558
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10550.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10550
Immune check-point inhibitors (ICPIs) serve as a method of cancer immunotherapy. At present, mult
Our Center has recently treated two patients with bloody and mucus stools. A 63-year-old woman received Sintilimab combined with Fluorouracil and Nedaplatin treatment for esophageal cancer. She had abdominal pain, mucus and bloody diarrhea 1 mo after the treatment. Another 69-year-old gent
Case 1: A 63-year-old female went to the doctor in May 2021 with chief complaints of abdominal pain and bloody diarrhea accompanied by vomiting, fatigue, anorexia and weight loss of about 10 kg for 1 mo.
Case 2: A 69-year-old gentleman went to the doctor in March 2021. The chief complaint was bloody diarrhea, accompanied by abdominal pain, fatigue, tenesmus and weight loss of about 10 kg for 3 mo.
Case 1: The patient described that there had been mucus and blood in her bowel movements with the symptom of tenesmus 10-15 times per day for 1 mo.
Case 2: The patient suffered diarrhea 3 mo ago with a frequency up to 10 times a day. His stool was watery with a little blood and the symptoms did not worsen after taking montmorillonite powder and mesalazine orally. As he described, there had been mucus and more blood in his bowel movements 20-30 times per day for 1 mo and he also had fecal incontinence.
Case 1: The patient was diagnosed with esophageal squamous cell carcinoma 3 mo ago and treated with Sintilimab in combination with Fluorouracil and Nedaplatin. The symptoms of abdominal pain and bloody diarrhea appeared about 1 mo later after the treatment.
Case 2: The patient was diagnosed with poorly differentiated lung adenocarcinoma 4 mo ago which was anaplastic lymphoma kinase (-) and received treatment with Camrelizumab combined with Pemetrexed and Carboplatin. The symptoms of diarrhea appeared about 20 d after the treatment and then the patient received the immunotherapy for four cycles.
Cases 1 and 2: The patient denied fever, chills, night sweats, skin rash, arthralgia, history of unclean diet, history of contact with those who had related diseases, recent travel history and no personal or family history of colitis.
Case 1: The patient's vital signs were stable. Physical examination results showed that the abdomen was flat, soft and tender without rebound pain or muscle tension. No skin rash or joint swelling was seen on the whole body.
Case 2: The patient's vital signs were stable. Physical examination results of the abdomen showed that the abdomen was flat, soft and there was generalized abdominal tenderness without rigidity. No skin rash or joint swelling was seen.
Case 1: Initial significant laboratory results showed a hemoglobin level of 106 × 1012/L, leukocytes of 8.3 × 109/L and a platelet count of 349 × 109/L; other test results included a creatinine level of 68.4 μmol/L, blood urea of 4.65 mmol/L, alanine transaminase of 18 U/L, aspartate aminotransferase of 26 U/L, albumin of 24.2 g/L, C-reactive protein of 40.5 mg/L, serum potassium of 2.87 mmol/L, prothrombin time of 15.7 s and a d-dimer of 283 ug/L. Fecal pathogen culture, Clostridioides difficile, and Calprotectin tests didn’t show any detected positive results.
Case 2: Initial significant laboratory results included a hemoglobin level of 126 × 1012/L, leukocytes of 3.4 × 109/L and a platelet count of 367 × 109/L; other test results included a creatinine level of 79 μmol/L, blood urea of 7.68 mmol/L, alanine transaminase of 29 U/L, aspartate aminotransferase of 36 U/L, albumin of 33.2 g/L, C-reactive protein of 60.7 mg/L, Procalcitonin of 0.168 ng/mL, serum potassium of 3.51 mmol/L, prothrombin time of 10.9 s, and a d-dimer of 465 ug/L. Fecal pathogen culture, Clostridioides difficile and Calprotectin tests didn’t show any detected positive results.
Case 1: Abdominal computed tomography scan revealed inflammatory changes involving the thickened walls of the colon and rectum. After the enhanced scan, layered enhancement of the tube wall could be seen with exudation observed.
Case 2: Computed tomography (CT) scan of the abdomen revealed inflammatory changes involving thickened walls of the large intestine with blood vessels around the mesentery increased in size.
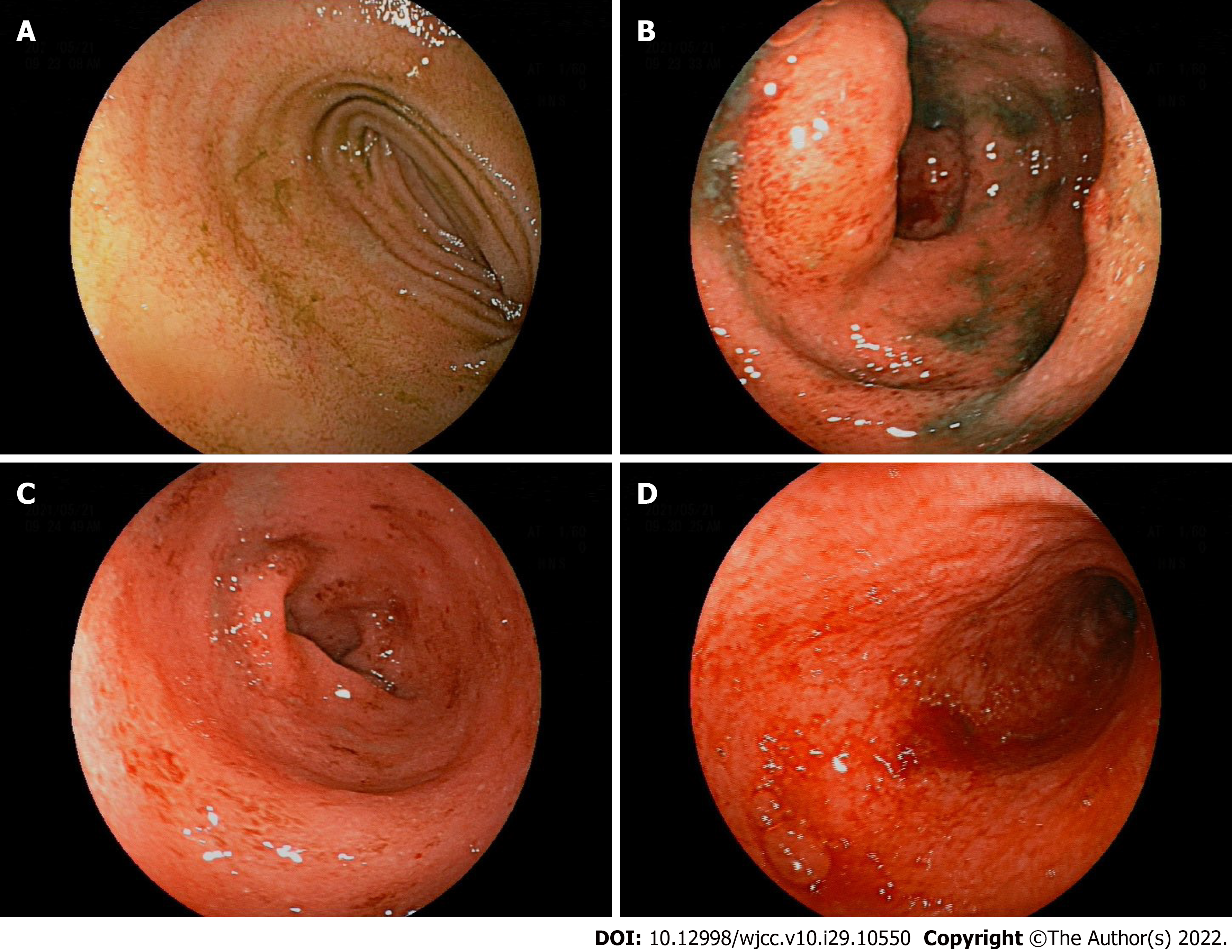
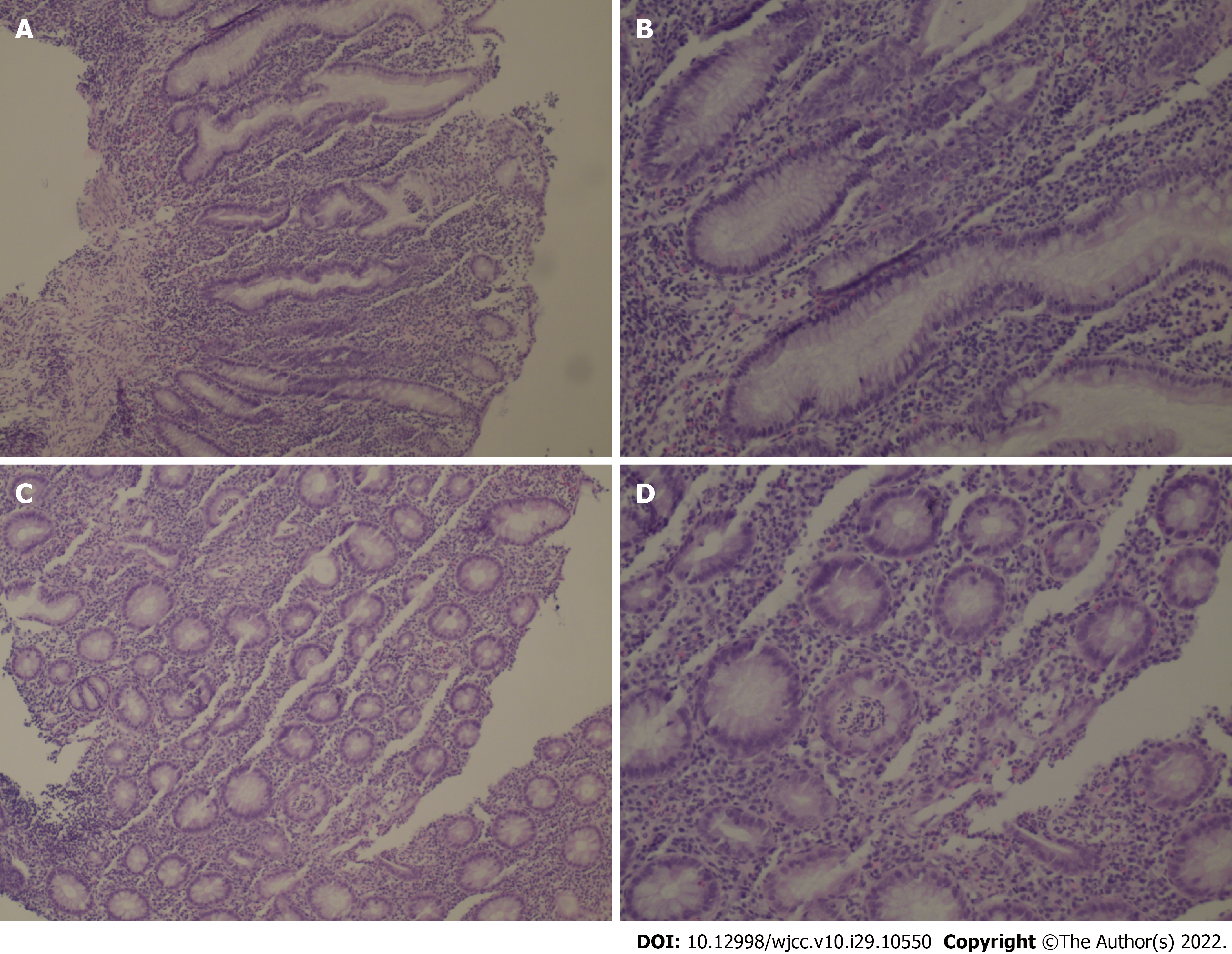
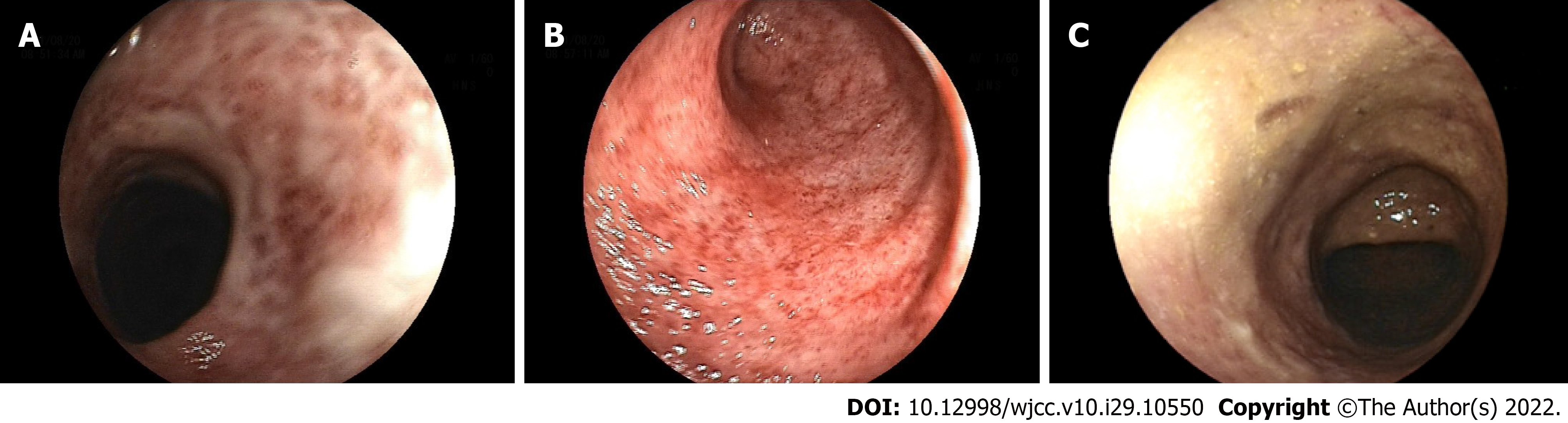
The patient was examined by colonoscopy upon specialist consultation in Gastroenterology, Oncology and Infectious disease. According to the results of the colonoscopy, the mucosa at 20 cm of the distal ileum was smooth and there was marked hyperemia and edema of the mucosa from the ileocecum to the rectum. Diffuse mucosal erosion and superficial ulcers were visible and the mucosa was brittle and easy to bleed when exposed (Figure 1). HE staining biopsy showed no atypia of intestinal mucosal glands, but found slightly irregular crypt with branches, crypt inflammation and crypt abscess, infiltration of such inflammatory cells as interstitial neutrophils and plasma cells, acid-fast mycobacterium fluorescence staining, Epstein-Barr virus encoded RNA's in situ hybridization, cytomegalovirus immunohistochemistry (-) (Figure 2).
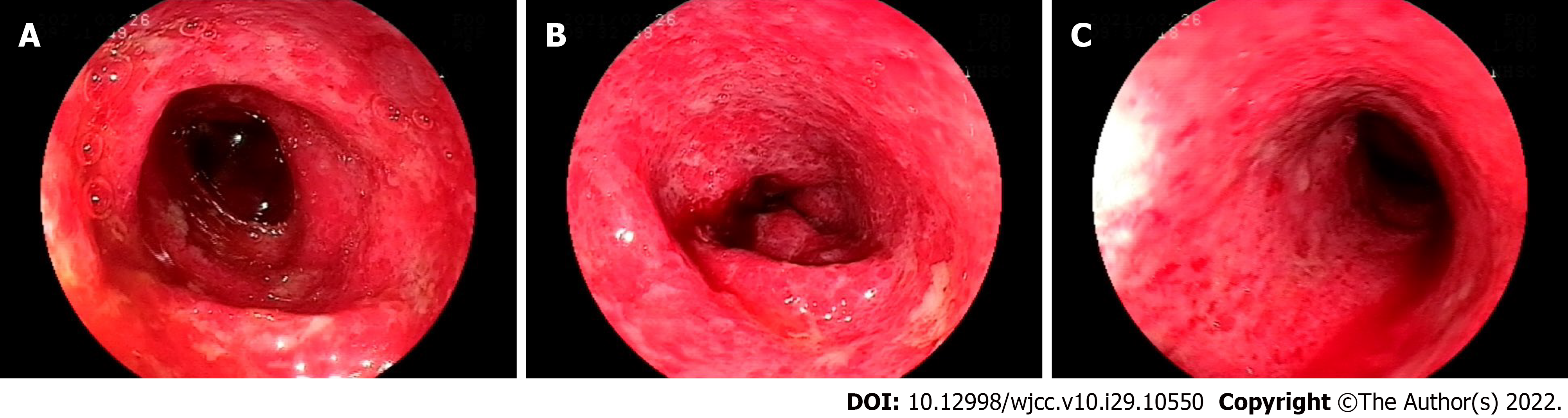
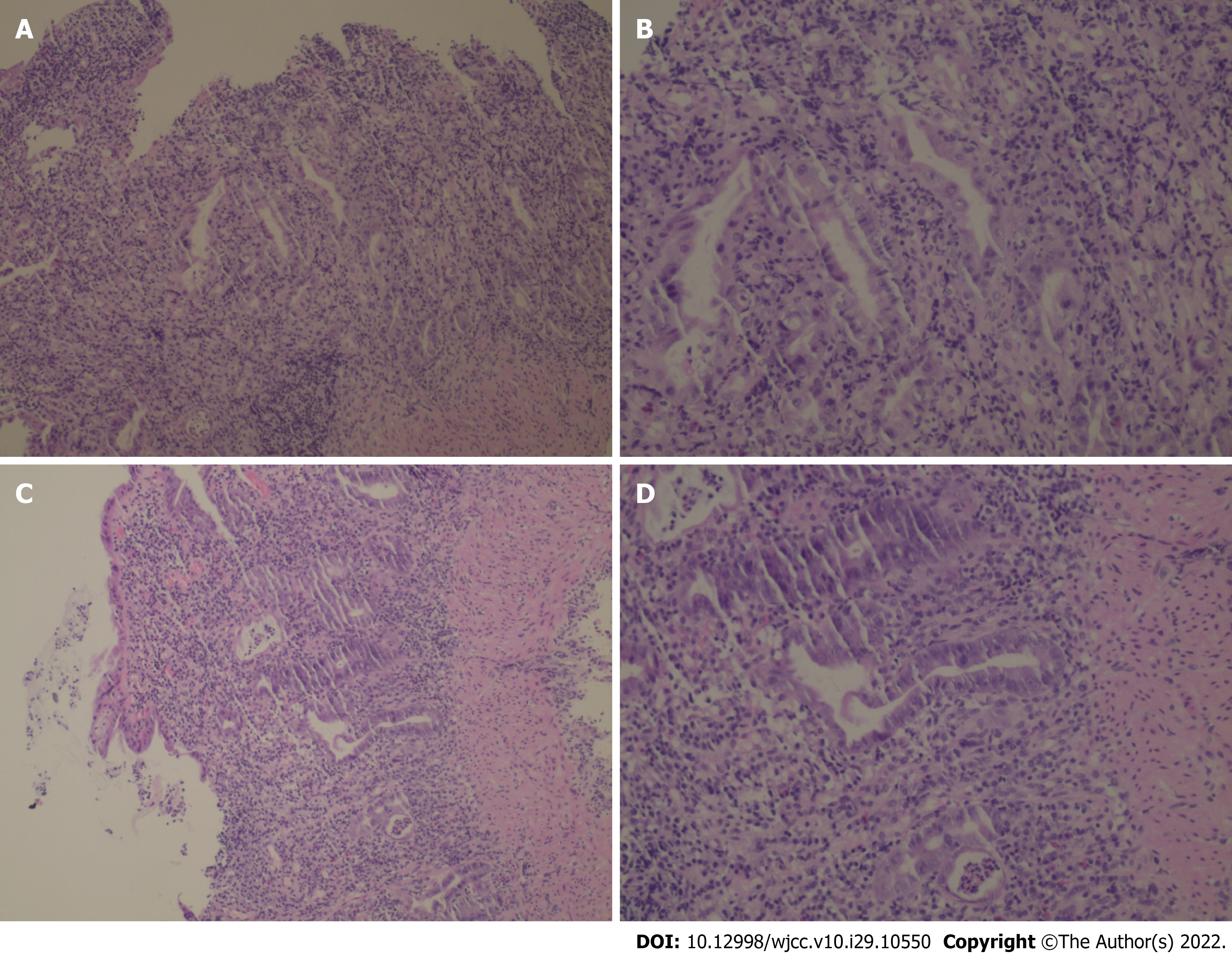
The patient was examined by colonoscopy upon specialist consultation in Gastroenterology, Oncology and Infectious disease. Due to the narrow intestinal stenosis caused by obvious hyperemia and edema of the intestinal mucosa, the colonoscope could only reach the transverse colon proximal splenic flexion of the colon, but observed obvious hyperemia and edema in the colon and rectum mucosa with diffuse mucosal erosion, ulcers and bleeding. The ulcers were covered with white fur and the intestinal mucosa was brittle and prone to bleeding when exposed (Figure 3). HE staining biopsy showed crypt inflammation and crypt abscess, infiltration of inflammatory cells, acid-fast mycobacterium fluorescence staining, Epstein-Barr virus encoded RNA's in situ hybridization, cytomegalovirus immunohistochemistry (-) (Figure 4).
Based on the history, imaging, endoscopic and histologic exams, the patient was diagnosed with grade IV colitis induced by immunotherapy.
According to the history, imaging, endoscopic and histological examination, the patient was diagnosed with grade IV immune-mediated colitis.
On admission, Mesalazine was administered orally, somatostatin was pumped intravenously to inhibit intestinal juice secretion, and empiric antibiotic coverage with Cefmenoxime combined with Metronidazole was given. Upon the diagnosis of grade IV colitis, the patient received an intravenous infusion of 60 mg/d methylprednisolone for 7 d, after which corticosteroids were not used immediately. At the same time, 300 mg Vedolizumab was injected and 300 mg was given again after 2 wk.
On admission, the patient underwent fasting, got parenteral nutrition and somatostatin injected to inhibit intestinal juice secretion and was empirically given Cefmenoxime and Metronidazole to control the infection. Upon the diagnosis of grade IV colitis, the patient received intravenous infusion of 60 mg/d of Methylprednisolone for 7 d and then corticosteroids were no longer administered immediately. At the same time, the patient was given 300 mg Vedolizumab intravenously and re-treated with 300 mg Vedolizumab at the 2nd and 4th week after the initial treatment with Vedolizumab.
The 2nd day after the treatment, the stool frequency of the patient was reduced from 10-15 times a day to 4-5 times a day and the decrease of bloody stool was also significantly improved. The frequency of stool turned normal about 1 wk later after treatment. Currently, the patient has no abdominal pain or bloody diarrhea. The patient refused to undergo colonoscopy reexamination. The immunotherapy cycles were discontinued due to the colitis event. The patient is not currently continuing anti-tumor therapy for esophageal cancer.
The patient's symptoms gradually improved after the treatment and the frequency of stool turned normal after 2 wk. The patient has no abdominal pain or bloody diarrhea right now. After 5 mo of treatment, the patient underwent colonoscopy, which showed visible mucosal erosion and a mucosal healing scar, and the hyperemia and edema of the colonic mucosa were less than before but still resulted in intestinal stenosis (Figure 5). Considering that the patient’s symptoms of hematochezia were relieved after treatment, the colonoscopy showed that intestinal mucosal erosion, ulcers and bleeding were alleviated but there were still symptoms of active colitis. 300 mg Vedolizumab was given intravenously, again. However, the immunotherapy cycles were discontinued due to the colitis. The patient is not currently continuing anti-tumor therapy for lung cancer.
It is proven that ICPIs are efficacious for treating cancers and improving the survival in metastatic malignancies[6]. ICPIs, by inhibiting checkpoints involved in regulating T-cell activation, enable augmentation of immunologic response against tumor cells which in turn has improved survival rates, particularly for patients with small cell lung carcinoma, renal cell carcinoma and melanoma[1,7,8]. ICPIs can cause a widespread activation of non-tumor-specific T-cells[9]. The disadvantage of this mechanism is the specific and uncontrolled activation of immune system cells which leads to excessive immune responses and autoimmune diseases. ICPIs also have an immune-mediated side-effect due to the action of aiding T-cell activation. The gastrointestinal (GI) tract, endocrine system, lungs, liver and skin are usually affected but GI tract Immune-Related Adverse Events (irAEs) typically presenting with diarrhea but are rarely affected. This could be either the only presenting symptom that is self-limiting or the part of ICPIs-induced colitis that requires hospitalization and treatment. The combination of CTLA-4 and PD-1 inhibitors resulted in a significantly higher incidence and severity of irAEs (including colitis) than single-agent PD-1 inhibition[10].
As a humanized monoclonal antibody against PD-1, Camrelizumab (SHR-1210) usually blocks the binding of PD-1 to PD-L1 and consequently inhibits the immune escape of tumor cells. It has been shown by clinical trials of Phase 1 that Camrelizumab was well tolerated in patients with advanced solid tumors showing some antitumor activity[11-14]. In a phase-3 clinical study for the treatment of advanced esophageal squamous cell carcinoma, 228 patients who received Camrelizumab were reported, and 44 (19%) of patients were reported undergoing treatment-related to adverse events of grade 3 or higher, including three cases of diarrhea[15]. Of patients treated with Decitabine combined with Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma, 30% underwent immune-related AEs and 6% of them had diarrhea[16]. Lickliter et al[17] found in a study of Camrelizumab for patients with advanced or metastatic cancer that a total of 6 patients reported 8 irAEs, including 1 with diarrhea (grade 3), and the results of the study suggested that there was no clear correlation between the incidence and severity of related irAEs and the dose of Camrelizumab.
As a fully human IgG4 monoclonal antibody, Sintilimab can bind to PD-1, block the interaction of PD-1 with its ligands and recover the anti-tumor response of T-cells. In addition, Sintilimab is undergoing the development of Phase I, II and III for various solid tumors including non-small cell lung cancer and esophageal cancer, in China[18]. The immune-related AEs of Sintilimab included diarrhea[19] in the official instructions. In fact, ICPIs-colitis induced by Sintilimab were rarely reported. IrAEs, as autoimmune entities should be reversed by immunosuppression, with corticosteroids as the first-line agent[4]. Infliximab is usually reserved for treating GI-irAEs refractory to steroids or of high severity[20]. However, the antitumor efficacy of ICPIs therapy may be adversely affected by immunosuppressive therapy[21]. Furthermore, various debilitating adverse events including infections may occur because of systemic immunosuppressive therapy[22].
Vedolizumab, a humanized monoclonal IgG1 antibody that selectively blocks gut lymphocyte trafficking by specifically recognizing the α4β7 integrin, is a cell surface glycoprotein variably expressed on T lymphocytes[23,24]. The α4β7 integrin interacts with the mucosal addressin cell adhesion molecule on intestinal vasculature to regulate the migration of leukocytes into inflamed intestinal tissue[25,26]. Vedolizumab is approved for the treatment of inflammatory bowel disease (IBD) because there are many similarities in the clinical manifestations including mucus and bloody diarrhea between ICPIs-induced colitis and IBD, and colonoscopy results show extensive and diffuse erosions and ulcers in the intestinal mucosa. Histology results suggest inflammatory cell infiltration, so the effective treatment for IBD may also be effective for ICPIs-induced colitis. Hamzah et al[27] found in their study that Vedolizumab can be appropriate for the treatment of steroid-refractory ICPIs-induced colitis, with favorable outcomes and safety. Bergqvist et al[28] had reported that the patients with ICPIs-induced colitis either steroid-refractory and/or steroid-dependent were given Vedolizumab treatment for two to four times, and most patients achieved steroid-free remission. Moreover, due to the gut-specific action mechanism of Vedolizumab, we speculate that the treatment for ICPIs-induced colitis may cause lesser affection on the treatment and development of cancer. However, due to the gradual effect of Vedolizumab[29] and the fact that our two patients had severe bloody diarrhea, we chose the therapy of Vedolizumab combined with short-term corticosteroids. Fortunately, the patients underwent rapid remission of symptoms.
For the symptoms of bloody diarrhea after the ICPIs treatment of cancer, the possibility of ICPIs-induced colitis should be considered. Colonoscopy, mucosal biopsies, abdominal CT scan and fecal calprotectin are necessary for the diagnosis. Cessation of the immunotherapy and the introduction of anti-inflammatory therapy can help to control the ICPIs-induced colitis. Vedolizumab combined with short-term corticosteroids may be appropriate for the safe and effective treatment of ICPIs-induced colitis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jabbarpour Z, Iran; Xu PF, China A-Editor: Liu X, China S-Editor: Xing YX L-Editor: Filipodia P-Editor: Xing YX
| 1. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4171] [Article Influence: 379.2] [Reference Citation Analysis (0)] |
| 2. | Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1913] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 3. | Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1565] [Article Influence: 173.9] [Reference Citation Analysis (0)] |
| 4. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119-iv142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 1495] [Article Influence: 186.9] [Reference Citation Analysis (1)] |
| 5. | Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists' perspective. J Clin Pathol. 2018;71:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor DR, Salama AK, Taylor MH, Ott PA, Horak C, Gagnier P, Jiang J, Wolchok JD, Postow MA. Combined nivolumab and ipilimumab vs ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 748] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 7. | Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov. 2014;13:883-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, Lum P, Raju G, Shuttlesworth G, Stroehlein J, Diab A. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 9. | Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 10. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6070] [Cited by in RCA: 6181] [Article Influence: 618.1] [Reference Citation Analysis (0)] |
| 11. | Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, Wang X, Lan B, Zhang H, Chi Y, Yang Q, Xu B. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119:538-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, Zhang Y, Chen L, Zhou N, Zhao Y, Hou X, Yang Q, Zhang L. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 351] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 13. | Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, Qu D, Wang X, Lan B, Yang B, Wang P, Zhang H, Yang Q, Jiao Y. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients with Advanced Esophageal Carcinoma. Clin Cancer Res. 2018;24:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 15. | Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, Chen J, Zhang H, Niu Z, Fan Q, Lin L, Gu K, Liu Y, Ba Y, Miao Z, Jiang X, Zeng M, Fu Z, Gan L, Wang J, Zhan X, Liu T, Li Z, Shen L, Shu Y, Zhang T, Yang Q, Zou J; ESCORT Study Group. Camrelizumab vs investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 410] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 16. | Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, Li X, Liu J, Ku W, Zhang Y, Chen M, An X, Shi L, Brock MV, Bai J, Han W. Addition of Low-Dose Decitabine to Anti-PD-1 Antibody Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma. J Clin Oncol. 2019;37:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | Lickliter JD, Gan HK, Voskoboynik M, Arulananda S, Gao B, Nagrial A, Grimison P, Harrison M, Zou J, Zhang L, Luo S, Lahn M, Kallender H, Mannucci A, Somma C, Woods K, Behren A, Fernandez-Penas P, Millward M, Meniawy T. A First-in-Human Dose Finding Study of Camrelizumab in Patients with Advanced or Metastatic Cancer in Australia. Drug Des Devel Ther. 2020;14:1177-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Hoy SM. Sintilimab: First Global Approval. Drugs. 2019;79:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (2)] |
| 19. | Instructions for Sintilimab Injection (2020). Available from: http://inn-qc.iqiaomai.com/5c89f09e539a2. |
| 20. | Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL Jr, Abdel-Wahab N, Anderson J, Davis JE, Joseph J, Uemura M, Noman A, Abu-Sbeih H, Yee C, Amaria R, Patel S, Tawbi H, Glitza IC, Davies MA, Wong MK, Woodman S, Hwu WJ, Hwu P, Wang Y, Diab A. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. 2018;6:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 21. | Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, Jiang J, Robert C. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol. 2017;35:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 876] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 22. | Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, Ayanian JZ, Nallamothu BK. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 526] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 23. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1851] [Article Influence: 154.3] [Reference Citation Analysis (1)] |
| 24. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1554] [Article Influence: 129.5] [Reference Citation Analysis (1)] |
| 25. | Marsal J, Agace WW. Targeting T-cell migration in inflammatory bowel disease. J Intern Med. 2012;272:411-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1130] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 27. | Abu-Sbeih Hamzah, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, Richards DM, Charabaty A, Wang Y. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer. 2018;6:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 28. | Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, Carneiro A, Marsal J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Bryant RV, Sandborn WJ, Travis SP. Introducing vedolizumab to clinical practice: who, when, and how? J Crohns Colitis. 2015;9:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |