Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9945
Peer-review started: May 21, 2022
First decision: July 29, 2022
Revised: August 12, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 117 Days and 20.6 Hours
Mitochondrial encephalomyopathy (ME) is a multisystem metabolic disease that primarily affects the central nervous system and skeletal muscle. It is caused by mutations in mitochondrial or nuclear DNA, resulting in abnormal mitochondrial structure and function and insufficient ATP synthesis. The most common subtype is mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episode (MELAS) syndrome. In recent years, reports of MELAS syndrome have increased but familial cases are rare.
We report a case of familial MELAS syndrome. Cases 2 and 3 are sisters and case 1 is their nephew. All are short in stature and showed stroke-like episodes with rapid onset and no obvious symptoms such as paroxysmal headache, aphasia, or blurred vision. After admission, blood lactate levels were significantly higher than normal. The patients underwent magnetic resonance imaging of the head. Cases 1 and 2 were considered to have ME, whereas case 3 was considered to have a space-occupying lesion in the left temporal lobe. Pathological evaluation showed no obvious tumor cells in the brain lesions of case 3. Muscle biopsy or genetic test results were consistent with ME. The patients were diagnosed with MELAS syndrome and their symptoms improved with intravenous infusions of coenzyme Q10, coenzyme A, vitamin B, and vitamin C. At the 6 mo follow-up, there was no recurrence or progression.
When a patient has MELAS syndrome, familial MELAS syndrome should be considered if related family members have similar symptoms.
Core Tip: Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episode (MELAS) syndrome is a hereditary metabolic disease with complex clinical manifestations, and is often misdiagnosed as cerebral infarction and encephalitis. When the patient has a family history of this disease, blood lactate level is higher than normal, and imaging examination suggests MELAS syndrome, the possibility of MELAS syndrome should be considered. The diagnosis should be confirmed by muscle biopsy and genetic testing.
- Citation: Yang X, Fu LJ. Familial mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episode syndrome: Three case reports. World J Clin Cases 2022; 10(27): 9945-9953
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9945.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9945
Mitochondrial encephalomyopathy (ME) is a multisystem metabolic disorder mainly caused by mutations in mitochondrial DNA (mtDNA) or nuclear DNA, resulting in abnormal mitochondrial structure and function and insufficient ATP synthesis[1,2]. ME has six main subtypes: ME, lactic acidosis, and stroke-like episode (MELAS) syndrome; Leigh syndrome (LS); Leber’s hereditary optic neuropathy; chronic progressive external ophthalmoplegia (CPEO); myoclonus epilepsy with ragged-red fiber (MERRF); and Keams-sayre syndrome. The most common subtype is MELAS syndrome[3], which is maternally inherited[4]. The main clinical manifestations are stroke-like episodes, hemiplegia, aphasia, cortical blindness, epilepsy, paroxysmal headache, limb weakness, and hearing loss[5,6]. The disease usually occurs before the age of 40, with the highest incidence between 10-years-old and 30-years-old, although late-onset MELAS syndrome in adults after age 40 has occasionally been reported[7]. Reports of MELAS syndrome have increased in recent years, but familial cases are rare.
Case 1: A 30-year-old male was admitted to the hospital because of headache, dizziness, nausea, vomiting, fever, blurred vision, paroxysmal dysphasia, and right strabismus for 10 d.
Case 2: A 50-year-old female was admitted to the hospital because of aphasia with a slow response for 7 d.
Case 3: A 55-year-old female was admitted to the hospital because of aphasia with memory loss for more than 2 mo.
Case 1: The patient had an acute attack. Ten days before hospital admission, the patient developed headache, dizziness, nausea, vomiting, fever, blurred vision, paroxysmal speech confusion, and right strabismus following a cold or diarrhea. After seeing a doctor in the local hospital, the patient’s symptoms did not improve. He was admitted to our hospital for further diagnosis and treatment.
Case 2: This patient is the aunt of case 1. Three years after case 1 was diagnosed with MELAS syndrome, case 2 developed a speech disorder but could understand the speech of others. Before admission, the symptoms had lasted for 7 d.
Case 3: This patient is the older sister of case 2. About 1 year after case 2 was diagnosed with MELAS syndrome, case 3 showed aphasia and memory loss. The patient could understand the speech of others but had difficulty speaking. These symptoms were accompanied by memory loss dominated by anterograde amnesia and paroxysmal pain in the left temporal region. Before admission, the symptoms had lasted for 2 mo.
Cases 1, 2 and 3: For all 3 patients, the personal history-taking yielded no remarkable information.
Case 1: The patient was born in Tianjin and has lived there almost all his life. The patient usually smokes 10 cigarettes a day, does not drink alcohol, works in a compressor factory, is unmarried, and is 165 cm in height and 45 kg in weight. His mother and brother are in good health and his father suffers from high blood pressure.
Case 2: The patient was born in Tianjin and has lived there almost all her life. She has no health-risk behaviors. The patient is short in stature, is 153 cm in height and 44 kg in weight, has had hearing loss for 5 years, got married at the age of 25, has a healthy husband, and had a son who died of uremia at the age of 18. Her nephew suffers from MELAS syndrome.
Case 3: The patient was born in Tianjin and has lived there almost all her life. She has no health-risk behaviors. The patient is short in stature, 150 cm in height and 41 kg in weight. Her husband and daughter are healthy. Her nephew suffers from MELAS syndrome.
Case 1: The patient’s body temperature was 37.7°C, respiratory rate was 20 breaths per min, heart rate was 95 beats per min, and blood pressure was 120/75 mmHg. The patient was conscious, and had sensory aphasia, right strabismus, was unable to cooperate with the instructions/movements for vision and visual field tests, normal muscle tone in the extremities, and bilateral superficial sensory symmetry on gross testing.
Case 2: The patient’s body temperature was 36.4°C, respiratory rate was 19 breaths per min, heart rate was 80 beats per min, and blood pressure was 95/64 mmHg. She was conscious but had sensory aphasia. She was uncooperative during the physical examination.
Case 3: The patient’s body temperature was 36.5°C, respiratory rate was 16 breaths per min, heart rate was 75 beats per min, and blood pressure was 110/69 mmHg. She was conscious but had sensory aphasia. The physical examination showed no abnormality.
Case 1: Laboratory test results showed that the white blood cell (WBC) count was as high as 16.75 × 109/L (normal range: 3.97-9.15 × 109/L), and the percentage of neutrophils was as high as 82.8% (normal range: 50%-70%). Cerebrospinal fluid (CSF) protein was as high as 73.2 mg/dL (normal range: 15-45 mg/dL), and CSF glucose was as high as 4.59 mmol/L (normal range: 2.5-4.5 mmol/L). There were no WBCs in the CSF, and the bacterial smear and tuberculosis antibody test were negative. The liver and kidney function indexes were normal.
Case 2: Laboratory test results showed that the WBC count was as high as 11.29 × 109/L (normal range: 3.97-9.15× 109/L), and the percentage of neutrophils was as high as 90.0% (normal range: 50%-70%). The liver and kidney function indexes were normal.
Case 3: Laboratory examinations showed that routine blood test, routine urine test and urinary sediment examination, routine fecal exam and fecal occult blood test, blood biochemistry, immune indexes, and infection indexes were normal. The liver and kidney function indexes were also normal.
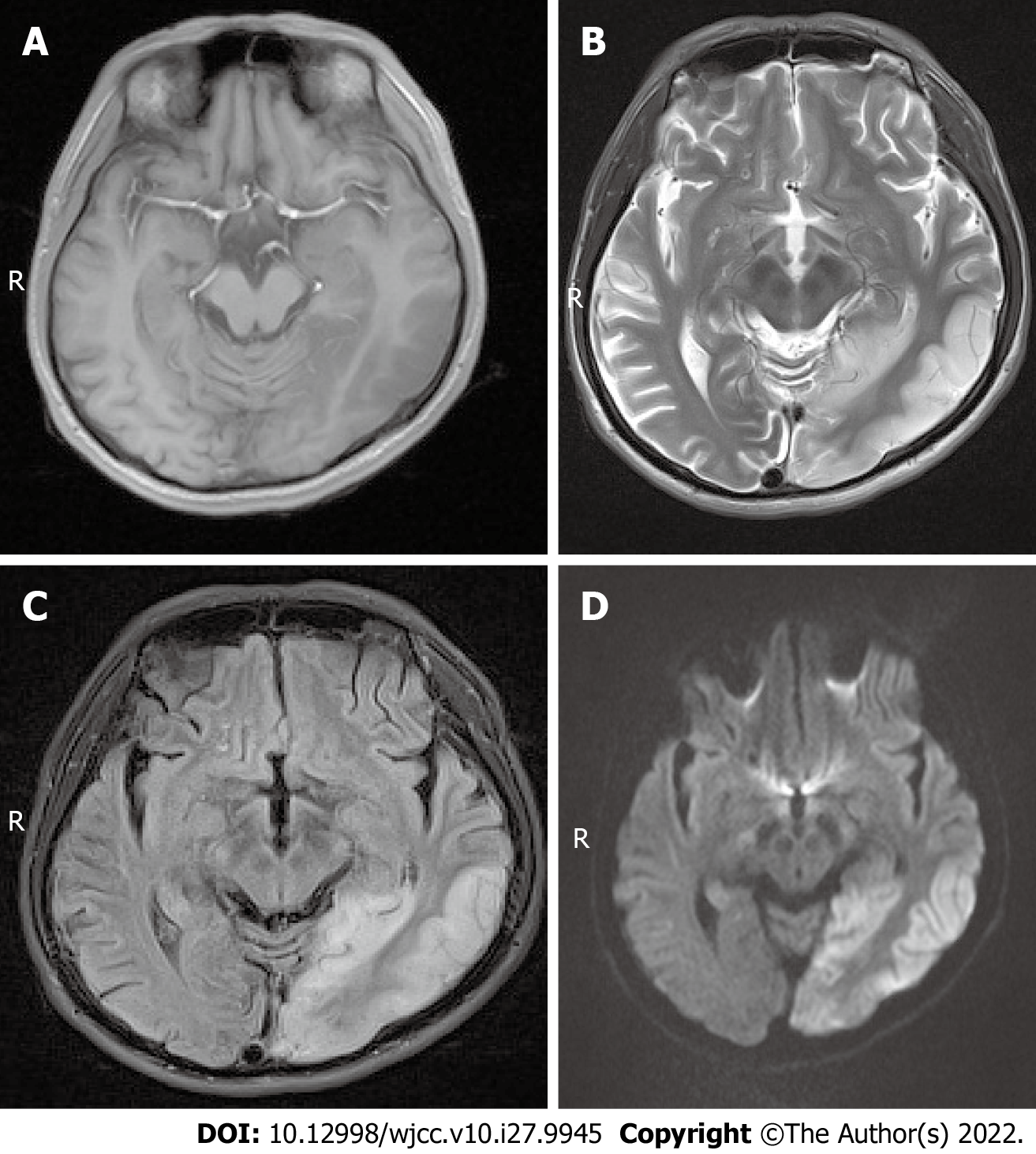
Case 1: Magnetic resonance imaging (MRI) scans showed hypointense T1 and hyperintense T2 signals in the left temporal lobe and occipital cortex (Figure 1A and B), and the corresponding regions showed slightly higher signal intensity in the fluid attenuated inversion recovery (FLAIR) sequence and diffusion-weighted imaging (DWI) (Figure 1C and D). MR spectroscopy (MRS) showed that the N-acetylaspartate peak of the left occipital lobe was significantly lower than that of the right side, and a few lactate peaks were locally visible. The diagnosis was local swelling of the left temporal lobe and occipital cortex, which was considered ME.
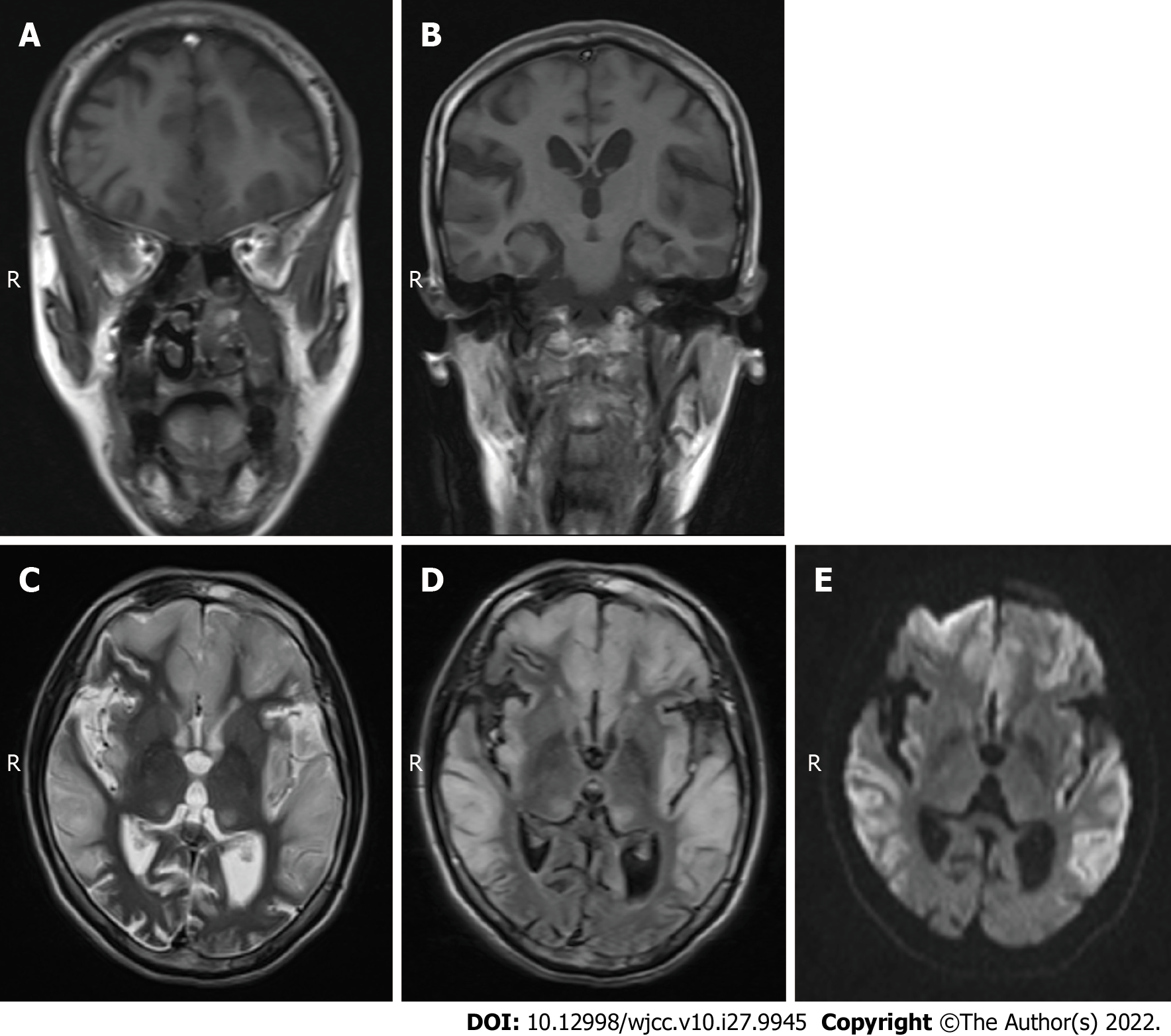
Case 2: MRI scans showed hypointense T1 and hyperintense T2 in the bilateral frontal lobe, temporal lobe, parietal lobe and insular cortices (Figure 2A-C), and mild hyperintensity in the FLAIR sequence and DWI in the corresponding regions (Figure 2D and E). The diagnosis was swelling of the bilateral frontal lobe, temporal lobe, parietal lobe and insular cortex. Based on these findings combined with the medical history and considering the possibility of ME, enhanced MRI and MRS examinations were recommended in addition to blood and CSF lactate tests.
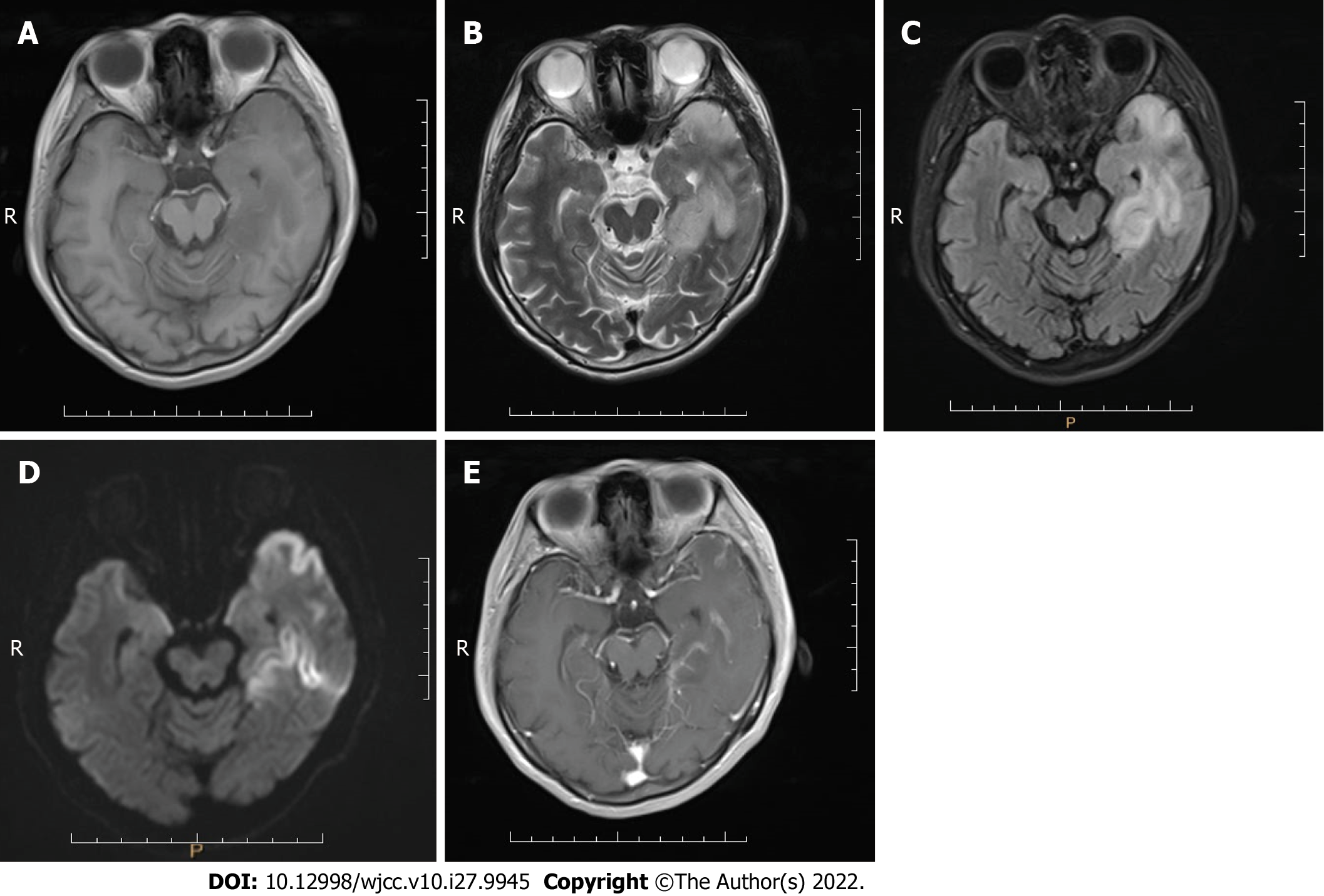
Case 3: MRI scans showed an abnormal signal in the left temporal lobe with irregular shape and unclear boundary. T1-weighted imaging (T1WI) showed low signal intensity (Figure 3A), and T2WI, FLAIR sequence, and DWI showed high signal intensity (Figure 3B-D). Contrast-enhanced imaging showed mild enhancement of the lesion (Figure 3E). The space-occupying lesion of the left temporal lobe was considered low-grade glioma.
The 3 patients were eventually diagnosed with MELAS syndrome.
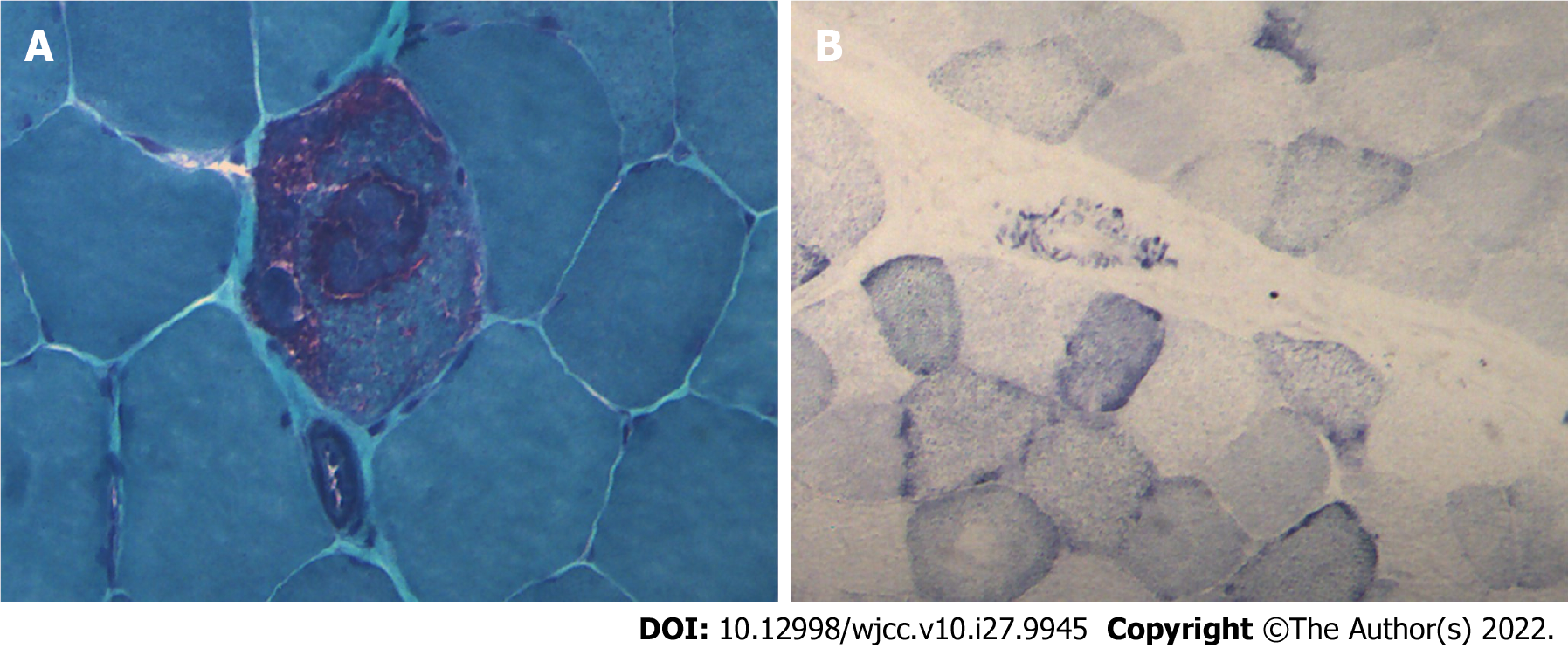
Case 1: Considering MELAS syndrome, on the 1st day after admission, the patient was administered 200 IU coenzyme A, 40 mg ATP, and 1 g levocarnitine by intravenous drip once a day. These drugs were used to improve energy metabolism. He took coenzyme Q10 three times a day, 1 tablet each time (10 mg per tablet). He took 10 mg vitamin B1 and 500 mg mecobalamin each time, three times a day. He was given 30 mL of Danhong injection by intravenous drip daily to improve the blood supply. Because of the patient’s clinical presentation of paroxysmal right strabismus and neck stiffness, symptomatic epilepsy was considered. He was prescribed 50 mg topiramate daily to improve his symptoms. On the 3rd day after admission, his blood lactate level was as high as 6.50 mmol/L. His symptoms improved, the seizures stopped, the headache disappeared, but the sensory aphasia still persisted. On the 8th day after admission, muscle biopsy results suggested that ME should be considered. Modified gomori trichromatic (MGT) staining of the muscle tissue showed red fiber breakage (Figure 4A). Succinate dehydrogenase (SDH) staining showed blue-stained fiber breakage and hyperstained small vessels (Figure 4B). Cytochrome c oxidase (COX) staining showed scattered COX-negative fibers.On the 15th day after admission, genetic testing showed no disease-causing gene mutation. During treatment, the treatment regimen remained unchanged.
Case 2: Considering MELAS syndrome, on the 1st day after admission, the patient was administered 100 IU coenzyme A, 20 mg ATP, 1 g vitamin C, 180 mg brain protein hydrolysate, and 1 g levocarnitine by intravenous drip once a day. To improve energy metabolism, she took 10 mg coenzyme Q10 and vitamin B2 three times a day. She was given 0.2 g Salvia miltiorrhiza polyphenol hydrochloride by intravenous drip daily to improve her intracranial blood circulation. She was given 3 g cefoperazone/sulbactam by intravenous drip every 12 h to control infection. On the 2nd day after admission, blood lactate level was as high as 5.90 mmol/L. The patient’s symptoms did not change significantly. On the 6th day after admission, the WBC count was normal. Throughout the treatment period, the treatment regimen remained unchanged.
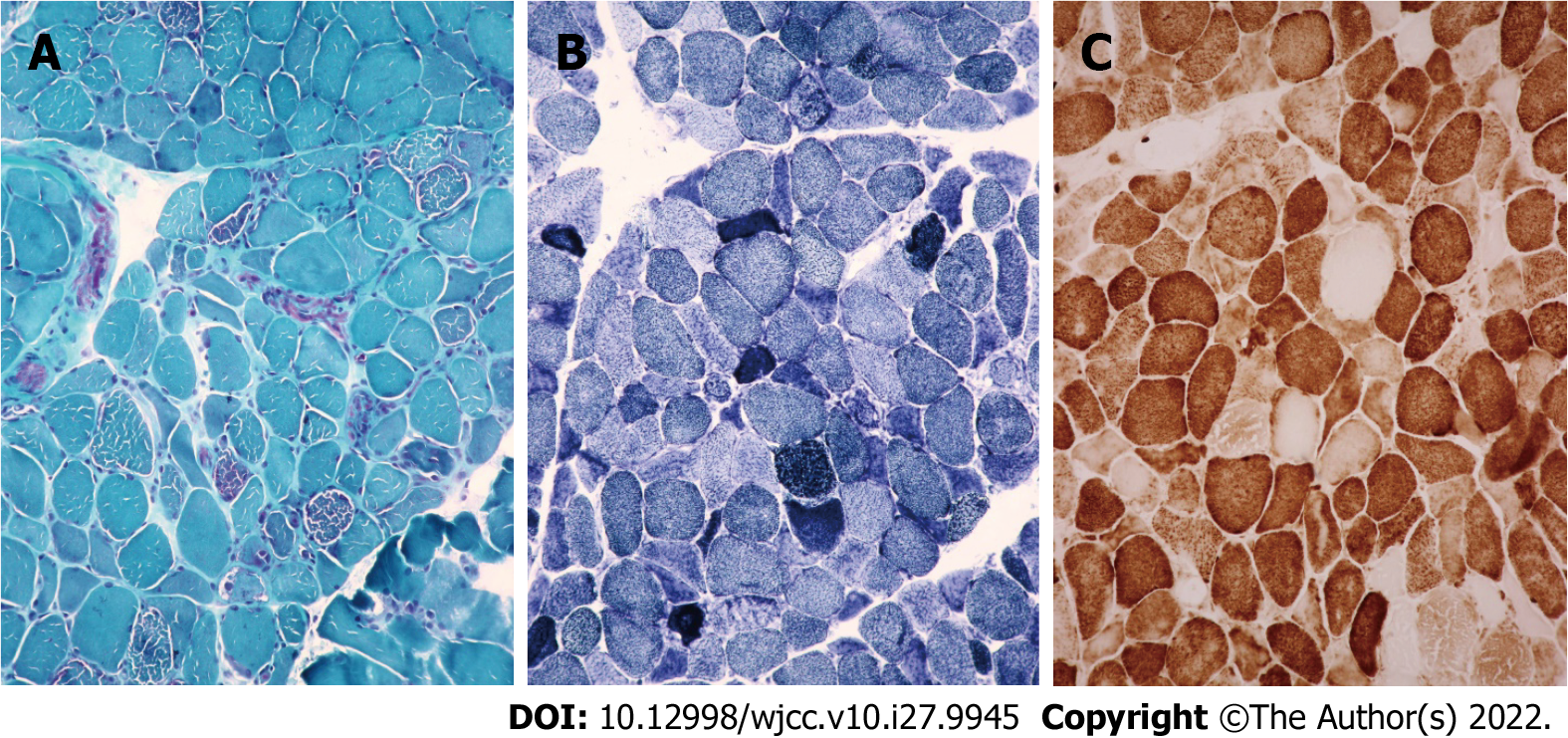
Case 3: Considering the left temporal lobe space-occupying lesion, the various preoperative examinations were performed and the results were normal. Craniotomy exploration was performed on the 5th day after admission, and no obvious tumor cells were found in postoperative pathology. After the operation, after repeatedly inquiring about the medical history, we learned that the patient’s nephew and sister suffer from MELAS syndrome. Therefore, on postoperative day 6, the patient underwent muscle biopsy, genetic testing, and lactate testing and the blood lactate level was as high as 5.25 mmol/L. Considering MELAS syndrome, coenzyme Q10 was added to routine postoperative treatment. She was asked to take 10 mg coenzyme Q10 once a day. On postoperative day 10, the muscle biopsy results suggested that ME should be considered. MGT staining of the muscle tissue showed red fiber breakage (Figure 5A). SDH staining showed blue-stained fiber breakage and hyperstained small vessels (Figure 5B). COX staining showed scattered COX-negative fibers (Figure 5C). Genetic testing suggested that there was a disease-causing gene mutation: tRNAm.3243A>G (about 9%). The patient’s symptoms were better than before surgery as manifested by decreased sensory aphasia. The patient continued to take coenzyme Q10.
Case 1: On the 17th day after admission, WBC count and the percentage of neutrophils were normal. The patient presented with blurred vision and mild sensory aphasia. After discharge, the patient continued to take 50 mg topiramate twice a day, and 500 mg mecobalamin, 5 mg folic acid, and 10 mg vitamin B1 three times a day. There was no recurrence at 6 mo after discharge.
Case 2: On the 8th day after admission, the patient’s symptoms improved as manifested by decreased sensory aphasia. The patient was discharged into the care of her family, who accompanied her to the community hospital for continued treatment. There was no recurrence at 6 mo after discharge.
Case 3: On postoperative day 15, the patient’s symptoms were completely relieved and she was discharged. She was prescribed 10 mg vinylamine and 0.8 g oxiracetam three times a day. She also continued to take 10 mg coenzyme Q10 once a day. There was no recurrence at 6 mo after discharge.
This case report of familial MELAS syndrome provides important experience regarding its diagnosis and treatment. For MELAS syndrome, there is a lack of evidence-based medicine on the effectiveness of drug therapy. Clinicians often use coenzyme Q10, vitamin B1, vitamin C, vitamin E, idebenone, riboflavin, lipoic acid, glutathione, L-carnitine, aspartic acid, folic acid, and taurine in combination to treat MELAS syndrome, known as “cocktail therapy”[8].
The core symptoms of MELAS syndrome are adolescent onset, recurrent stroke-like episodes, and lactic acidosis. MRI findings of MELAS syndrome are characteristic. The lesions are mostly located in the cortex and subcutaneous white matter areas of the occipital and parietal lobes, showing hypointense T1 and hyperintense T2 areas, and the corresponding areas show high signal intensity in DWI sequences, especially cortical involvement, which is characterized by changes in lace-like patterns. No enhancement or mild enhancement can be seen in contrast-enhanced examination. When the clinical manifestations are complex and the conventional MRI findings are not specific, MELAS syndrome is easily misdiagnosed as cerebral infarction, encephalitis, or brain neoplasms[9]. At this time, introducing functional MR imaging is particularly important. For example, MR angiography shows lesions that are not in accordance with the distribution of a single intracranial aorta. MRS shows high lactate peaks in the lesions and CSF[10]. MR perfusion-weighted imaging (PWI) shows increased cerebral blood flow to the lesions, and the hyperperfused regions persist for more than 3 mo[11,12].
Case 1 was given a CSF test for encephalitis because of fever on admission and onset of illness following a cold or diarrhea. No WBCs were found in the CSF, ruling out encephalitis. The patient was a young male with a clinical manifestation of stroke-like seizures. MRI and MRS findings were characteristic of MELAS syndrome. Case 2 was diagnosed with MELAS syndrome due to her short stature, family history, high blood lactate level, and MRI findings. Case 3 was misdiagnosed with a space-occupying lesion, possibly related to the absence of preoperative PWI, MRS, and blood lactate tests.
Clinical symptoms and imaging findings are important for the diagnosis of MELAS syndrome and can be confirmed by muscle biopsy or genetic testing (the positive rate of genetic testing is as high as 95%)[6,13]. We can first screen for hot spot gene mutations in Chinese MELAS syndrome patients such as mtDNA3243A>G, mtDNA13513G>A and mtDNA3271T>C, or perform full-length mtDNA sequencing and/or related nuclear gene detection[13]. The mutated gene in most patients with MELAS syndrome is mtDNA3243A>G[2,5]. The region where the mtDNA3243A locus is located encodes a transfer RNA. This locus is highly conserved and has an important impact on the function of its coding products after occurrence of the A3243G mutation. The mutation corresponds to a variety of clinical phenotypes, which can cause different diseases in different individuals. In addition to MELAS syn
Compared with MELAS syndrome, the T8993G locus mutation of mtDNA is more common in neuropathy, ataxia, and retinitis pigmentosa syndrome[14], a maternally inherited ME. The main clinical manifestations are: (1) Peripheral neuropathy with sensory nerve involvement or neurogenic myasthenia gravis; the main manifestation of neurogenic myasthenia gravis is proximal myasthenia with morbid fatigue; (2) Cerebellar ataxia, which can be the first symptom; and (3) Retinitis pigmentosa, which can be the only clinical symptom. Patients often progress from night blindness to vision loss. Fundus examination shows osteocyte-like pigmentation in the retina.
These gene mutations lead to functional defects in the mitochondrial respiratory chain enzyme complex protein, resulting in mitochondrial dysfunction, which leads to decreased production of ATP, increase of oxygen free radicals, and accumulation of lactic acid. These changes affect the central nervous system and skeletal muscle system, which consume more energy.
Typical pathological changes in MELAS syndrome are MGT-stained red fiber breakage, SDH-stained blue fiber breakage, and/or hyperstained small vessels[9]. Muscle biopsy results in case 1 were consistent with ME, but no disease-causing gene mutation was found in genetic testing. There are two possible reasons. First, only mtDNA A3243G, A8344G, and T8993C (G) were detected in the patient’s genetic testing. Other disease-causing gene mutations might go undetected. Second, the mutation rate of mtDNA varies significantly in different tissues, especially in adults. The positive rates of muscle tissue, urinary sediment cells, and hair follicles were higher than those of peripheral blood cells. Therefore, no disease-causing gene mutation was detected in the patient’s blood. The genetic test results of cases 2 and 3 and the muscle biopsy results of case 3 were consistent with MELAS syndrome.
The clinical manifestations of the 3 patients were different, manifested as blurred vision in case 1, hearing loss in case 2, and normal hearing and vision in case 3, which was due to the “heterogeneity” and “threshold effect” of mtDNA mutations. Threshold effect means that when the mtDNA mutation in mitochondria reaches a certain proportion, it will lead to the dysfunction of corresponding cells, resulting in organ damage. Heterogeneity refers to significant differences in the proportion of mutated and non-mutated mtDNA in different organs of the same patient and in the cells of the same organ of different patients, resulting in different degrees of involvement of different organs and different clinical manifestations[5].
According to the law of genetics, when a mutated gene is located in mtDNA, it follows maternal inheritance. In this paper, cases 2 and 3 are siblings, and the mutated disease-causing genes are located in the mitochondria, so their mother and sister (the mother of case 1) should have a certain proportion of disease-causing gene mutations, and almost all children born to patients should have disease-causing gene mutations. However, the sister and daughter of case 3 are in good health, which may be related to the “threshold effect” of mtDNA mutations. Since the immediate family members of the patient did not undergo genetic testing, this cannot be confirmed.
For patients with MELAS syndrome, the risk of familial disease can be reduced by providing marital and reproductive guidance to patients and their families.
We are grateful to the patients’ families for their consent to publish this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belosludtseva NV, Russia; Gokce E, Turkey S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Liu K, Zhou Z, Pan M, Zhang L. Stem cell-derived mitochondria transplantation: A promising therapy for mitochondrial encephalomyopathy. CNS Neurosci Ther. 2021;27:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (5)] |
| 2. | Dawod PGA, Jancic J, Marjanovic A, Brankovic M, Jankovic M, Samardzic J, Gamil Anwar Dawod A, Novakovic I, Abdel Motaleb FI, Radlovic V, Kostic VS, Nikolic D. Mutational Analysis and mtDNA Haplogroup Characterization in Three Serbian Cases of Mitochondrial Encephalomyopathies and Literature Review. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Hou Y, Zhao XT, Xie ZY, Yuan Y, Wang ZX. Mitochondrial encephalopathy, lactic acidosis and stroke-like episodes / myoclonus epilepsy with ragged-red fibers /Leigh overlap syndrome caused by mitochondrial DNA 8344A>G mutation. Beijing Da Xue Xue Bao Yi Xue Ban. 2020;52:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lahiri D, Sawale VM, Banerjee S, Dubey S, Roy BK, Das SK. Chorea-ballism as a dominant clinical manifestation in heteroplasmic mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes syndrome with A3251G mutation in mitochondrial genome: a case report. J Med Case Rep. 2019;13:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Gagliardi D, Mauri E, Magri F, Velardo D, Meneri M, Abati E, Brusa R, Faravelli I, Piga D, Ronchi D, Triulzi F, Peverelli L, Sciacco M, Bresolin N, Comi GP, Corti S, Govoni A. Can Intestinal Pseudo-Obstruction Drive Recurrent Stroke-Like Episodes in Late-Onset MELAS Syndrome? Front Neurol. 2019;10:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Wang S, Song T, Wang S. Mitochondrial DNA 10158T>C mutation in a patient with mitochondrial encephalomyopathy with lactic acidosis, and stroke-like episodes syndrome: A case-report and literature review (CARE-complaint). Medicine (Baltimore). 2020;99:e20310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Dias SP, Sequeira J, Almeida M. Spastic paraparesis and sensorineural hearing loss: keep brucellosis in mind. J Neurol Sci. 2018;385:144-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Tragni V, Primiano G, Tummolo A, Cafferati Beltrame L, La Piana G, Sgobba MN, Cavalluzzi MM, Paterno G, Gorgoglione R, Volpicella M, Guerra L, Marzulli D, Servidei S, De Grassi A, Petrosillo G, Lentini G, Pierri CL. Personalized Medicine in Mitochondrial Health and Disease: Molecular Basis of Therapeutic Approaches Based on Nutritional Supplements and Their Analogs. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Zhang Q, Sun YL, Zhang CP, Qu BQ, Zhang ZQ. Ultrastructural and clinical findings of mitochondrial encephalomyopathy:report of 27 cases. Zhonghua Bing Li Xue Za Zhi. 2019;48:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Gramegna LL, Evangelisti S, Di Vito L, La Morgia C, Maresca A, Caporali L, Amore G, Talozzi L, Bianchini C, Testa C, Manners DN, Cortesi I, Valentino ML, Liguori R, Carelli V, Tonon C, Lodi R. Brain MRS correlates with mitochondrial dysfunction biomarkers in MELAS-associated mtDNA mutations. Ann Clin Transl Neurol. 2021;8:1200-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Wang R, Hu B, Sun C, Geng D, Lin J, Li Y. Metabolic abnormality in acute stroke-like lesion and its relationship with focal cerebral blood flow in patients with MELAS: Evidence from proton MR spectroscopy and arterial spin labeling. Mitochondrion. 2021;59:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Cheng W, Zhang Y, He L. MRI Features of Stroke-Like Episodes in Mitochondrial Encephalomyopathy With Lactic Acidosis and Stroke-Like Episodes. Front Neurol. 2022;13:843386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Chen H, Hu Q, Raza HK, Chansysouphanthong T, Singh S, Rai P, Cui G, Zhang Z, Ye X, Xu C, Liu Y, Jiang H. An analysis of the clinical and imaging features of mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Somatosens Mot Res. 2020;37:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Licchetta L, Ferri L, La Morgia C, Zenesini C, Caporali L, Lucia Valentino M, Minardi R, Fulitano D, Di Vito L, Mostacci B, Alvisi L, Avoni P, Liguori R, Tinuper P, Bisulli F, Carelli V. Epilepsy in MT-ATP6 - related mils/NARP: correlation of elettroclinical features with heteroplasmy. Ann Clin Transl Neurol. 2021;8:704-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |