Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10266
Peer-review started: May 3, 2022
First decision: June 7, 2022
Revised: June 13, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: October 6, 2022
Processing time: 146 Days and 23.5 Hours
Kawasaki disease (KD) is a self-limiting febrile illness and an acute vasculitis with an unknown origin. It predominantly affects children aged < 5 years. KD is the common cause of acquired heart disease in children. We here report a case of KD in an asymptomatic young female patient diagnosed with multiple coronary aneurysms with calcification.
A 29-year-old female patient admitted to Hangzhou First People's Hospital with coronary artery abnormality identified for 1 wk. The patient was asymptomatic; however, chest computed tomography occasionally revealed strip-like dense shadows in the coronal sulcus. After coronary angiography and Doppler echocardiography, the final diagnosis was coronary artery aneurysms (CAAs) caused by KD. Although the patient was asymptomatic with no history of KD in childhood, the definitive diagnosis was CAAs caused by KD. The patient was administered anticoagulant, and surgical treatment was recommended.
KD potentially causes CAAs in 25% of untreated cases, primarily occurring in the proximal portions of the coronary arteries.
Core Tip: Kawasaki disease (KD) is the self-limited febrile illness and predominantly affects children < 5 years of age. Here, we report a case of KD in a young girl with coronary artery aneurysms, but with no symptoms. Coronary artery aneurysms occur primarily in the proximal portions of the major coronary arteries in KD, which may result in myocardial infarction. Patients should be diagnosed and treated immediately to obtain a favorable prognosis.
- Citation: He Y, Ji H, Xie JC, Zhou L. Coronary artery aneurysms caused by Kawasaki disease in an adult: A case report and literature review. World J Clin Cases 2022; 10(28): 10266-10272
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10266.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10266
Kawasaki disease (KD) is an acute vasculitis with an unknown cause and predominantly affects children under five years[1]. More than 60 countries have reported cases of KD. Notably, KD has a significant ethnic variation. For instance, Asian/Pacific Islanders have the highest incidence of 29.8 in 100000 children under five years. Nevertheless, the incidence in white children is only 13.7 in 100000[2].
Moreover, according to a 2015 Japanese KD survey, the incidence rate of KD was 330.2 in 100000 children[3,4]. Typical clinical features of KD include fever persistence for five days or more, bilateral conjunctival congestion, changes in lips and oral cavity, polymorphous exanthema, the changes of peripheral extremities, as well as acute non-purulent cervical lymphadenopathy[5]. In the acute phase, erythema and edema manifest in the hands, whereas feet and periungual desquamation was remarkable[1]. Nonetheless, patients diagnosed in adulthood are asymptomatic with no history of KD in childhood. They instead present coronary disease without other findings. Coronary artery aneurysms (CAAs) are another KD complication, mostly occurring in the proximal coronary artery. Most KD patients with CAAs are symptomatic. We here report a rare case of KD in an asymptomatic young female with CAAs, and discuss the diagnosis and treatment of KD.
A 29-year-old female patient was admitted to our hospital on April 3, 2019, due to the presence of coronary artery abnormality for one week.
When the patient had a medical check-up one week earlier, a computed tomography (CT) scan of the lungs revealed postoperative cardiac changes. The patient had no obvious discomfort. One day earlier, at the outpatient department of Hangzhou First People's Hospital, echocardiography was performed, and showed coronary artery changes associated with KD. The patient was hospitalized at the Department of Cardiology for further diagnosis and treatment.
The patient reported no history of KD hypertension, diabetes, coronary heart disease, and neurodevelopmental disorders, no history of surgery, and no family history of related genetic disorders.
The patient had no relevant personal and family history.
On examination, the patient had a temperature of 36.7°C, blood pressure of 131/70 mmHg (1 mmHg = 0.133 kPa), and heart rate of 73 beats/min. The heart rhythm was regular, the heart boundary was not enlarged, and there were no murmurs in each valve area. The whole abdomen was flat without rebound tenderness. Also, no edema was observed in both lower limbs.
Blood routine examination, and liver function, kidney function, coagulation function and autoantibody tests were normal.
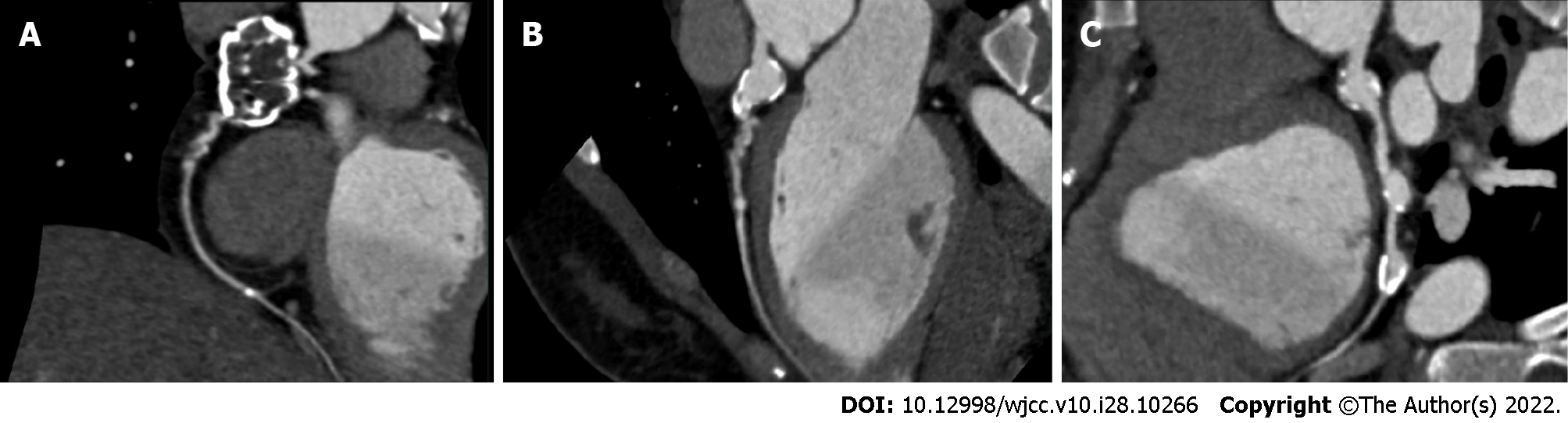
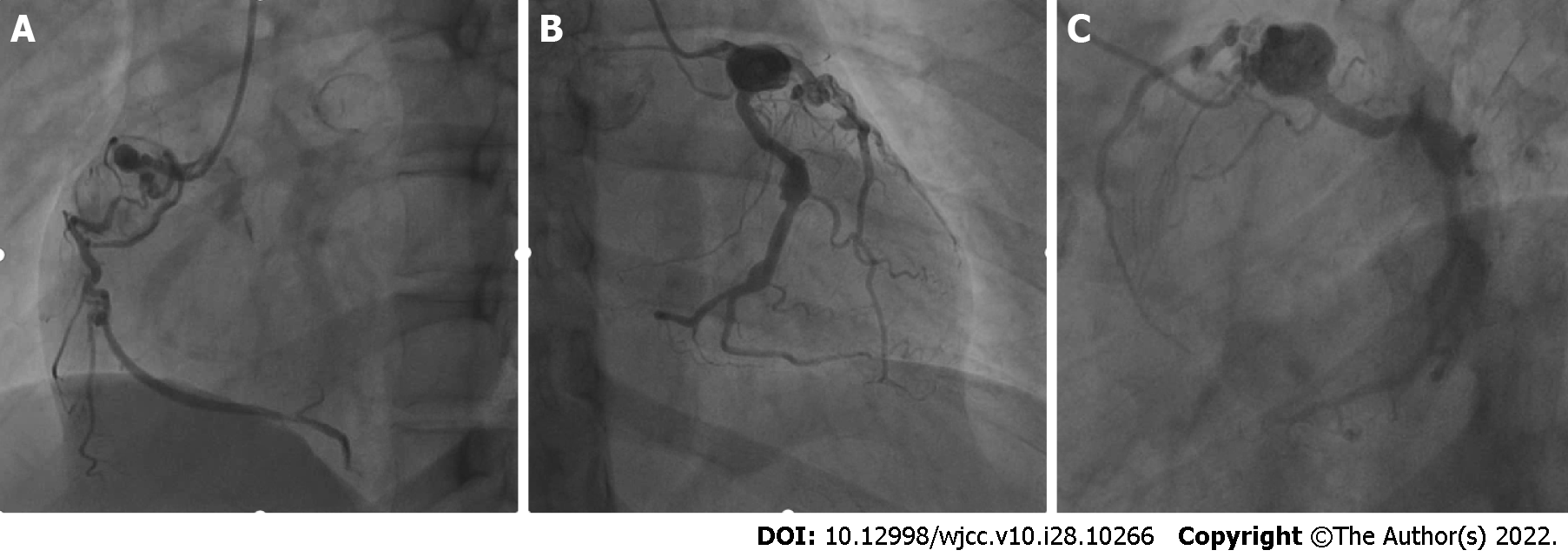
A CT scan of the lungs showed occasional strip-like dense shadows at the coronal sulcus. An electrocardiogram showed sinus arrhythmia and wandering heart rate in the sinoatrial node. Exercise treadmill test showed negative outcomes. To further establish the cause of coronary artery abnormality, the patient underwent Doppler echocardiography and coronary artery computed tomography angiography (CTA). Irregular widening and enhanced wall echo at the beginning of the coronary artery, and multiple CAAs with thrombogenesis were observed (Figure 1). The cause of coronary artery ectasia remained to be determined, and coronary artery changes were associated with KD. And then, CAG revealed CAAs in multiple branches of coronary arteries with thrombosis and calcification (Figure 2). Coronary artery ectasia was observed at the extremity of left main coronary artery. The vessels in the descending proximal left anterior were tortuous with thrombus. The distal vessels were in the myocardial bridge. Moreover, two hemangiomas were observed at the extremity of the left circumflex artery with calcification. In addition, a huge coronary artery aneurysm was in a proximal segment of the right coronary artery with an organized thrombus. The vascular wall was calcified with curved residual blood vessels. There was arteriosclerosis in the distal vessels, narrowing by 30%-40%.
The final diagnosis was CAAs caused by KD based on coronary angiography and other examinations.
The patient was administered 0.1 g acetylsalicylic acid (ASA) and 75 mg Clopidogrel Hydrogen Sulphate Tablets daily to resist platelets. The patient was also administered Metoprolol Succinate Sustained-release Tablets to control ventricular rate. Further surgical treatment was recommended. However, the patient refused it. Post-discharge medication was adjusted to Rivaroxaban and Metoprolol Succinate Sustained-release Tablets.
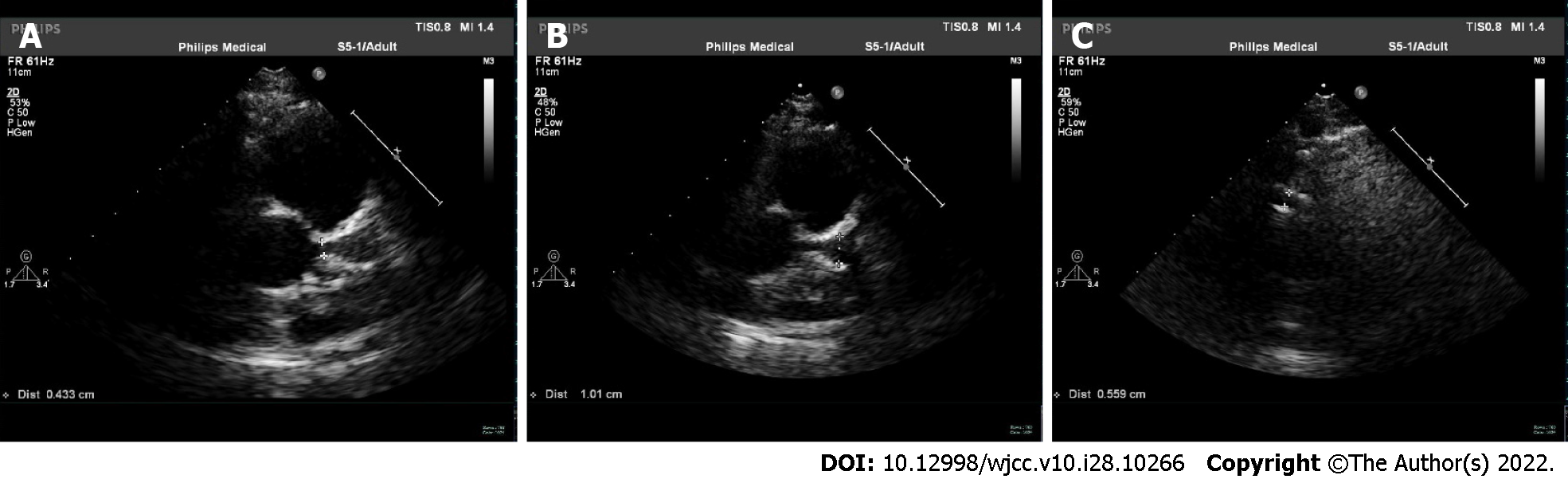
The patient was followed up for nearly three years. The patient was effectively improved without apparent discomfort. Doppler echocardiography was performed one year after discharge. The inner diameter of the left main coronary artery was 0.4 cm; the inner diameter of the aneurysm near the cross of vessels was 1.0 cm; the inner diameter of the right coronary artery was 0.56 cm (Figure 3). We found no significant changes in coronary arteries with an ejection fraction (EF) value of 0.69. Doppler echocardiography two years after discharge showed no significant changes in coronary arteries with an EF value of 0.64.
KD is an acute vasculitis with unknown origin and predominantly affects children under five years, resulting in multi-system inflammatory syndrome[1]. It is also known as mucocutaneous lymph node syndrome. KD may be caused by pathogen infection, vaccination, environmental factors, inherited genetic susceptibility, and immune response[6]. The pathological vascular changes of KD are subdivided into three processes[7]. In the early stages of KD, coronary arteries undergo mixed inflammatory cell infiltration. The second is the primary stage of coronary artery injury and aneurysm formation. Fibrosis of blood vessels and myocardium is the third and final stage of coronary artery disease[6]. Over 60 countries have reported cases of KD. Typical clinical features include fever which persists for five days or more, bilateral conjunctival congestion, changes in lips and oral cavity, polymorphous exanthema, changes in peripheral extremities, and acute non-purulent cervical lymphadenopathy[5]. Table 1 shows a review of Clinical Characteristics, Management, and Outcome of Coronary Artery Aneurysm (CAAs) caused by KD; various symptoms were observed[8-20].
| Ref. | Age (yr) | Sex | Indication | Sites | Sizes | FU | Operation | Antithrombotic therapy | Adjuvant drug | Outcome |
| Hu et al[8], 2014 | 8 | Male | Ruptured coronary aneurysm | LAD/RCA | 3.7 mm/5.2 mm | 12 d | NO | ASA | IVIG (2 g/kg), ramipril | Dead |
| Sato et al[9], 2014 | 35 | Male | AMI | LAD | 2.3 mm × 2.0 mm | 9 yr | PCI | NO | NO | Stable |
| Matsushita et al[10], 2014 | 32 | Male | AMI | LAD/RCA | NA | 30 yr | PCI | ASA | NO | Stable |
| Ekici et al[11], 2014 | 4 mo | NA | MI | LAD/RCA | 6.5 mm/6.7 mm | 51 d | NO | ASA, LMWH | IVIG (2 g/kg), acetylsalicylic acid | Dead |
| Luu et al[12], 2015 | 17 | Male | MI | LAD/LCX/RCA | NA | 18 mo | PCI | ASA, clopidogrel | Bisoprolol, ramipril | Stable |
| Chong et al[13], 2018 | 9 | Female | Severe respiratory failure | LAD | 7 mm | 8 mo | NO | ASA, Enoxaparin, warfarin | IVIG (2 g/kg) | Stable |
| Takai et al[14], 2019 | 3 | Male | Fever | RCA | 8.3 mm | 3 mo | NO | ASA, ticlopidine, warfarin, | IVIG (2 g/kg), urinastatin, infliximab, enalapril | Stable |
| Tsuda et al[15], 2020 | 58 | Female | Palpitate | LAD | NA | NA | Implantable defibrillator, Implantation, radiofrequency catheter ablation | ASA | Beta-blocker, verapamil | Stable |
| Chen et al[16], 2020 | 22 | Male | AMI | LMCA | 18 - 20 mm | 2 mo | Heart transplant | Rivaroxaban, clopidogrel | Metoprolol, rosuvastatin, spironolactone | Stable |
| Fujioka et al[17], 2021 | 33 | Female | Postpartum | RCA | 25 mm | 5 mo | Resection, CABG | ASA, ticlopidine hydrochloride | NA | Stable |
| Wang et al[18], 2021 | 5 mo | Male | Cerebral infarction | LAD/RCA | 11 mm × 9 mm/19 mm × 14 mm | 15 mo | NA | ASA, clopidogrel | IVIG (2 g/kg) | Dead |
| Almeshary et al[19], 2021 | 4 mo | NA | Fever | LMCA/LAD/RCA | 4.6 mm/3.8 mm/4.2 mm | 1 mo | NA | ASA | IVIG (2 g/kg) | Stable |
| Toyoshima et al[20], 2022 | 14 | Female | AMI | LMCA/LAD | 7.2 mm/4.0 mm | 1 yr | CABG | warfarin, clopidogrel | Carvedilol, enalapril | Stable |
Nevertheless, a few patients are asymptomatic with no history of KD in childhood. We here report an asymptomatic 29-year-old female patient who had CAAs caused by KD. After carefully reviewing the coronary artery CTA and coronary angiography images, the cause of CAAs was KD. Therefore, attention should be paid to asymptomatic patients by conducting Doppler echocardiography and coronary angiography to confirm the KD diagnosis. CAAs caused by KD primarily occur in the proximal coronary artery. The diagnostic tests include Doppler echocardiography, magnetic resonance angiography, coronary artery CTA, and coronary arteriography. KD diagnostic indicators include a fever that persists for five days or more with at least 4 of the 5 principal clinical features[21]. These principal clinical features include bilateral conjunctival congestion, changes in lips and oral cavity, polymorphous exanthema, changes in peripheral extremities, and acute non-purulent cervical lymphadenopathy[5].
In addition, incomplete KD is evaluated in patients without complete clinical features of classic KD, and diagnosis is confirmed if coronary artery abnormalities are detected[21]. Thus, our patient conforms to the diagnosis of incomplete KD. Regular Doppler echocardiography is also important in the diagnosis. The coronary artery CTA and coronary arteriography show the location and extent of CAAs. We performed the coronary artery CTA and coronary arteriography and confirmed that KD caused CAAs. Noteworthy, CAAs and thrombus in the lumen are severe complications of KD. These complications result in myocardial infarction and ischemic heart disease. The diameter of CAAs greater than or equal to 5 mm has a higher risk of thrombosis[22]. The patient experienced no discomfort; however, CAAs had calcification and thrombosis.
Primary therapy includes intravenous immunoglobulin (IVIG) and ASA. The refractory cases require corticosteroids, tumor necrosis factor (TNF) inhibition, interleukin 1 inhibition, calcineurin inhibition, etc[1]. IVIG is most effective when used within 10 days of fever onset. Therefore, the risk of CAAs decreases from 20%-25% to 3%-5% in patients with appropriate treatment[23,24]. Additionally, ASA should be administered at a moderate dose (30-50 mg/kg/d)[25]. By early adjunctive corticosteroid therapy, patients with a higher risk of poor coronary outcomes can significantly benefit from corticosteroids[26,27]. TNF inhibitors, interleukin 1 inhibition, and calcineurin inhibition are uncommonly used. Primary prevention of thrombosis was fundamental in this patient. ASA and Clopidogrel Hydrogen Sulphate Tablets were administered to resist platelets. We adjusted post-discharge medication to Rivaroxaban and Metoprolol Succinate Sustained-Release Tablet. Although this therapy did not yield a complete cure, it provided a reference for subsequent treatment strategies, i.e., heart bypass surgery.
In addition, the maximum Z score of proximal LCA or RCA (maximum Z of CA) can be used as an index for long-term follow-up to evaluate the ability of KD patients to achieve coronary perfusion during exercise[28]. Compared with normal children, KD children have a higher prevalence of epilepsy and Tourette's syndrome[29]. Other functional impairments have also been mentioned, such as facial paralysis, sensorineural hearing and visual loss, ataxia, and behavioral disorders[30].
The most significant clinical outcome of KD is inflammation of the coronary arteries. KD can be classified into complete KD and incomplete KD. KD may lead to CAAs in 25% of untreated cases. CAAs occur primarily in the proximal portions of the major coronary arteries in KD, which further results in myocardial infarction. Patients should be diagnosed and treated immediately to obtain a favorable prognosis. More research attention should be paid to asymptomatic KD patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Moretti A, Italy S-Editor: Liu JH L-Editor: Ma JY P-Editor: Liu JH
| 1. | Rife E, Gedalia A. Kawasaki Disease: an Update. Curr Rheumatol Rep. 2020;22:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 2. | Holman RC, Shahriari A, Effler PV, Belay ED, Schonberger LB. Kawasaki syndrome hospitalizations among children in Hawaii and Connecticut. Arch Pediatr Adolesc Med. 2000;154:804-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Makino N, Nakamura Y, Yashiro M, Kosami K, Matsubara Y, Ae R, Aoyama Y, Yanagawa H. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015-2016. Pediatr Int. 2019;61:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 4. | Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, Kojo T, Uehara R, Kotani K, Yanagawa H. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25:239-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Haider KH, Alshoabi SA, Qurashi AA, Hamid AM. Incidentally discovered Kawasaki disease in an adult man. Pak J Med Sci. 2021;37:2032-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Yuan F, Li Y, Xiao X, Yinfei Z, Xiaohui Gong. Advances in etiology of kawasaki disease and injury mechanism of coronary artery, Molecular Cardiology of China, 2021; 21: 4365-4370. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Takahashi K, Oharaseki T, Yokouchi Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int J Rheum Dis. 2018;21:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Hu P, Wang J, Fan XC, Hu B, Lu L. Hypertension triggers the rupture of coronary artery aneurysm in an 8-year-old boy with Kawasaki disease. J Clin Hypertens (Greenwich). 2014;16:766-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Sato K, Latib A, Costopoulos C, Panoulas VF, Naganuma T, Miyazaki T, Colombo A. A case of Kawasaki's disease with extensive calcifications needing rotational atherectomy with a 2.5mm burr. Cardiovasc Revasc Med. 2014;15:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Matsushita K, Tamura T, Nishiga M, Kaitani K, Izumi C, Nakagawa Y. Acute myocardial infarction and 30-year coronary aneurysm follow-up by serial angiography in a young adult with Kawasaki disease. Cardiovasc Interv Ther. 2015;30:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ekici F, Varan B, Kocabaş A, Erdoğan İ, Eminoğlu S, Aktaş D. Multiple giant aneurysms and stenoses of the coronary and systemic arteries in an infant with kawasaki disease at the early stage of convalescent period. Echocardiography. 2014;31:E147-E150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Luu B, Esmaeili A, Schranz D, Fichtlscherer S. Bioresorbable Vascular Scaffold Implantation for Successful Treatment of a Symptomatic Coronary Lesion in a 17-Year-Old Boy After Kawasaki Disease. Pediatr Cardiol. 2015;36:1539-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Chong CH, Lee SJ, Bullock A, Harris L, Loh R, Knight G, Rueter K. Kawasaki disease: An ongoing challenge. J Paediatr Child Health. 2018;54:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Takai S, Takasawa K, Doi S. Atypical coronary artery aneurysms due to Kawasaki disease in Noonan syndrome with a novel PTPN11 mutation. Cardiol Young. 2019;29:228-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Tsuda E, Noda T, Noguchi T. Two females with coronary artery occlusion caused by presumed Kawasaki disease would have delivered without recognition of ischaemic heart disease. Cardiol Young. 2020;30:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Chen T, Li J, Xu Q, Li X, Lv Q, Wu H. Antithrombotic Therapy of a Young Adult with Giant Left Main Coronary Artery Aneurysm. Int Heart J. 2020;61:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Fujioka T, Asakawa N, Suzuki T, Kobayashi J, Takahashi K, Tsuchiya K. Giant coronary artery aneurysm associated with Kawasaki disease showing progressive dilation over 30 years. J Cardiol Cases. 2021;23:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Wang L, Duan H, Zhou K, Hua Y, Liu X, Wang C. Kawasaki Disease Complicated by Late-Onset Fatal Cerebral Infarction: A Case Report and Literature Review. Front Pediatr. 2021;9:598867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Almeshary MZ, Alanazi SA, Almoosa KM, Bassrawi RK. Kawasaki disease in an infant after administration of hexavalent vaccine. Saudi Med J. 2021;42:790-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Toyoshima Y, Tsuda E, Kato Y, Iwasa T, Sakaguchi H, Shimahara Y, Tabata S, Ikedo T, Shiraishi I, Kurosaki K. Coronary artery aneurysms of unknown origin in a 14-year-old girl. J Cardiol Cases. 2022;25:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Correction to: Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2019;140:e181-e184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Minich LL, Tani LY, Pagotto LT, Young PC, Etheridge SP, Shaddy RE. Usefulness of echocardiography for detection of coronary artery thrombi in patients with Kawasaki disease. Am J Cardiol. 1998;82:1143-1146, A10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Wu MH, Chen HC, Yeh SJ, Lin MT, Huang SC, Huang SK. Prevalence and the long-term coronary risks of patients with Kawasaki disease in a general population <40 years: a national database study. Circ Cardiovasc Qual Outcomes. 2012;5:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 966] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 25. | Zheng X, Yue P, Liu L, Tang C, Ma F, Zhang Y, Wang C, Duan H, Zhou K, Hua Y, Wu G, Li Y. Efficacy between low and high dose aspirin for the initial treatment of Kawasaki disease: Current evidence based on a meta-analysis. PLoS One. 2019;14:e0217274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Wardle AJ, Connolly GM, Seager MJ, Tulloh RM. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2017;1:CD011188. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Kimura M, Harazaki M, Fukuoka T, Asakura I, Sakai H, Kamimaki T, Ohkawara I, Akiyama N, Tsurui S, Iwashima S, Shimomura M, Morishita H, Meguro T, Seto S. Targeted use of prednisolone with the second IVIG dose for refractory Kawasaki disease. Pediatr Int. 2017;59:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Tuan SH, Li MH, Hsu MJ, Tsai YJ, Chen YH, Liao TY, Lin KL. Cardiopulmonary Function, Exercise Capacity, and Echocardiography Finding of Pediatric Patients With Kawasaki Disease: An Observational Study. Medicine (Baltimore). 2016;95:e2444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Lin CH, Lin WD, Chou IC, Lee IC, Hong SY. Heterogeneous neurodevelopmental disorders in children with Kawasaki disease: what is new today? BMC Pediatr. 2019;19:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Moretti A. Are TNF-α blockers effective and safe for Kawasaki disease in children? Int J Rheum Dis. 2020;23:1252-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |