Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10366
Peer-review started: July 9, 2022
First decision: August 1, 2022
Revised: August 5, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 79 Days and 21 Hours
Cronkhite-Canada syndrome (CCS) is a rare non-hereditary disease with a poor prognosis and a mortality rate of up to 55%. Currently, there is no standard treatment for CCS. The department of gastroenterology of our hospital admitted a patient with CCS whose symptoms improved significantly after treatment with thalidomide combined with endoscopy, and there was no obvious adverse reaction during the 2-year follow-up.
A 47-year-old Chinese man presented with diarrhea for more than 4 mo, acco
The patient’s symptoms were significantly relieved by thalidomide 2 years after treatment, proposing it as a potential treatment for CCS.
Core Tip: Cronkhite-Canada syndrome (CCS) is a rare non-hereditary disease with a poor prognosis and a mortality rate of up to 55%. Currently, there is no standard treatment for CCS. The symptoms of the patient in this case were significantly improved after treatment with thalidomide combined with endoscopy, and they were followed up for 2 years. No obvious adverse reactions were observed. Thalidomide may be a new potential therapeutic drug for CCS, and we will continue to follow up to determine its long-term efficacy.
- Citation: Rong JM, Shi ML, Niu JK, Luo J, Miao YL. Thalidomide combined with endoscopy in the treatment of Cronkhite-Canada syndrome: A case report. World J Clin Cases 2022; 10(28): 10366-10374
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10366.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10366
Cronkhite-Canada syndrome (CCS), also known as polyp pigmentation alopecia fingernail dystrophy syndrome, is characterized by multiple polyps of the gastrointestinal tract, hair loss, nail dystrophy, and abnormal skin pigmentation. It was first reported by Cronkhite and Canada in 1955[1]. The patho
A 47-year-old male patient presented with diarrhea for more than four months.
The patient had diarrhea without obvious incentives for more than 4 mo. The number of times a day was 8-10, and the stool was yellow paste. The amount was approximately 100-200 mL/time. There was no mucus, pus, or blood and intermittent pain around the umbilical cord. The diarrhea was usually accompanied by loss of taste, fatigue, and occasional nausea and vomiting. The vomit included stomach contents, and there was no obvious abdominal distension or hair loss.
He had a previous medical history of hyperthyroidism, Grave’s eye disease, and diabetes mellitus.
The patient smoked about 360 packs per year of cigarettes for more than 20 years and drank an average of 4 double liquors of alcohol per day for more than 10 years while denying any relevant family history.
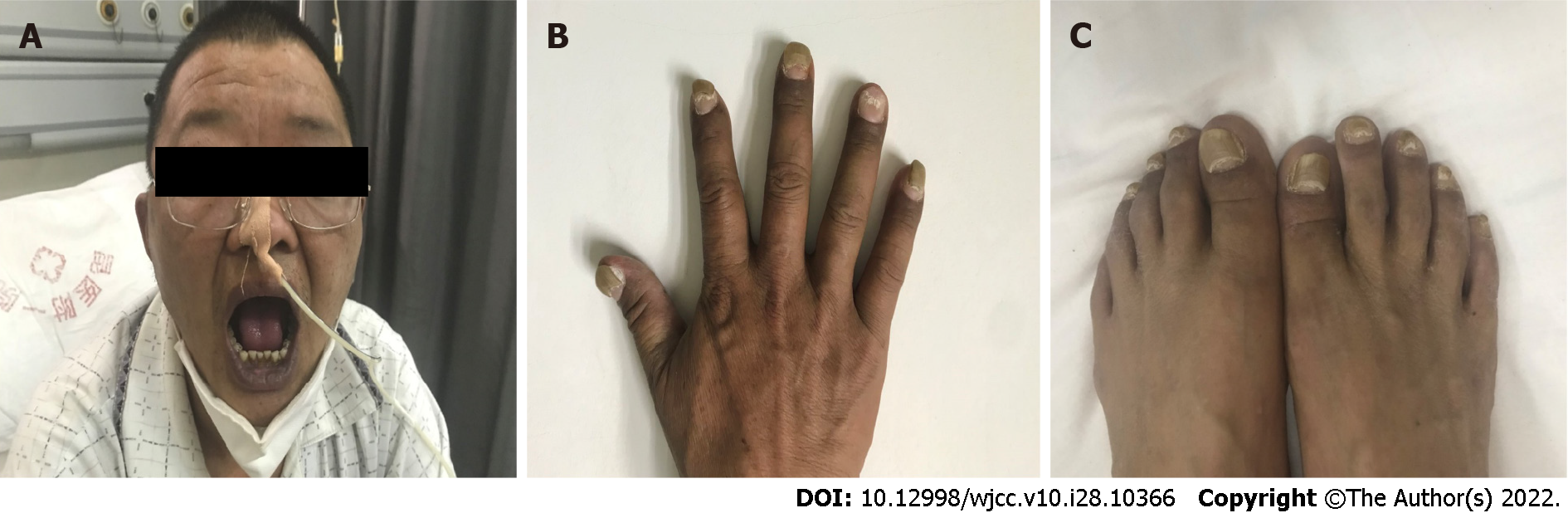
On physical examination, the vital signs were as follows: body temperature, 36.3 ℃; blood pressure, 111/88 mmHg; heart rate, 110 beats per min; respiratory rate, 22 breaths per min. His height was 170 cm, and his weight was 75 kg. Furthermore, the skin around his lips and hands was pigmented, the anterior edge of the nails of both his hands was significantly thickened and yellow, and his nails were partially atrophied (Figure 1).
Pertinent laboratory findings included an increased platelet count (5.01 × 1011/L) and hypoalbuminemia (serum albumin of 27.3 g/L) with a reduced total protein (50 g/L). The level of the calcium correction amount was (2.164 mM) along with his IgM and C3 levels (0.25 g/L and 0.82 g/L, respectively), but his anti-neutrophil cytoplasmic antibody, antinuclear antibody, IgG and IgG4 findings were normal.
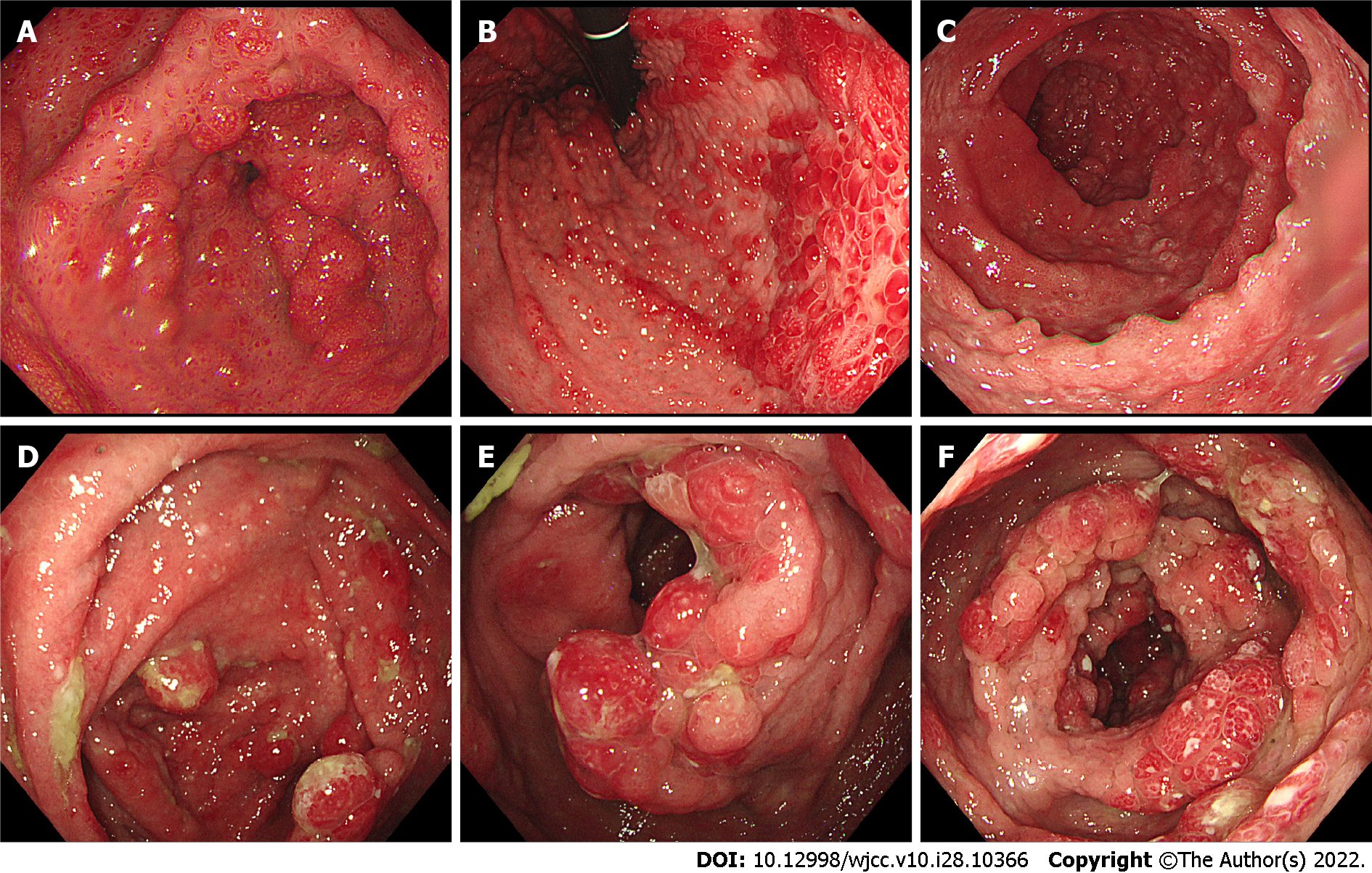
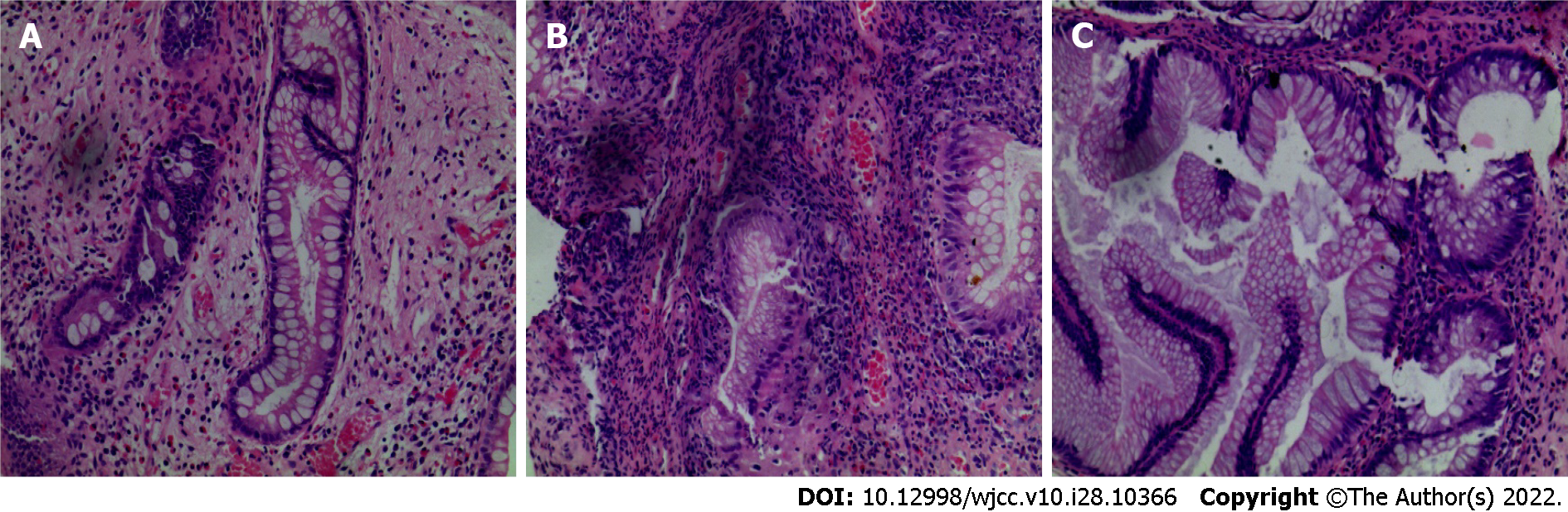
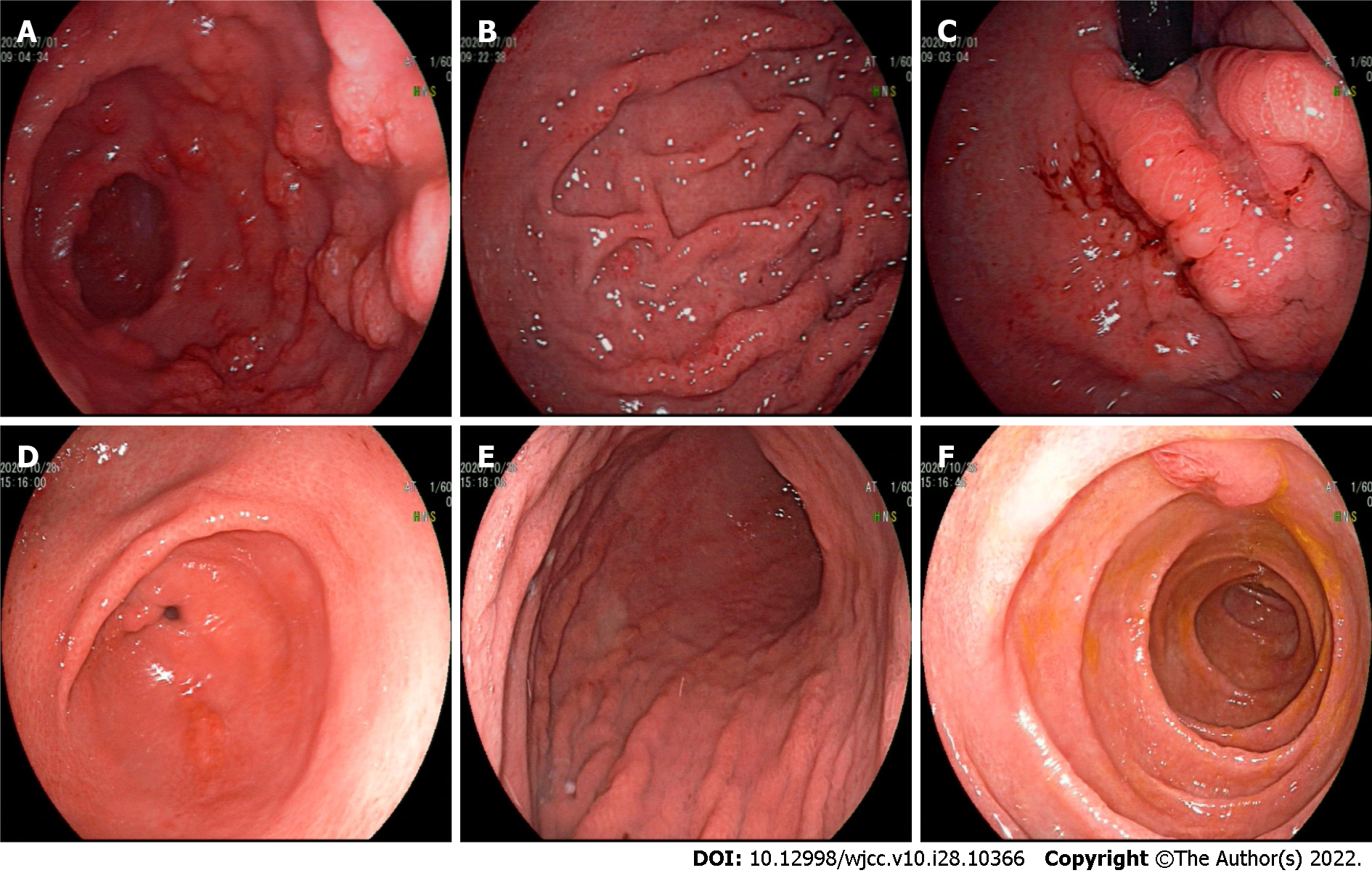
Gastroscopy imaging identified the esophageal mucosa to be smooth without any abnormalities. The mucosa of the whole stomach, pylorus, and duodenum bore a diffuse polypoid bulge appearance, and the surface mucosa was congested (Figure 2A-C). Pathological examination of the tissue sent for gastric biopsy showed the existence of hyperplastic polyp changes. The colonoscopy performed identified mucosal hyperemia, edema, and granular bulge in the lower segment of the ileum (about 30 cm away from the ileocecal valve), and a diffuse polypoid bulge of the mucosa could be observed from about 15 cm from the end of the ileum to the rectum, some of which was nodular with mucosal hyperemia and erosion on the surface. Rectal lesions were lighter than in other intestinal segments (Figure 2D-F). Pathological examination of the descending colon, transverse colon, ascending colon, terminal ileum, lower segment of the ileum, rectum, and sigmoid colon identified proliferative and juvenile polyps under the microscope (Figure 3).
The diagnosis of CCS was finally made.
A thalidomide dose of 150 mg per day (two tablets each time, three times a day) was administered orally to regulate immunity, alongside enteral nutritional support to regulate the intestinal flora, stop the diarrhea, and ameliorate the remaining symptoms. After one week of treatment, the patients’ diarrhea was relieved, and the taste loss, abnormal pigmentation, and malnourished nails were gradually improved. The patient continued the treatment after his discharge from the hospital.
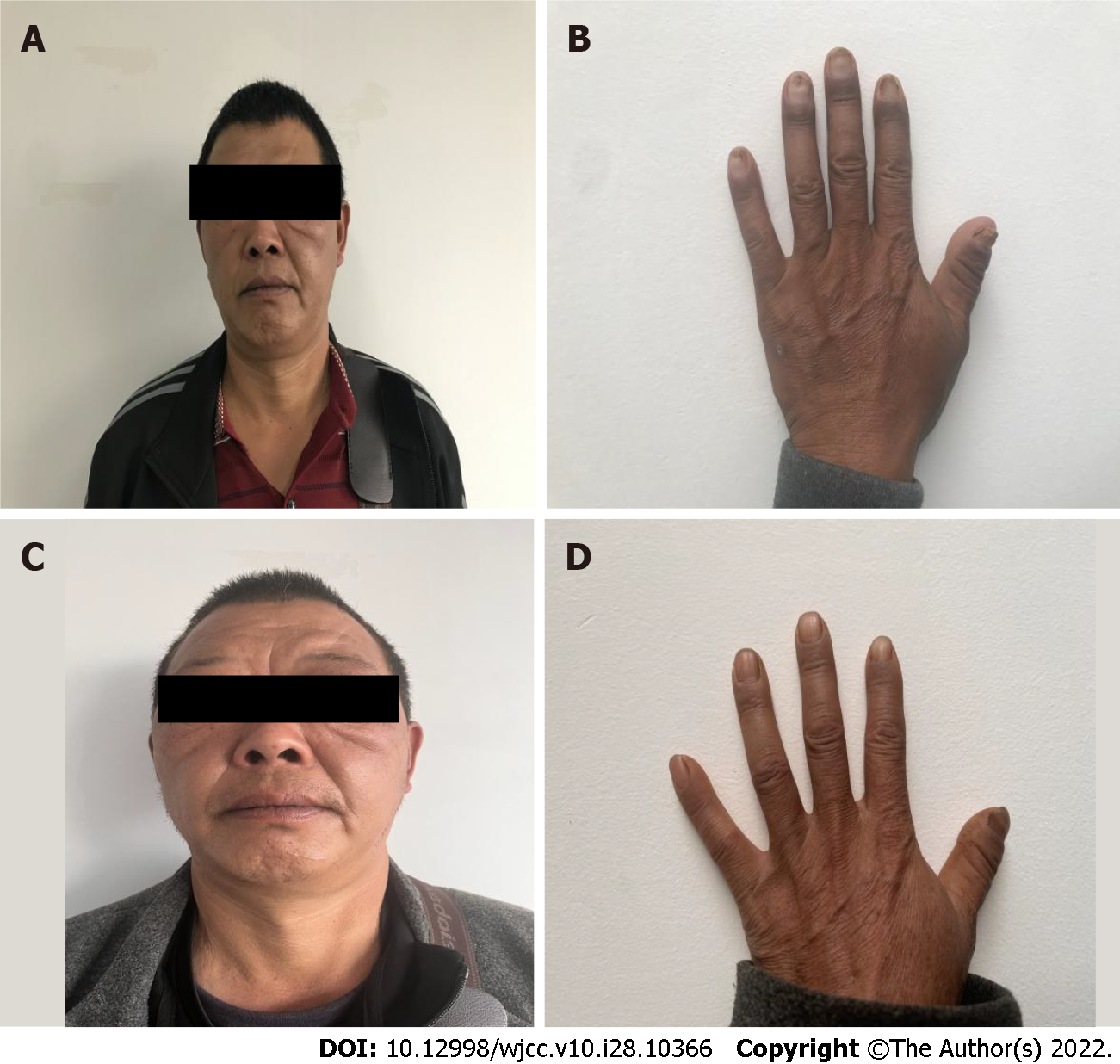
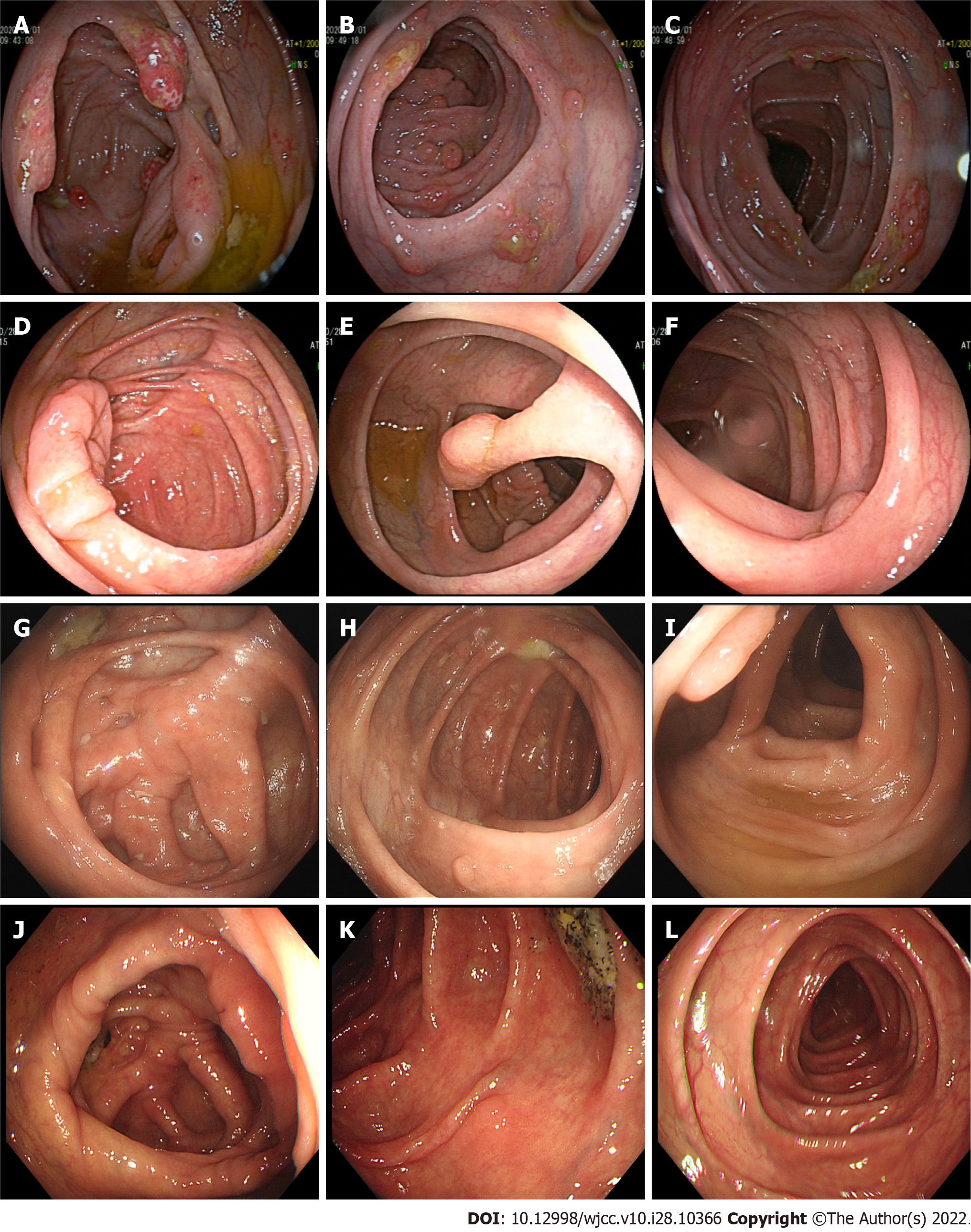
At the first follow-up visit (July 1, 2020), the symptoms of fatigue, diarrhea, and taste loss were significantly improved, the pigmentation around the lips and the back of the hand had improved, and the nail development was close to normal (Figure 4A and B). He bore an albumin level of 38.8 g/L and a platelet count of 5.13 × 1011/L. Gastroscopic examination revealed a diffuse polypoid protuberance of the mucosa of the whole stomach, pylorus, and duodenum and congestion of the surface mucosa (Figure 5A-C). During enteroscopic examination from the ileocecal part to the rectum, a diffuse polypoid protrusion of the mucosa could be observed, some of which was nodular, with congestion and erosion of surface mucosa. The rectal lesions were lighter than other intestinal segments. This examination showed a notable improvement as compared to the previous examination (Figure 6A-C).
At the second follow-up visit (October 28, 2020), the patient felt no discomfort, and the nails had returned to normal without pigmentation (Figure 4C and D). He bore an albumin concentration of 42.4 g/L and a platelet count of 3.35 × 1011/L. Gastroscopic examination of the rough mucosa of the gastric fundus and body identified congestion and edema, and the same was true for the mucosa of the gastric horn, antrum, and duodenum. The lesion improved significantly (Figure 5D-F). Colonoscopy identified > 10 polyps with a diameter of about 0.3-1.0 cm, varying in size and shape throughout the large intestine, and with the remaining mucosa being smooth (Figure 6D-F). Thalidomide administration was adjusted to 100 mg per day (two tablets each time, two times a day) for treatment maintenance.
At the third follow-up visit (June 8, 2021), the patient felt no discomfort, and his nails had returned to normal without pigmentation. His albumin level was 47.1 g/L, and his platelet count was 2.46 × 1011/L. Gastroscopic examination demonstrated congestion and edema of the gastric fundus, gastric body, gastric horn, gastric antrum, and duodenal mucosa. Colonoscopy identified > 10 polyps of different sizes and shapes with a diameter of about 0.3-1.0 cm throughout the whole large intestine. Since the larger polyps had not subsided after drug treatment and there was a risk of tumorigenesis, the polyps were removed by endoscopic high-frequency electrocoagulation. The pathological examination showed the presence of tubular adenoma of the colon with low-grade intraepithelial neoplasia (Figure 6G-I). After successful recovery and discharge, the patient was followed up in the outpatient department, and thalidomide treatment was reduced to 75 mg per day (three tablets each time, once a day).
After the fourth follow-up visit (January 14, 2022), the patient felt no discomfort, and the nails had returned to normal without pigmentation. His albumin level was 50 g/L, and his platelet count was 2.74 × 1011/L. Gastroscopic examination revealed the presence of congestion and edema of the gastric fundus, gastric body, and duodenal mucosa. Enteroscopy identified three polyps with a diameter of about 0.5-1.0 cm in the hepatic flexure, descending colon, and sigmoid colon (Figure 6J-L). The patient was instructed to adjust thalidomide to 100 mg per day at present and is still under follow-up.
CCS is a rare and non-hereditary disease characterized by multiple polyps and ectodermal changes in the digestive tract. A retrospective study of 103 cases of CCS in China in 2020[6] found that the incidence rate of CCS among people aged 50-70 years was high (62.62%), most of them were men (72.82%), and 50 patients (51.02%) received corticosteroid treatment, which is the treatment most frequently deployed. The etiology and pathogenesis of CCS are not clear and may be related to diverse etiologies, such as immunity, infection, inflammation, lack of growth factors, arsenic poisoning, fatigue, stress response, or mental stress[7,8]. Increasing evidence supports autoimmune diseases as an underlying cause of CCS pathogenesis (Hashimoto’s thyroiditis, membranous nephropathy, rheumatoid arthritis, systemic lupus erythematosus[9]) accompanied by potentially increased levels of blood antinuclear antibody and IgG4. The typical characteristics of CCS include abdominal pain, diarrhea, hair loss, loss of finger or toenails, abnormal skin pigmentation, decreased libido and taste, weight loss due to insufficient food intake, malabsorption and gastrointestinal tract loss[10], hypoproteinemia, hypokalemia, and hypocalcemia, to name a few. Under endoscopy, it is often manifested by the presence of multiple polyps in the digestive tract below the esophagus. The diagnosis of CCS should be implemented comprehensively. At present, there is no unified diagnostic standard. Endoscopic features include diffuse polyps throughout the entire gastrointestinal tract, except for the esophagus. Pathological types of polyps in CCS mainly include inflammatory, hyperplastic, hamartomatous, and adenomatous polyps. Observed under the microscope, CCS polyps in different parts show some common features with relatively specific morphological manifestations, including edema and widening of the muscularis propria, inflammatory cell infiltration, local cystic expansion of glands, and filling with proteins. Fluid or condensed mucus; even if normal mucosa is observed under endoscopy, biopsy often has abnormal manifestations, such as edema with a chronic inflammatory reaction, etc. Generally, when other gastrointestinal polyp syndromes are excluded and the patient presents typical endoscopic and histopathological manifestations, as well as gastrointestinal symptoms, such as diarrhea and ectodermal changes, CCS should be considered.
This case has been of an older man with chronic onset, mainly manifested by diarrhea, fatigue, decreased taste, and significant weight loss, with pigmentation around his lips and hands, as well as thickening and atrophy of his finger and toenails. The patient had a history of hyperthyroidism, Grave’s eye disease, and other diseases but bore no significant family history. His symptoms were accompanied by hypoalbuminemia and hypocalcemia. Gastroenteroscopy revealed diffuse mucosal polyp-like protuberances. The disease was diagnosed as proliferative polyps and tubular adenoma. After excluding familial genetic diseases with multiple intestinal polyps as main manifestations, such as familial adenomatous polyposis, Peutz-Jeghers syndrome, and other diseases, the diagnosis of CCS was comprehensively considered.
At present, there is no standard treatment for CCS, and few patients recover without treatment[11]. The currently used CCS treatments include glucocorticoids, antibiotics, 5-aminosalicylic acid, H2 receptor antagonists, calcineurin inhibitors, cyclosporine, azathioprine and anti-tumor necrosis factor antagonists, Helicobacter pylori eradication, fecal bacteria transplantation, nutritional support, and other symptomatic treatments[12-19]. Glucocorticoids are a commonly used treatment method that can quickly and effectively elicit disease remission. However, with the reduction or even withdrawal of glucocorticoids, some patients will relapse or even aggravate the disease. More than 35% of patients fail to achieve long-term clinical remission after taking glucocorticoids[20]. So far, there is no relevant report on thalidomide in the treatment of CCS.
Thalidomide is an effective tumor necrosis factor inhibitor with immunosuppressive, immunomodulatory, anti-inflammatory, and potentially anti-tumor activities[21]. It has been widely used in the clinic to treat autoimmune diseases, such as leprosy nodular erythema, vasculitis, ankylosing spondylitis, rheumatoid arthritis, Behcet’s disease, and inflammatory bowel disease[22-24]. Thalidomide can inhibit TNF-α transcription and the cytochrome pathway by binding α1-acid glycoproteins and inhibiting the secretion of TNF-α. Studies have shown that TNF-α is strongly positive in macrophages and lymphocytes in CCS patients[25]. In fact, CCS could be characterized as an immune disorder syndrome mediated by IgG4 plasma cells[26]. Previous studies have reported the effect of thalidomide on hormone and immunosuppressant treatment of IgG4-related diseases. Therefore, we speculated that thalidomide, an immunosuppressant, may be effective in the treatment of CCS. At the same time, compared with hormones, thalidomide acts faster and presents a lower risk of causing infertility. Its use also allows for avoiding other side effects such as osteoporosis, ischemic osteonecrosis, and peptic ulcer caused by hormones. Therefore, we chose thalidomide for its immunomodulatory properties. The long-term follow-up results showed that after taking thalidomide, the clinical symptoms of CCS were quickly relieved, could be maintained for a long time, had only a small economic burden, and were effective and convenient.
CCS prognosis is generally poor. If there is no treatment or treatment delay, the mortality rate of CCS can be as high as 55%. Malnutrition, hypoproteinemia, repeated infection, sepsis, heart failure, and gastrointestinal bleeding are the common causes of death from the disease[26]. Patients with this disease also bear the risk of malignant tumors. For example, intestinal polyps can be adenomatous polyps and serrated adenomas, both of which are precancerous lesions of colorectal cancer. Therefore, regular monitoring and follow-up are required during the treatment of CCS. If polyps that cannot be subsided by drug administration are found, they need to be removed under endoscopy in time. Experts generally believe that endoscopic monitoring should be carried out every 6-12 mo in order to reduce the mortality rate due to CCS. The patient, in this case, was followed up regularly. Polyps that had not subsided with thalidomide treatment during the follow-up period were timely combined with endoscopic resection, and the postoperative pathological examination was atypical hyperplasia, suggesting that we could early identify precancerous lesions, reduce the risk of polyp malignancies, and obtain a good prognosis.
Our case report is limited to only one patient, and the follow-up time has not been long enough to provide meaningful statistical results. The benefit of thalidomide combined with endoscopy in the treatment of CCS has not been confirmed using a larger sample. Therefore, further clinical studies are needed to determine the dose and duration of treatment and evaluate the long-term efficacy and side effects of thalidomide.
At present, the etiology, pathogenesis, and reasonable treatment plan of CCS disease are still in the exploratory stage. The clinical data, diagnosis, and treatment of thalidomide combined with endoscopic therapy in this patient with CCS suggest that thalidomide may be an effective treatment for CCS and thus provide a reference for clinicians.
We would like to thank the patients and the members of the team for their participation in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin MS, Taiwan; Mirazimi SMA, Iran S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | CRONKHITE LW Jr, CANADA WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med. 1955;252:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 247] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Vashistha N, Chakravarty S, Singhal D. Cronkhite-Canada syndrome. Gastrointest Endosc. 2017;86: 922-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Oba MS, Murakami Y, Nishiwaki Y, Asakura K, Ohfuji S, Fukushima W, Nakamura Y, Suzuki Y. Estimated Prevalence of Cronkhite-Canada Syndrome, Chronic Enteropathy Associated With SLCO2A1 Gene, and Intestinal Behçet's Disease in Japan in 2017: A Nationwide Survey. J Epidemiol. 2021;31:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Watanabe C, Komoto S, Tomita K, Hokari R, Tanaka M, Hirata I, Hibi T, Kaunitz JD, Miura S. Endoscopic and clinical evaluation of treatment and prognosis of Cronkhite-Canada syndrome: a Japanese nationwide survey. J Gastroenterol. 2016;51:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Chakrabarti S. Cronkhite-Canada Syndrome (CCS)-A Rare Case Report. J Clin Diagn Res. 2015;9:OD08-OD09. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Lu Y, Huang F, Wang Y, Zhou J, Zhao Q, Liu L. Clinical and Endoscopic Characteristics of Chinese Cronkhite-Canada Syndrome Patients: A Retrospective Study of 103 Cases. Dig Dis. 2021;39:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Takeuchi Y, Yoshikawa M, Tsukamoto N, Shiroi A, Hoshida Y, Enomoto Y, Kimura T, Yamamoto K, Shiiki H, Kikuchi E, Fukui H. Cronkhite-Canada syndrome with colon cancer, portal thrombosis, high titer of antinuclear antibodies, and membranous glomerulonephritis. J Gastroenterol. 2003;38:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Ward EM, Wolfsen HC. Pharmacological management of Cronkhite-Canada syndrome. Expert Opin Pharmacother. 2003;4:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Firth C, Harris LA, Smith ML, Thomas LF. A Case Report of Cronkhite-Canada Syndrome Complicated by Membranous Nephropathy. Case Rep Nephrol Dial. 2018;8:261-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Slavik T, Montgomery EA. Cronkhite-Canada syndrome six decades on: the many faces of an enigmatic disease. J Clin Pathol. 2014;67:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | She Q, Jiang JX, Si XM, Tian XY, Shi RH, Zhang GX. A severe course of Cronkhite-Canada syndrome and the review of clinical features and therapy in 49 Chinese patients. Turk J Gastroenterol. 2013;24: 277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Daniel ES, Ludwig SL, Lewin KJ, Ruprecht RM, Rajacich GM, Schwabe AD. The Cronkhite-Canada Syndrome. An analysis of clinical and pathologic features and therapy in 55 patients. Medicine (Baltimore). 1982;61:293-309. [PubMed] |
| 13. | Kim SY, Shin J, Park JS, Cha B, Seo Y, Park SH, Lee JH, Kim JS, Kwon G. The first report on effect of fecal microbiota transplantation as a complementary treatment in a patient with steroid-refractory Cronkhite-Canada syndrome: A case report. Medicine (Baltimore). 2022;101:e29135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Lê KA, Li Y, Xu X, Yang W, Liu T, Zhao X, Tang YG, Cai D, Go VL, Pandol S, Hui H. Alterations in fecal Lactobacillus and Bifidobacterium species in type 2 diabetic patients in Southern China population. Front Physiol. 2012;3:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Nagy A, Tóth L, Theisz J, Bajkó N, Zolnai Z, Varga M, Igaz I. Cronkhite-Canada syndrome. Orv Hetil. 2021;162:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Okamoto K, Isomoto H, Shikuwa S, Nishiyama H, Ito M, Kohno S. A case of Cronkhite-Canada syndrome: remission after treatment with anti-Helicobacter pylori regimen. Digestion. 2008;78:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Sweetser S, Alexander GL, Boardman LA. A case of Cronkhite-Canada syndrome presenting with adenomatous and inflammatory colon polyps. Nat Rev Gastroenterol Hepatol. 2010;7:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Watanabe D, Ooi M, Hoshi N, Kohashi M, Yoshie T, Ikehara N, Yoshida M, Yanagita E, Yamasaki T, Itoh T, Azuma T. Successful treatment of Cronkhite-Canada syndrome using anti-tumor necrosis factor antibody therapy. Endoscopy. 2014;46 Suppl 1 UCTN:E476-E477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Yu X, Wang C, Wang M, Wu Y, Zhang L, Yang Q, Chen L. Cronkhite-Canada syndrome: a retrospective analysis of four cases at a single medical center. Scand J Gastroenterol. 2022;57:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Liu S, You Y, Ruan G, Zhou L, Chen D, Wu D, Yan X, Zhang S, Zhou W, Li J, Qian J. The Long-Term Clinical and Endoscopic Outcomes of Cronkhite-Canada Syndrome. Clin Transl Gastroenterol. 2020;11:e00167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Tjiu JW, Hsiao CH, Tsai TF. Cutaneous Rosai-Dorfman disease: remission with thalidomide treatment. Br J Dermatol. 2003;148:1060-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Lehman TJ, Schechter SJ, Sundel RP, Oliveira SK, Huttenlocher A, Onel KB. Thalidomide for severe systemic onset juvenile rheumatoid arthritis: A multicenter study. J Pediatr. 2004;145:856-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Letsinger JA, McCarty MA, Jorizzo JL. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J Am Acad Dermatol. 2005;52:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Yang C, Singh P, Singh H, Le ML, El-Matary W. Systematic review: thalidomide and thalidomide analogues for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1079-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Martinek J, Chvatalova T, Zavada F, Vankova P, Tuckova I, Zavoral M. A fulminant course of Cronkhite-Canada syndrome. Endoscopy. 2010;42 Suppl 2:E350-E351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Sweetser S, Ahlquist DA, Osborn NK, Sanderson SO, Smyrk TC, Chari ST, Boardman LA. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci. 2012;57:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |