Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10136
Peer-review started: February 8, 2022
First decision: March 23, 2022
Revised: April 5, 2022
Accepted: July 27, 2022
Article in press: July 27, 2022
Published online: October 6, 2022
Processing time: 230 Days and 22.5 Hours
Malignant melanoma is becoming more common among middle-aged individuals all over the world. Melanoma metastasis can be found in various organs, although metastases to the spleen and stomach are rare. Herein we present a rare metastatic multifocal melanoma, clinically and histologically mimicking lymphoma, with metastases of multiple organs.
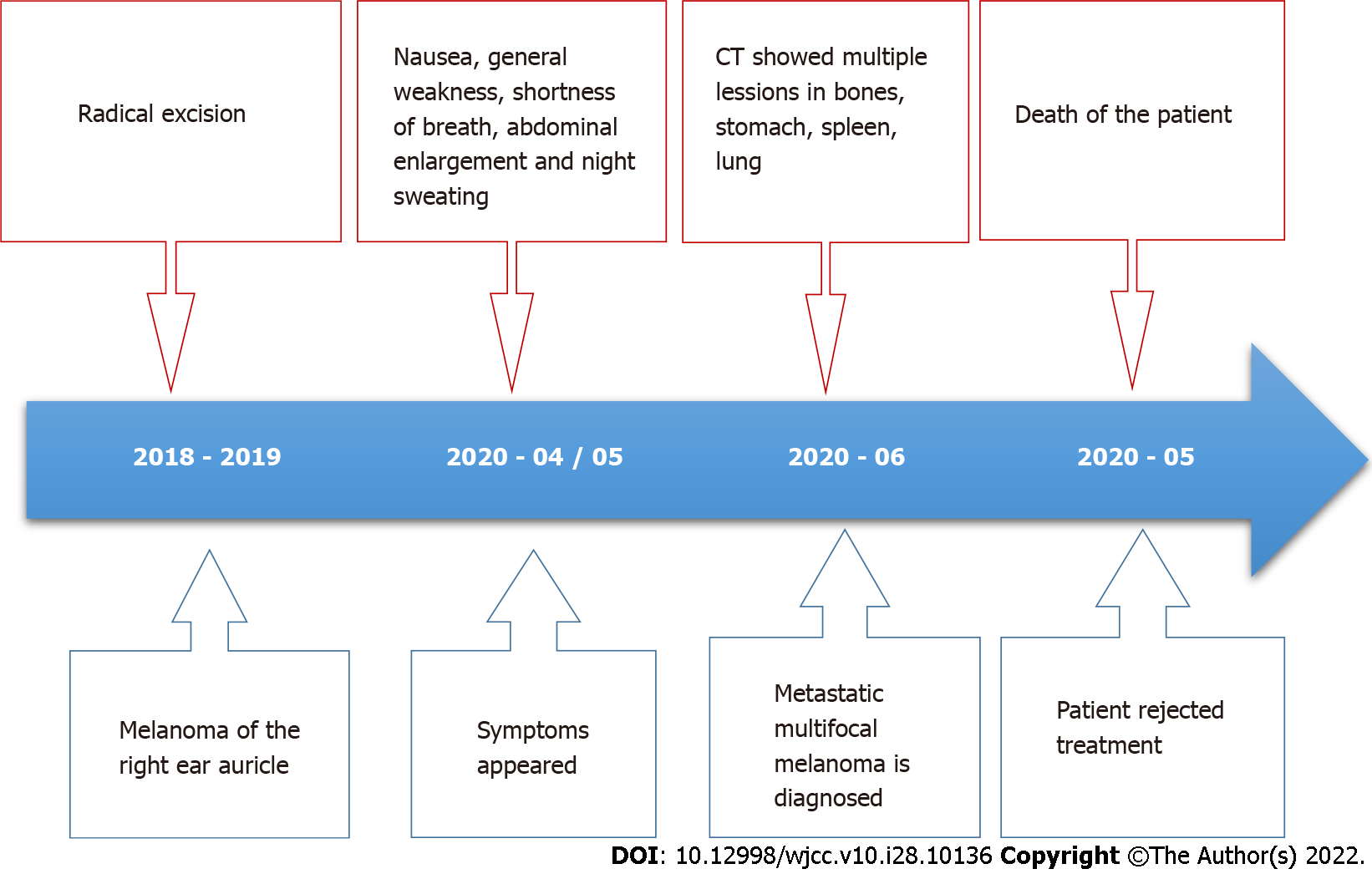
A 46-year-old Caucasian male with a history of nodular cutaneous malignant melanoma was presented with nausea, general weakness, shortness of breath, abdominal enlargement, and night sweating. The abdominal ultrasound revealed enlarged liver and spleen with multiple lesions. Computed tomography demon
This case report highlights the clinical relevance of rare metastatic multifocal melanoma of multiple organ systems.
Core Tip: Malignant melanoma is becoming more common among middle-aged individuals all over the world. Melanoma metastasis can be found in various organs, although multiple metastases to the spleen and stomach are rare. Herein we present a rare metastatic multifocal melanoma, clinically and histologically mimicking lymphoma, with metastases of multiple organs including spleen, stomach, bones, lungs, liver, lymph nodes. This case report highlights the clinical relevance of metastatic multifocal melanoma.
- Citation: Maksimaityte V, Reivytyte R, Milaknyte G, Mickys U, Razanskiene G, Stundys D, Kazenaite E, Valantinas J, Stundiene I. Metastatic multifocal melanoma of multiple organ systems: A case report. World J Clin Cases 2022; 10(28): 10136-10145
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10136.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10136
The incidence rate of melanoma is continuously increasing among middle-aged adults[1]. According to the American Cancer Society, approximately 106110 new cases of melanoma will be diagnosed in the United States for the 2021. Since metastatic melanoma is highly associated with increased mortality, the prognosis for metastatic disease is extremely poor. Approximately 90 percent of patients diagnosed with metastatic melanoma of 3 or more metastases die within one year[2]. The most common metastatic sites of the cutaneous malignant melanoma are lymph nodes, lungs, brain and liver[3]. Though melanoma metastases can be found in almost any part of the body, metastases found in the stomach or spleen are very uncommon[4]. In this case report, we present a patient suffering from malignant cutaneous melanoma with metastases to multiple organs, including stomach, spleen, duodenum, liver, lungs, bones, and lymph nodes.
A 46-year-old Caucasian male was referred to the Department of Hepatology and Gastroenterology complaining of nausea, general weakness, shortness of breath, abdominal enlargement, and night sweating.
Patient’s symptoms started about a month ago.
He had a history of localized cutaneous malignant melanoma of the right ear auricle, status after excision with surgery carried out 1 year ago. The excision of the tumor and histological examination was performed in other institution. The primary tumor was nodular melanoma with ulceration and a Breslow thickness of 3.55 mm. High mitotic activity (58 mitosis per square millimeter) was also mentioned in the pathological report. The sentinel lymph node was negative for metastatic melanoma.
He had no family health history of cancer.
The enlarged and painless lymph node of the neck was found upon physical examination. No other abnormal findings were found.
The initial laboratory tests performed in the Emergency Department showed the increased aspartate transaminase 110 U/L, alanine transaminase 55 U/L (< 40 U/L), alkaline phosphatase 451 U/L (< 40 U/L), lactate dehydrogenase (LDH) 1200 U/L (125-243 U/L), gamma- glutamyl transferase 802 U/L (< 36 U/L), C-reactive protein 168.8 mg/L (0-5 mg/L).
Procalcitonin 7.76 ug/L (< 0.05 L ug/L) levels. Complete blood count revealed anemia with hemoglobin count of 115 g/L and leukocytosis with white blood cells count of 34.87 × 109 per L (4-9.8 × 109 per L) (Table 1).
| Characteristics | Result | Units | Normal value |
| ALT | 110 | U/L | < 40 |
| AST | 55 | U/L | < 40 |
| ALP | 451 | U/L | < 40 |
| LDH | 1200 | U/L | 125-243 |
| GGT | 802 | U/l | < 36 |
| CRP | 168.8 | mg/L | 0-5 |
| PCT | 7.76 | ug/L | < 0.051 |
| Hgb | 115 | g/L | 138-172 |
| WBC | 34.87 | × 109/L | 4-9.8 |
The abdominal ultrasound revealed enlarged liver (308 mm length) and spleen (157 mm length), with multiple hyperechogenic lesions with hypoechogenic shell, the largest with a diameter of 24 mm in the liver. A small amount of ascites was seen in the abdominal cavity.
The patient was referred to the Department of Hepatology and Gastroenterology with a suspected diagnosis of metastatic liver disease, and additional tests were performed.
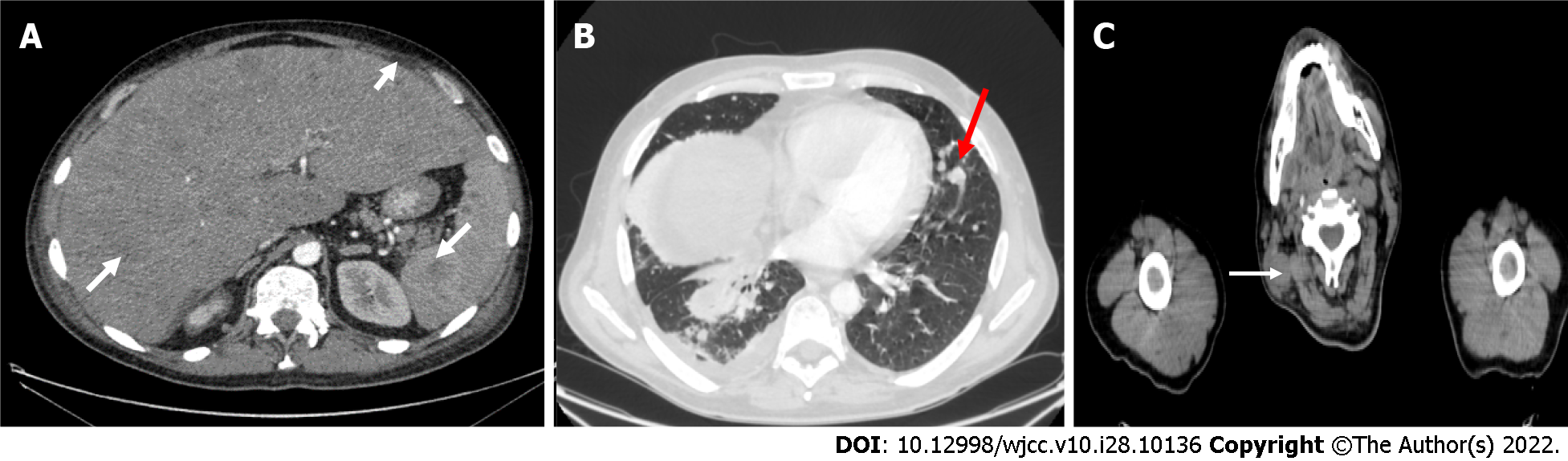
Computed tomography (CT) was performed and demonstrated multiple lesions in the lungs, liver, spleen, subcutaneous tissue, and bones. CT also showed a pathological lymphadenopathy of the neck and the right lung. Fluid was present in both abdominal and pleural cavity (Figure 1).
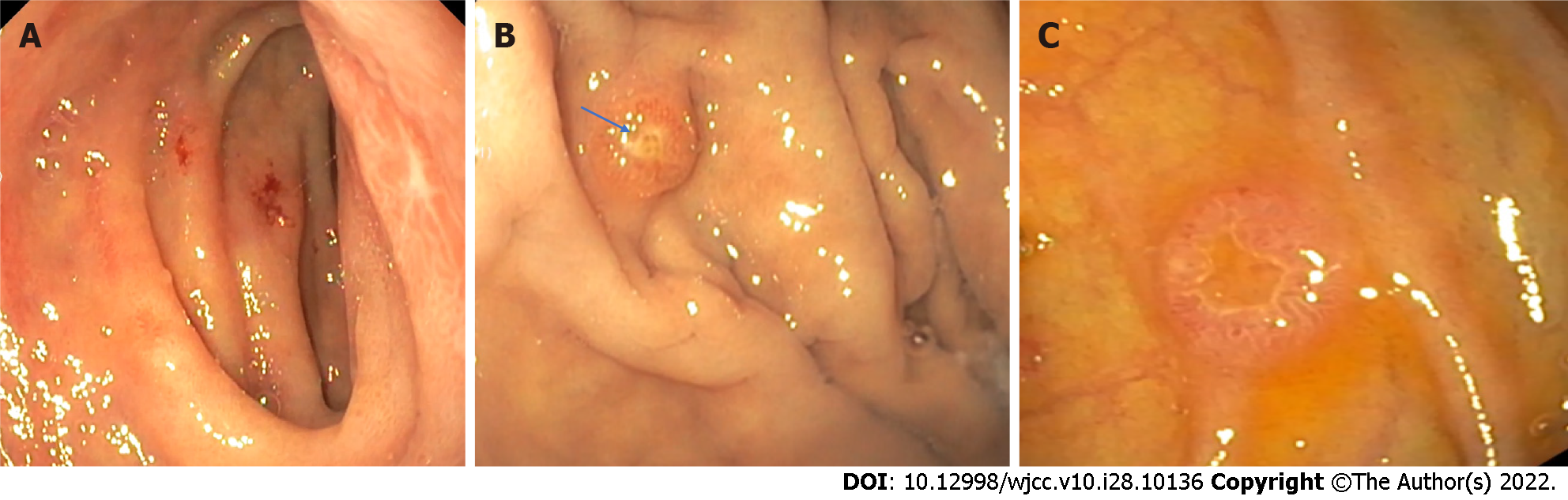
Due to anemia and unknown primary cancer site, an upper endoscopy was performed. Gastroscopy revealed atypical ulcers of the duodenum (Figure 2A). It also showed several 0.5 cm red polyps in gastric body and fundus, also in the descending part of the duodenum (Figure 2B and C). Biopsies were taken and sent for histological evaluation.
Considering the typical symptoms (night sweats, fatigue), high leukocyte count and lesions in multiple organs, lymphoma was suspected. Trephine biopsy and the biopsy from the enlarged lymph node in the neck were taken.
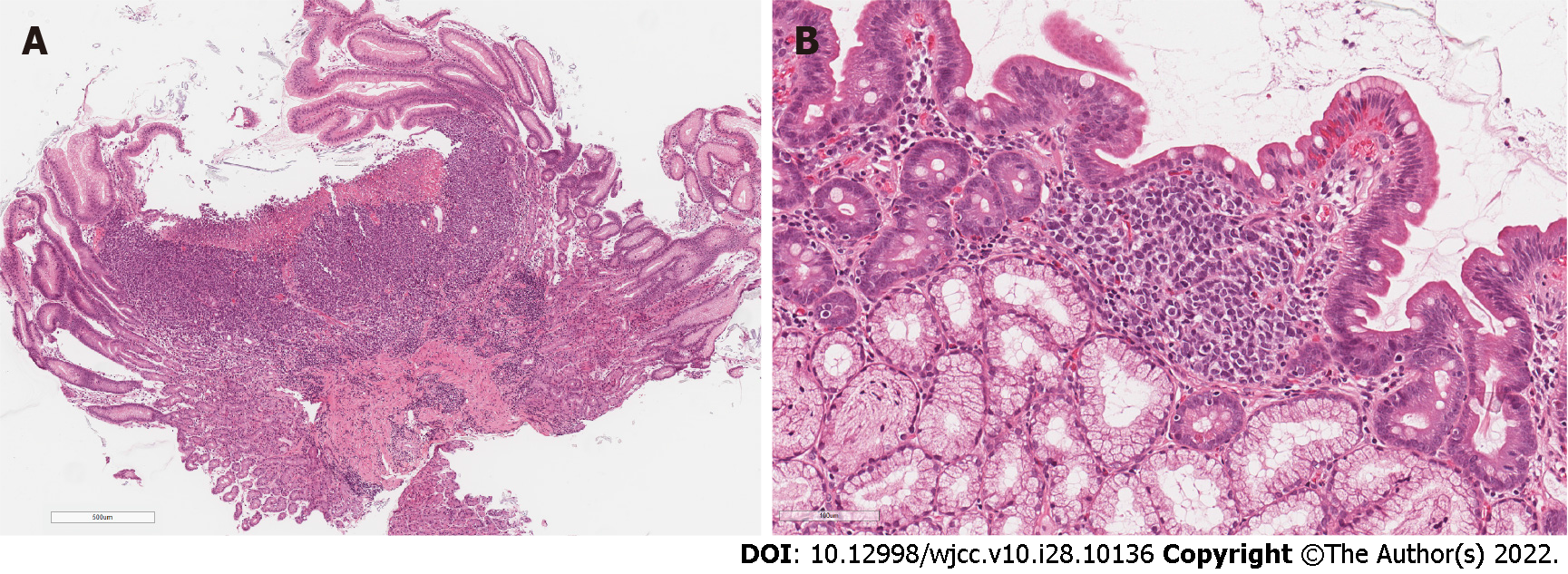
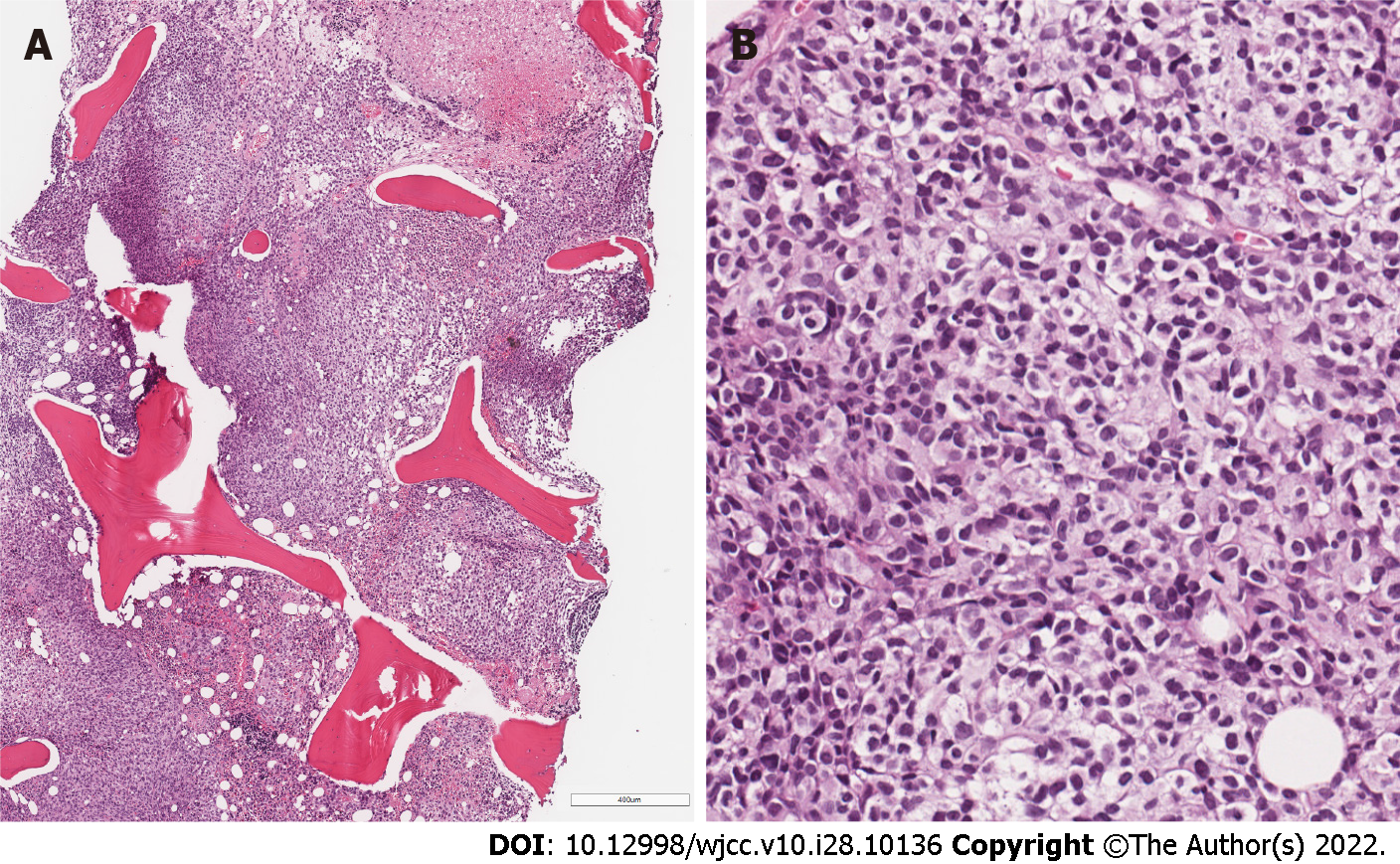
Microscopically biopsies from stomach and duodenum lesions showed focally ulcerated, dense infiltrate in lamina propria, composed of discohesive medium-sized cells with pale or clear cytoplasm and hyperchromatic, oval, or irregularly shaped nuclei with small nucleoli (Figure 3A and B). Due to the lack of clinical information about prior melanoma, the suspicion of hematologic malignancy and histological features, such as multifocality and cell discohesion, our initial differential diagnosis was between myeloid leukemia or enteropathy-associated T- cell lymphoma.
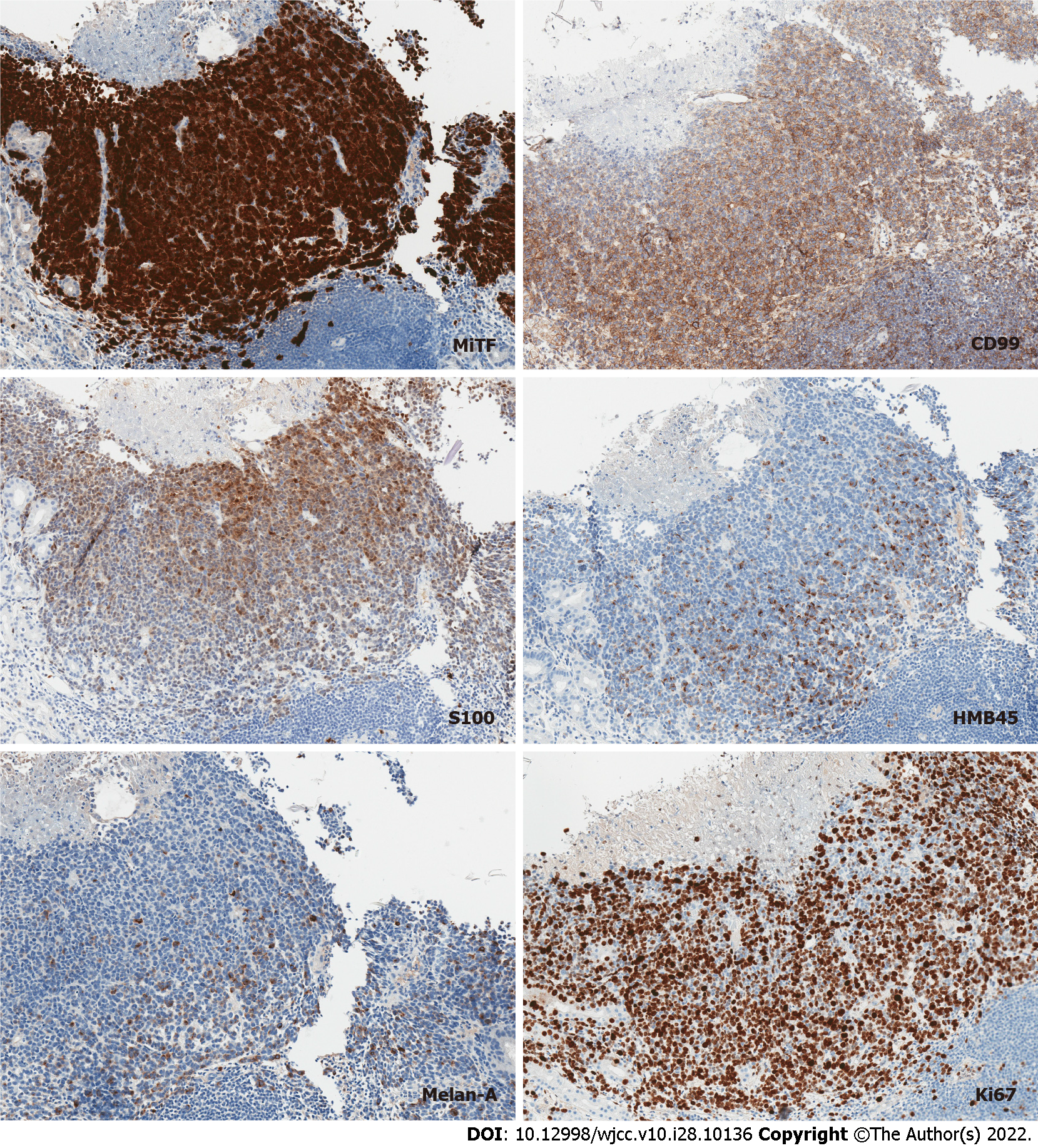
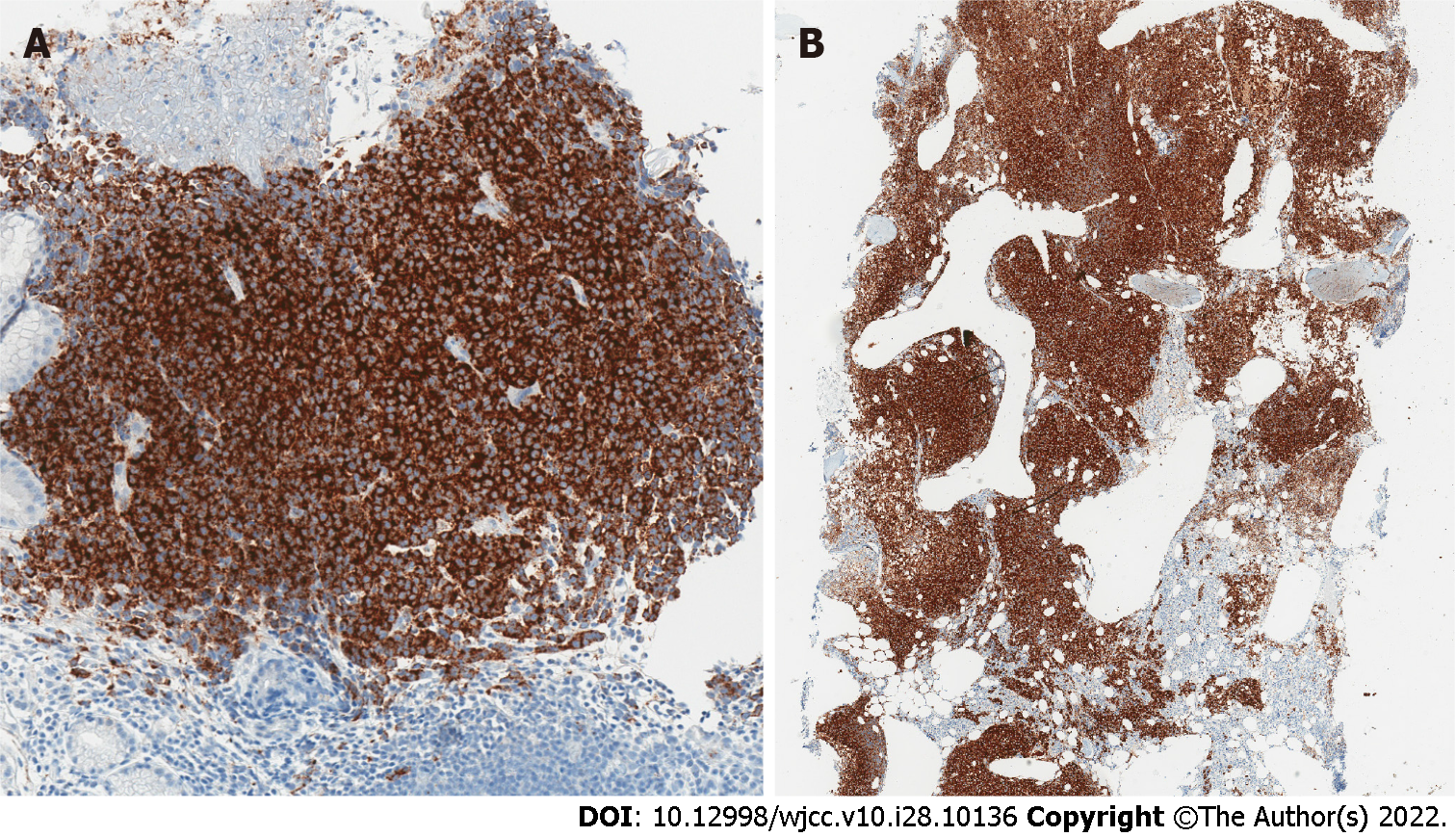
However, staining for myeloid and lymphoid markers, such as LCA, CD34, CD117, MPO, CD68, CD123, CD7, CD4, CD3, CD20, CD30 were all negative. On the contrary, tumor cells showed diffuse positivity for CD99 - a non-specific immunohistochemical marker, expressed in a wide variety of malignancies, including melanoma. All the subsequently stained melanocytic markers showed diffuse or focal positivity (Figure 4).
The following bone marrow trephine biopsy revealed metastatic lesion with focal necrosis, composed of densely arranged tumor cells (Figure 5), analogous to previously seen in duodenal and gastric mucosa. Similar diffuse infiltrate with interspersed melanophages was seen in a subsequent core biopsy of the enlarged cervical lymph node.
Tumor cells showed diffuse immunohistochemical labelling for BRAF V600E (Figure 6). Moreover, BRAF V600E mutation was confirmed by real-time polymerase chain reaction test.
The final diagnosis of the presented case is a metastatic multifocal melanoma of the stomach, duodenum, liver, spleen, lungs, lymph nodes and bones.
The patient was referred to the Oncology Clinic for chemotherapy admission.
Having combined all the findings a metastatic multifocal melanoma of the stomach, duodenum, liver, spleen, lungs, lymph nodes and bones was diagnosed.
As the disease was too advanced the surgical treatment was not applicable. After the diagnosis, the patient was referred to the Oncology Clinic for chemotherapy admission, but unfortunately, the patient refused treatment for personal reasons and died a week later at the hospital, due to malignancy (Figure 7).
Cutaneous melanoma is a malignant tumor arising from melanocytes usually due to effusive ultraviolet exposure[5]. The very first case of melanoma in European literature can be found in the publication of Dr. Highmore and Dr. Bonet, published in 1651. They portrayed melanoma as fatal dark lesions on their patients’ bodies[6].
Nodular melanoma is an aggressive, rapidly growing type of cutaneous melanoma, that lacks radial growth phase[7]. Nodular melanoma is repeatedly associated with worse outcome in comparison to other types of cutaneous melanoma[8-10]. Even then adjusted for tumor thickness, nodular melanoma vs superficial spreading melanoma demonstrate higher rate of regional metastasis[10] and substantially worse disease-free survival (DFS)[8]. Ulceration status for cutaneous melanoma is not only a de
Although mitotic rate is no longer used as a one of the determinants of T classification[12], it should still be evaluated and noted in pathological report, due to its predictive value[13,14]. The presence of any mitoses in the dermis is associated with positive sentinel lymph node and poorer survival[15]. Interestingly, sentinel lymph node of the primary cutaneous tumor in the current case was negative for metastatic melanoma, despite high tumor thickness and a brisk mitotic activity.
Consistent with study of Hugdahl et al[16], the metastatic melanoma of the current case report demonstrated high BRAF V600E expression, associated with aggressive features in nodular type melanoma group, such as increased thickness, ulceration and higher mitotic count.
Melanoma can metastasize to almost every human organ. The most frequent sites of metastasis are nearby skin, lymph nodes and subcutaneous tissue. Also, melanoma can metastasize to distant organs, most commonly to the brain, liver, lungs, bones, and intestines[2,17]. The most common sites of meta
It should be mentioned that metastases of melanoma to the stomach are rare. According to the literature, the typical location of melanoma metastases in the stomach is the body and fundus of the stomach, and metastases are usually arranged as single derivatives rather than multiple ones[20]. Clinically gastric metastases can be silent for a long time or mimic the symptoms of gastritis and are often detected in the end stages of the disease. The prognosis of patients with melanoma metastasis in the gastrointestinal tract (GI) is poor and the median survival time is 4 to 6 mo[21].
As well as to the stomach, metastases to the spleen are rare, especially solitary metastasis. In our case report CT demonstrated multiple lesions in the spleen. Splenic metastases are usually asymptomatic and incidentally found[22]. If isolated and suitable for resection, surgery is the most effective treatment for metastatic spleen melanoma[23]. However multiple metastases like in this case report, are a sign of aggressive disease with a dismal prognosis.
Ultrasound, CT, magnetic resonance imaging and positron emission tomography are used to determine the location of melanoma metastasis, but they are not specific enough to identify the disease[24]. Endoscopy is the “gold standard” for diagnosing GI melanoma. Blecker et al[25] described three forms of endoscopic melanoma metastases presentation: ulcerated melanotic nodules growing on normal rugae, mass lesions with necrosis and melanosis as well as submucosal masses with ulcerations. In our presented case, gastric melanoma metastases were atypical and were presented as nonspecific red polyps. It is important to note that GI melanoma metastases can look like primary gastric tumors, metastases from other sites as solid tumors or even hematologic malignancies, as in the current case. Therefore, the immunohistochemical stains, such as S100 and HMB45, must be applied to make a final diagnosis[26].
Treatment of the metastatic melanoma relies on the site and the number of metastases. According to literature, when possible, surgery is the best treatment option and can prolong patients’ lives[27]. Radiotherapy is not a method of choice because melanoma is known as radio resistant. Systemic chemotherapy is now used as palliative treatment option for relapsing and resistant to other treatments melanomas[28].
The new studies focus on immunotherapy and target - therapy. Either alone or in combination with chemotherapy, immunotherapy has revolutionized how this malignancy is treated. The most usually applied medications in clinical practice are Nivolumab, Ipilimumab and Pembrolizumab[29]. In the longest follow - up study - Keynote - 001 (phase Ib) study, 655 patients with advanced melanoma received Pembrolizumab. This study showed that for patients, who were treated with Pembrolizumab overall survival rate is higher than those who received treatment with Ipilimumab (28.4% and 12.3%)[30].
In a study by De Luca et al[31], nivolumab showed to be effective and tolerated treatment for metastatic melanoma in patients with BRAF V600E mutation. In our case, BRAF V600E mutation was verified for the patient, but unfortunately the patient declined the proposed treatment. According to clinical studies, BRAF mutation detection is also seen elevated in patients with melanoma. Since 50% of cutaneous melanomas have mutation in BRAF immunotherapy alone or immunotherapy in combination can prolong patients’ lives[31,32].
Since melanoma has a poor prognosis for late stages, there are several biomarkers for the diagnosis and prognosis to help determine which patients are at risk of melanoma and what type of treatment do they need. Unfortunately, majority of melanoma diagnostic indicators rely on melanocyte detection rather than melanoma identification.
Serum LDH was the first serological marker to be included in the American Joint Committee on Cancer (AJCC) staging system. In metastatic melanoma, increased serum LDH is amongst the most significant independent prognostic markers[33]. A high blood LDH level has also been demonstrated to be a poor predictor of treatment response[33]. In recent studies, increased baseline LDH has also been consistently linked to low survival and response to immunotherapy rates[34]. However, an elevation in serum LDH is not specific only to melanoma[33]. S100B, a tumor marker, is more specific to melanoma even if its levels can be elevated in other diseases[35]. In a study by Mocellin et al[36], higher S100B levels in the blood were linked to a worse chance of survival in melanoma patients. In a study by Wagner et al[34], when compared to individuals with normal S100B and LDH, patients with high initial S100B and LDH had considerably worse survival rates. Furthermore, the biggest systematic review yet discovered that serum S100B has a higher accuracy than LDH in predicting melanoma recurrence[37]. However, to this day there is no agreement on the use of blood testing to detect disease relapse in individuals who have had their melanoma excised.
Nowadays, there are several clinical trials that are about circulating melanoma cells detection and new strategies of screening for early detection of melanoma in population[38]. However, current evidence is limited; therefore, further longitudinal studies looking for early melanoma detection are needed.
In this case report, we present a rare case of melanoma metastasizing to multiple organs: GI tract, bones, spleen, lungs, and lymph nodes. This case highlights the importance of suspicion for metastatic melanoma in patients with a history of melanoma in the past. When the diagnosis is confirmed via immunohistochemical staining, combination treatment of surgical resection, chemotherapy or immunotherapy should be considered as it may prolong survival rate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng J, China; Zhou S, China; Li H, China S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Sacchetto L, Zanetti R, Comber H, Bouchardy C, Brewster DH, Broganelli P, Chirlaque MD, Coza D, Galceran J, Gavin A, Hackl M, Katalinic A, Larønningen S, Louwman MWJ, Morgan E, Robsahm TE, Sanchez MJ, Tryggvadóttir L, Tumino R, Van Eycken E, Vernon S, Zadnik V, Rosso S. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer. 2018;92:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3397] [Article Influence: 212.3] [Reference Citation Analysis (0)] |
| 3. | FANGER H, ROBERTS WF. Malignant melanoma; a clinicopathological study. N Engl J Med. 1952;246:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 430] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20:1366-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 570] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 6. | Rebecca VW, Sondak VK, Smalley KS. A brief history of melanoma: from mummies to mutations. Melanoma Res. 2012;22:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Liu W, Dowling JP, Murray WK, McArthur GA, Thompson JF, Wolfe R, Kelly JW. Rate of growth in melanomas: characteristics and associations of rapidly growing melanomas. Arch Dermatol. 2006;142:1551-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Green AC, Viros A, Hughes MCB, Gaudy-Marqueste C, Akhras V, Cook MG, Marais R. Nodular Melanoma: A Histopathologic Entity? Acta Derm Venereol. 2018;98:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Cherobin ACFP, Wainstein AJA, Colosimo EA, Goulart EMA, Bittencourt FV. Prognostic factors for metastasis in cutaneous melanoma. An Bras Dermatol. 2018;93:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Dessinioti C, Dimou N, Geller AC, Stergiopoulou A, Lo S, Keim U, Gershenwald JE, Haydu LE, Ribero S, Quaglino P, Puig S, Malvehy J, Kandolf-Sekulovic L, Radevic T, Kaufmann R, Meister L, Nagore E, Traves V, Champsas GG, Plaka M, Dreno B, Varey E, Ramirez DM, Dummer R, Mangana J, Hauschild A, Egberts F, Peris K, Del Regno L, Forsea AM, Zurac SA, Vieira R, Brinca A, Zalaudek I, Deinlein T, Linos E, Evangelou E, Thompson JF, Scolyer RA, Garbe C, Stratigos AJ. Distinct Clinicopathological and Prognostic Features of Thin Nodular Primary Melanomas: An International Study from 17 Centers. J Natl Cancer Inst. 2019;111:1314-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Faut M, Wevers KP, van Ginkel RJ, Diercks GF, Hoekstra HJ, Kruijff S, Been LB, van Leeuwen BL. Nodular Histologic Subtype and Ulceration are Tumor Factors Associated with High Risk of Recurrence in Sentinel Node-Negative Melanoma Patients. Ann Surg Oncol. 2017;24:142-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Bajaj S, Donnelly D, Call M, Johannet P, Moran U, Polsky D, Shapiro R, Berman R, Pavlick A, Weber J, Zhong J, Osman I. Melanoma Prognosis: Accuracy of the American Joint Committee on Cancer Staging Manual Eighth Edition. J Natl Cancer Inst. 2020;112:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Hartman RI, Lin JY. Cutaneous Melanoma-A Review in Detection, Staging, and Management. Hematol Oncol Clin North Am. 2019;33:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4402] [Article Influence: 550.3] [Reference Citation Analysis (4)] |
| 15. | Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venerol. 2021;156:300-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Hugdahl E, Kalvenes MB, Puntervoll HE, Ladstein RG, Akslen LA. BRAF-V600E expression in primary nodular melanoma is associated with aggressive tumour features and reduced survival. Br J Cancer. 2016;114:801-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Damsky WE, Rosenbaum LE, Bosenberg M. Decoding melanoma metastasis. Cancers (Basel). 2010;3:126-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Wysocki WM, Komorowski AL, Darasz Z. Gastrointestinal metastases from malignant melanoma: report of a case. Surg Today. 2004;34:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Iliev S, Vladova P, Popovska S. Gastrointestinal Metastases of Malignant Skin Melanoma - Report of 2 Cases and Review of Literature. Int J Surg Med. 2016;2:89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Goral V, Ucmak F, Yildirim S, Barutcu S, Ileri S, Aslan I, Buyukbayram H. Malignant melanoma of the stomach presenting in a woman: a case report. J Med Case Rep. 2011;5:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Liang KV, Sanderson SO, Nowakowski GS, Arora AS. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc. 2006;81:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Giovagnoni A, Giorgi C, Goteri G. Tumours of the spleen. Cancer Imaging. 2005;5:73-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Lopez Monclova J, Targarona Soler E, Peraza Solis Y, Vidal Gonzalez P, Balague Ponz C, Rodriguez Luppi C, Trias Folch M. Laparoscopic approach for isolated splenic metastasis: comprehensive literature review and report of 6 cases. Surg Laparosc Endosc Percutan Tech. 2013;23:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Ettahri H, Elomrani F, Elkabous M, Rimani M, Boutayeb S, Mrabti H, Errihani H. Duodenal and gallbladder metastasis of regressive melanoma: a case report and review of the literature. J Gastrointest Oncol. 2015;6:E77-E81. [PubMed] |
| 25. | Blecker D, Abraham S, Furth EE, Kochman ML. Melanoma in the gastrointestinal tract. Am J Gastroenterol. 1999;94:3427-3433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Shinohara MM, Deubner H, Argenyi ZB. S100, HMB-45, and Melan-A negative primary melanoma. Dermatol Online J. 2009;15:7. [PubMed] |
| 27. | Gutman H, Hess KR, Kokotsakis JA, Ross MI, Guinee VF, Balch CM. Surgery for abdominal metastases of cutaneous melanoma. World J Surg. 2001;25:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Amabile S, Roccuzzo G, Pala V, Tonella L, Rubatto M, Merli M, Fava P, Ribero S, Fierro MT, Queirolo P, Quaglino P. Clinical Significance of Distant Metastasis-Free Survival (DMFS) in Melanoma: A Narrative Review from Adjuvant Clinical Trials. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Ralli M, Botticelli A, Visconti IC, Angeletti D, Fiore M, Marchetti P, Lambiase A, de Vincentiis M, Greco A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J Immunol Res. 2020;2020:9235638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 30. | Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, Dronca R, Mitchell TC, Patnaik A, Zarour HM, Joshua AM, Zhao Q, Jensen E, Ahsan S, Ibrahim N, Ribas A. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 579] [Cited by in RCA: 679] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 31. | De Luca R, Meraviglia S, Blasi L, Maiorana A, Cicero G. Nivolumab in metastatic melanoma: good efficacy and tolerability in elderly patients. Curr Oncol. 2020;27:e75-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Larkin J, Minor D, D'Angelo S, Neyns B, Smylie M, Miller WH Jr, Gutzmer R, Linette G, Chmielowski B, Lao CD, Lorigan P, Grossmann K, Hassel JC, Sznol M, Daud A, Sosman J, Khushalani N, Schadendorf D, Hoeller C, Walker D, Kong G, Horak C, Weber J. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator's Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol. 2018;36:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 33. | Petrelli F, Ardito R, Merelli B, Lonati V, Cabiddu M, Seghezzi S, Barni S, Ghidini A. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res. 2019;29:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Wagner NB, Forschner A, Leiter U, Garbe C, Eigentler TK. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br J Cancer. 2018;119:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Aceti A, Margarucci LM, Scaramucci E, Orsini M, Salerno G, Di Sante G, Gianfranceschi G, Di Liddo R, Valeriani F, Ria F, Simmaco M, Parnigotto PP, Vitali M, Romano Spica V, Michetti F. Serum S100B protein as a marker of severity in Covid-19 patients. Sci Rep. 2020;10:18665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 36. | Mocellin S, Zavagno G, Nitti D. The prognostic value of serum S100B in patients with cutaneous melanoma: a meta-analysis. Int J Cancer. 2008;123:2370-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Janka EA, Várvölgyi T, Sipos Z, Soós A, Hegyi P, Kiss S, Dembrovszky F, Csupor D, Kéringer P, Pécsi D, Solymár M, Emri G. Predictive Performance of Serum S100B Versus LDH in Melanoma Patients: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:772165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Aya-Bonilla CA, Morici M, Hong X, McEvoy AC, Sullivan RJ, Freeman J, Calapre L, Khattak MA, Meniawy T, Millward M, Ziman M, Gray ES. Detection and prognostic role of heterogeneous populations of melanoma circulating tumour cells. Br J Cancer. 2020;122:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |