Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9921
Peer-review started: May 17, 2022
First decision: June 16, 2022
Revised: June 24, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 122 Days and 9.2 Hours
Various types of drug-induced liver injury are induced by Polygonum multiflorum (PM); however, it rarely causes neutropenia. Herein, we report the case of a 65-year-old woman with concurrent severe hepatotoxicity and agranulocytosis induced by PM.
A 65-year-old woman reported with severe hepatotoxicity and agranulocytosis 17 d after ingestion of PM. The results of the Roussel Uclaf Causality Assessment Method demonstrated a highly probable relationship between hepatotoxicity and PM, with a total score of 10. The Naranjo algorithm results indicated that agranulocytosis had a probable relationship with PM, with an overall score of 6. Granulocyte colony-stimulating factor (for once), a steroid, compound glycyrr
Concurrent hepatotoxicity and agranulocytosis are rare and critical adverse drug reactions of PM, which should be highly valued.
Core Tip: Polygonum multiflorum is a common traditional Chinese medicine and is commonly used as a dietary supplement. However, severe idiosyncratic hepatotoxicity in certain individuals has been reported. Moreover, if idiosyncratic agranulocytosis occurs simultaneously, it may be fatal. Roussel Uclaf Causality Assessment Method scale and Naranjo algorithm are useful tools for the assessment of drug-induced liver injury and adverse drug reactions, respectively. Early discontinuation can prevent disease progression, facilitating recovery. The combination therapy of glucocorticoids, anti-inflammatory medications, and liver protection is beneficial for idiosyncratic drug reactions.
- Citation: Shao YL, Ma CM, Wu JM, Guo FC, Zhang SC. Concurrent severe hepatotoxicity and agranulocytosis induced by Polygonum multiflorum: A case report. World J Clin Cases 2022; 10(27): 9921-9928
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9921.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9921
As a commonly used traditional Chinese medicine, Polygonum multiflorum (PM) is used to treat various diseases through medicinal or dietary supplementation[1]. Unfortunately, PM is the most common cause of herbal medicine-related drug-induced liver injury (DILI)[2]. PM-induced liver injury was first reported in Hong Kong in 1996[3]. Since then, PM hepatotoxicity has attracted attention worldwide[4]. Although concurrent hepatotoxicity and neutropenia induced by chemotherapy have been presented frequently[5], neutropenia caused by PM has rarely been reported. Moreover, the simultaneous occurrence of these two complications owing to the use of PM has not been reported to date. This paper presents a case of concurrent hepatotoxicity and agranulocytosis induced by PM to emphasize the importance of timely diagnosis and treatment of these complications.
A 65-year-old woman was admitted with a history of yellowish pigmentation of the skin or whites of the eyes for 10 d on March 4, 2022.
On recording history, the patient reported a 17-d history of consecutive use of PM (30 g/day) owing to insomnia and dreaminess from February 11, 2022. She had fatigue, loss of appetite, and jaundice; however, she had no nausea and vomiting, abdominal pain, or fever. There was no history of trauma, surgery, drug and alcohol abuse, or blood transfusions, without recent travel history or family history of liver or blood system disorders.
Nine years ago, the patient suffered drug-induced liver injury caused by taking traditional Chinese medicine. After 3 wk of treatment, her liver function returned to normal and was maintained until this episode (the last liver function test was on October 12, 2021).
The patient had no history of trauma, surgery, drug and alcohol abuse, or blood transfusions, without recent travel history or family history of liver or blood system disorders.
The patient’s vital signs were stable. Skin and scleral jaundice were evident. Auscultation of both lungs and heart was clear, with regular heart rate and rhythm. No abdominal tenderness or rebound tenderness was noted, with a negative Murphy’s sign and mild percussion in the liver area. No flapping tremor was detected.
Liver function tests revealed severe acute liver injury. Complete blood count revealed agranulocytosis (erythrocytes 4.07 × 109 cells/L, platelets 159 × 109 cells/L, leukocytes 1.17 × 109 cells/L, and absolute neutrophil count 0.02 × 109 cells/L). Other possible causes of liver damage were ruled out by checking hepatitis B virus surface antigen, hepatitis A, C, D, and E virus antibodies, Epstein-Barr virus antibodies, cytomegalovirus antibodies, autoimmune liver disease antibodies, immunoglobulins, thyroid function, ceruloplasmin, etc. The results are summarized in Table 1.
| Parameter | Result | Normal range |
| Liver and kidney function | ||
| Alanine aminotransferase | 1442.8 | 7-40 U/L |
| Aspartate aminotransferase | 1565 | 13-35 U/L |
| Alkaline Phosphatase | 217 | 50-135 U/L |
| γ-glutamyl transferase | 183.2 | 7-45 U/L |
| Total bilirubin | 110 | 3.4-22 μmol/L |
| Direct bilirubin | 91.1 | 1.7-10.3 μmol/L |
| Albumin | 36.9 | 40-55 g/L |
| Globulin | 22.3 | 20-40 g/L |
| Serum creatinine | 43.6 | 35-80 μmol/L |
| Serum urea | 4.74 | 2.9-8.2 mmol/L |
| Serum lipids | ||
| Total cholesterol | 3.67 | 3.4-5.8 mmol/L |
| Low-density lipoprotein cholesterol | 0.6 | 0.78-2 mmol/L |
| High-density lipoprotein cholesterol | 1.91 | 0-3.7 mmol/L |
| Triglycerides | 1.88 | 0.56-1.7 mmol/L |
| Coagulation function | ||
| Prothrombin Time | 13.7 | 11.0-15.0 sec |
| Prothrombin activity | 86.56 | 75%-160% |
| International normalized ratio | 1.02 | 0.8-1.5 |
| Complete blood count | ||
| Leukocyte | 1.17 | 4.5-10 × 109 cells/L |
| Neutrophils | 0.02 | 1.8-6.3 × 109 cells/L |
| Eosinophils | 0 | 0.02-0.52 × 109 cells/L |
| Basophils | 0 | 0-0.06 × 109 cells/L |
| Lymphocytes | 0.98 | 1.1-3.2 × 109 cells/L |
| Monocytes | 0.17 | 0.1-0.6 × 109 cells/L |
| Erythrocyte | 4.07 | 3.8-5.1 × 1012 cells/L |
| Platelet | 159 | 125-350 × 109 cells/L |
| Inflammatory markers | ||
| C-reactive protein | 10.28 | 0-5 mg/L |
| Procalcitonin | 0.192 | 0-0.05 ng/mL |
| Screening for causes of acute liver injury | ||
| Autoimmune liver diseases | ||
| Immunoglobulin A | 1.4 | 0.72-4.29 g/L |
| Immunoglobulin G | 13.3 | 8-17 g/L |
| Immunoglobulin G4 | 0.427 | 0.05-1.54 g/L |
| Immunoglobulin M | 1.2 | 0.29-3.44 g/L |
| Anti-nuclear antibody | Negative | |
| Anti-smooth muscle antibody | Negative | |
| Anti-liver kidney microsome-1 | Negative | |
| Anti-soluble liver antigen/liver pancreas antigen | Negative | |
| Anti-liver cytosol-1 | Negative | |
| Anti-centromere antibody | Negative | |
| Anti-Mitochondrial-M2 antibody | Negative | |
| Anti-gp210 antibodies | Negative | |
| Anti-Sp100 antibodies | Negative | |
| Virology test | ||
| Hepatitis A IgM | Negative | |
| Hepatitis B surface antigen | Negative | |
| Hepatitis B core antibody IgM | Negative | |
| Hepatitis C antibody | Negative | |
| Hepatitis E IgM | Negative | |
| Anti-CMV IgM | Negative | |
| Anti-EBV viral capsid antigen IgM | Negative | |
| Anti-EBV early antigen IgM | Negative | |
| COVID-19 RNA | Negative | |
| HBV DNA | < 100 | < 100 IU/mL |
| Thyroid function | ||
| Thyroid-stimulating hormone | 0.8 | 0.56-5.91 uIU/mL |
| Free triiodothyronine | 4.4 | 3.53-7.37 pmol/L |
| Free Thyroxine | 14.68 | 7.98-16.02 pmol/L |
| Other | ||
| Ceruloplasmin | 0.36 | 0.16-0.45 g/L |
| Alpha-fetoprotein | 2.9 | 0.0-9.0 ng/mL |
The patient’s liver ultrasound showed normal echotexture and liver outline and non-dilated intrahepatic and extrahepatic bile ducts.
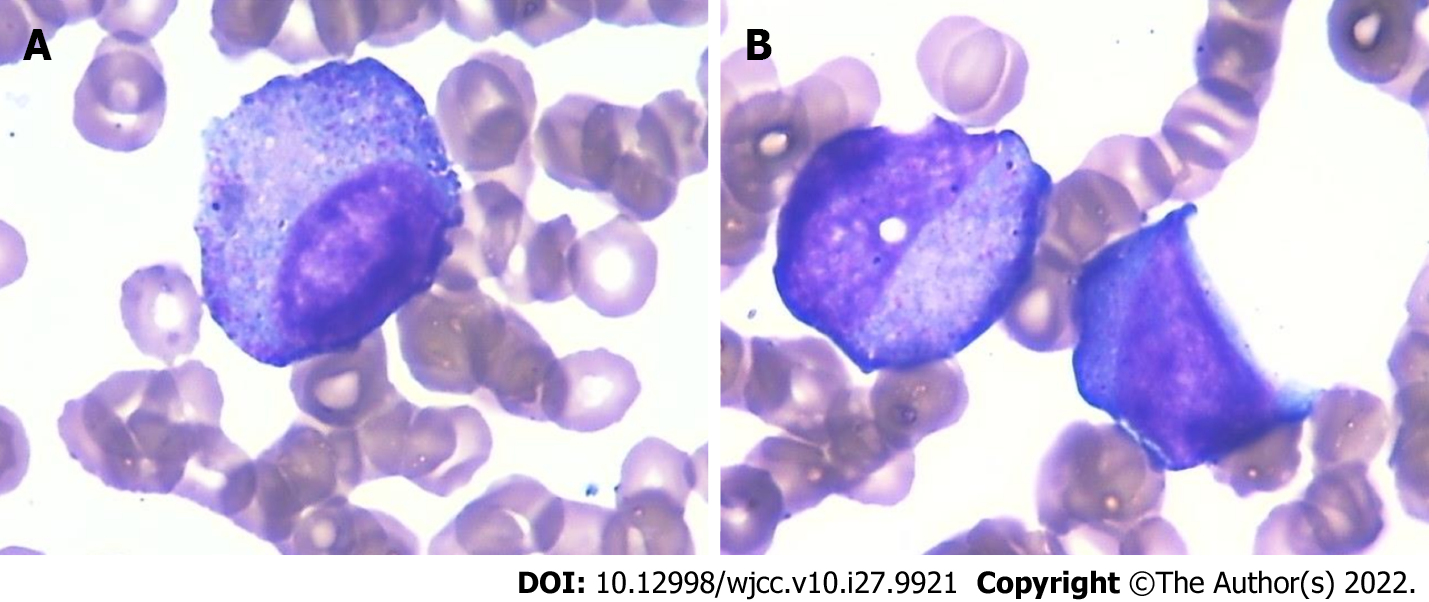
Cytological evaluation of bone marrow puncture revealed a myeloid/erythroid ratio of 0.16, and the erythrocyte and myeloid series cells were 47% and 7.5% of all nucleated cells, indicating severe agranulocytosis (Figure 1 and Table 2).
| Cell type | % | Reference value range |
| Myeloblasts | 1.5 | 0.31-0.97 |
| Promyelocytes | 1.5 | 1.51-1.63 |
| Neutrophilic myelocytes | 2.5 | 4.45-8.53 |
| Neutrophilic metamyelocytes | 2 | 5.93-9.87 |
| Neutrophilic stab granulocytes | 0 | 20.22-27.22 |
| Neutrophilic segmented granulocytes | 0 | 6.52-12.36 |
| Eosinophils | 0 | 0.15-0.61 |
| Basophils | 0 | 0.00-0.07 |
| Pronormoblasts | 0.5 | 0.27-0.87 |
| Early erythroblasts | 0.5 | 0.51-1.33 |
| Polychromatic normoblasts | 12.5 | 5.5-9.32 |
| Orthochromatic normoblasts | 33.5 | 8.39-13.11 |
| Lymphocytes | 42.5 | 15.71-29.82 |
| Monocytes | 2.5 | 2.12-3.88 |
| Plasmacytes | 0.5 | 0.29-1.13 |
| Total | 100 | |
| Myeloid:erythroid ratio | 0.16 | 2-4:1 |
The updated Roussel Uclaf Causality Assessment Method (RUCAM)[6] was used to assess whether PM was associated with acute liver injury in this patient. The results of RUCAM demonstrated a highly probable relationship between liver injury and PM, with a total score of 10 (RUCAM score: ≥ 9 = highly probable, 6–8 = probable, 3–5 = possible, 1–2 = unlikely; ≤ 0 = excluded). The hepatocellular injury was noted with an R-value of 22.44. Owing to the use of PM before the disease onset, the Naranjo algorithm[7] was used to score for PM. The result indicated that agranulocytosis had a probable relationship with PM, and the overall score was 6 (Naranjo score: 9–10 = definitely, 5–8 = probable, 1–4 = possible, score ≤ 1 = doubtful).
PM intake was discontinued 3 d before admission, and treatment was initiated immediately after admission. The following treatments were administered: Granulocyte colony-stimulating factor (300 μg/d, subcutaneous injection) for once, hydrocortisone sodium succinate (200 mg/d, 5 d → 100 mg/d, 5 d, intravenous infusion), compound glycyrrhizin (100 mL/d), and polyene phosphatidylcholine (465 mg/d) for 15 d by intravenous drip.
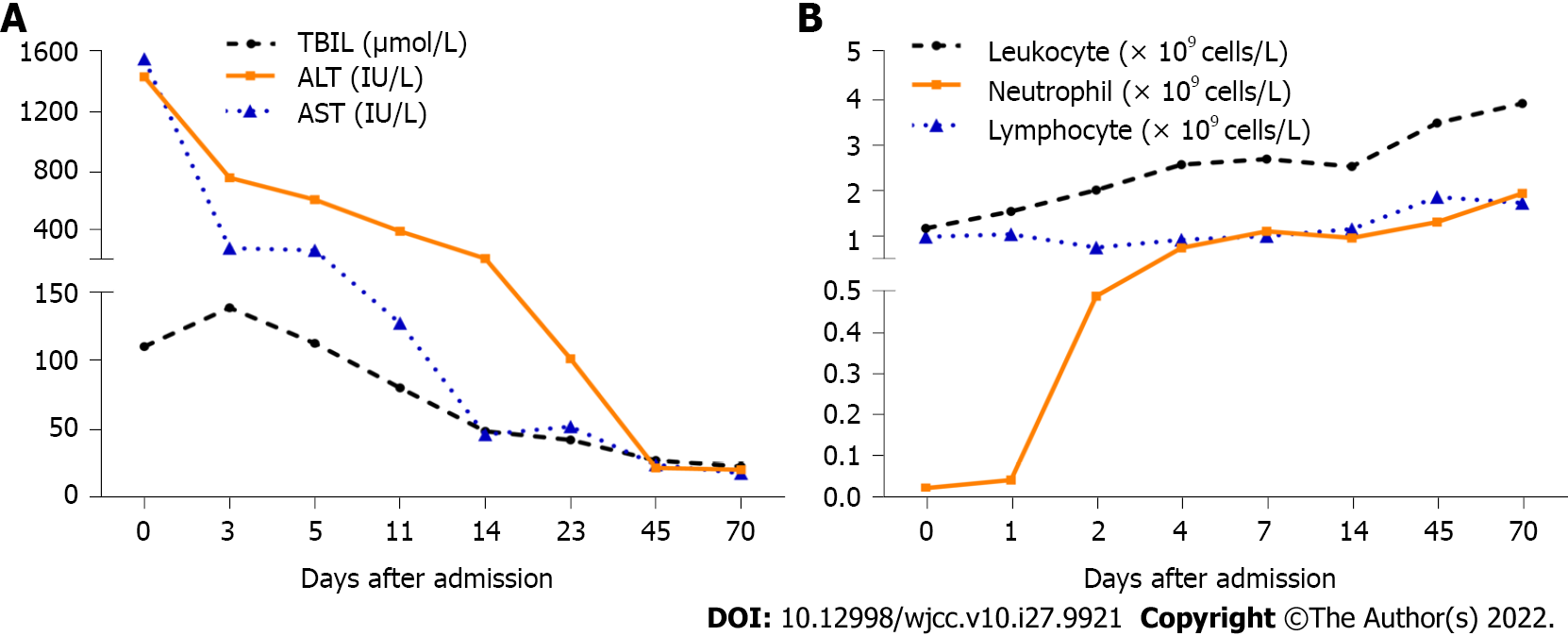
The patient’s liver biochemistry, leukocytes, and neutrophils levels improved gradually (Figure 2). Following this, the patient was discharged on day 15 after admission, and her liver biochemistry and granulocytes returned to normal on day 45. To avoid the recurrence of adverse drug reactions (ADRs), the patient was advised to avoid taking PM again.
The present case report is unique as the co-occurrence of DILI and agranulocytosis caused by PM have been poorly characterized. RUCAM is an established scoring tool used to assess the likelihood of DILI. A RUCAM score of 10 may be interpreted as the PM being a “highly probable” cause of the patient’s hepatocellular injury. In contrast, the Naranjo algorithm is a scoring tool used to assess the likelihood of ADRs. A Naranjo score of 6 may be interpreted as PM being a “probable” cause of the patient’s agranulocytosis.
Unpredictable immune-mediated adverse reactions to drugs or their reactive metabolites are known as idiosyncratic drug reactions. Idiosyncratic ADRs can generally occur at any dose within the normal therapeutic range. Idiosyncratic ADRs are extremely rare (1 in 10000 approximately 1 in 100000). Life-threatening idiosyncratic ADRs include DILI, serious myelosuppression, and cutaneous reactions[8]. DILI is the most common among these[9].
Idiosyncratic drug reactions owing to traditional Chinese drugs and dietary supplements are a major cause of DILI in China. PM is widely used in traditional Chinese medicine and dietary supplements; however, it is a major contributor to herbal DILI[10,11]. PM-induced hepatotoxicity occurs only in certain individuals[12]. PM can induce various types of DILI, such as 59.7%, 15.4%, and 24.9% of hepatocellular, cholestatic, and mixed types, respectively[13]. Despite a significant rise in the number of liver injuries caused by PM, such injuries occur only in a small proportion of individuals ingesting PM and are associated with idiosyncratic hepatotoxicity[4]. Hepatotoxicity does not occur in the majority of patients taking recommended therapeutic doses of PM, suggesting that an idiosyncratic response may be the primary mechanism of PM-induced DILI[4]. The following are the mechanisms of PM-related DILI[14]: (1) Cholestasis, leading to lipid peroxidation causing liver damage; (2) Affecting drug transport or metabolism through the CYP450 enzyme system; (3) Causing mitochondrial dysfunction through oxidative stress causing liver damage; and (4) Genetic susceptibility[15]. In the present case, liver function gradually improved after the administration of a glucocorticoid, compound glycyrrhizin, and polyene phosphatidylcholine was used to suppress inflammation and protect the liver. Although no pharmacological therapy for DILI has been adequately tested in randomized clinical trials, corticosteroids may be beneficial[9,16]. Compound glycyrrhizin is a safe and effective treatment for patients with DILI[17].
In addition to hepatotoxicity, agranulocytosis is another common adverse drug reaction[18]. In blood, absolute neutrophil count < 1.5 × 109 cells/L was defined as neutropenia and < 0.5 × 109 cells/L as agranulocytosis. Individuals with absolute neutrophil count < 0.1 × 109 cells/L had a significantly increased risk of morbidity and death owing to infection[18]. The clinical manifestations of idiosyncratic drug-induced agranulocytosis range from asymptomatic to various infections, and serious infections are often life-threatening[5]. There is approximately 5% of mortalities associated with idiosyncratic drug-induced neutropenia[19]. Poor prognosis is associated with individuals aged ≥ 65 years, absolute neutrophil count < 0.1 × 109 cells/L, severe infection, and comorbidities[20]. At present, the mechanism of PM-induced granulocytopenia is unknown, which is speculated to be related to idiosyncratic ADR. The most likely immune mechanisms for idiosyncratic drug-induced neutropenia are the hapten hypothesis and the danger signal hypothesis, which are related to the class I and II HLA genes[18]. In general, drug hepatotoxicity and hematological toxicity occur independently, and the co-occurrence of the two is rare, among which the mostly reported were antithyroid drugs[21,22], clozapine[23], methotrexate[24], and fusidic acid[25]. Regardless of the hepatotoxicity or hematologic toxicity of the drug, the primary treatment is immediate withdrawal. Despite the lack of prospective controlled randomized trials, two-thirds of reported cases of drug-related neutropenia received granulocyte-colony stimulating factor (G-CSF)[26]. G-CSF at 300 μg/d helped reduce the time to recovery of blood counts without causing any major toxicity or adverse effects[27]. Our patient was a 65-year-old woman with a minimum neutrophil count of 0.02 × 109 cells/L. Fortunately, after receiving a dose of 300 μg of G-CSF, her leukocyte and neutrophil counts improved rapidly, and she did not develop any infection even without antibiotics.
To the best of our knowledge, this is the first case report of concurrent hepatotoxicity and agranulocytosis with PM. It is a sudden, insidious disease that progresses rapidly and needs attention. Early discontinuation can prevent disease progression and facilitate recovery. The early elevation of granulocytes is essential to avoid infection; combination therapy of glucocorticoids, anti-inflammatory drugs, and protection of the liver is beneficial for idiosyncratic drug reactions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin MS, Taiwan; Shariati MBH, Iran; Soldera J, Brazil S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, Qu C, Ni J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J Ethnopharmacol. 2015;159:158-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Ballotin VR, Bigarella LG, Brandão ABM, Balbinot RA, Balbinot SS, Soldera J. Herb-induced liver injury: Systematic review and meta-analysis. World J Clin Cases. 2021;9:5490-5513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (2)] |
| 3. | But PP, Tomlinson B, Lee KL. Hepatitis related to the Chinese medicine Shou-wu-pian manufactured from Polygonum multiflorum. Vet Hum Toxicol. 1996;38:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Rao T, Liu YT, Zeng XC, Li CP, Ou-Yang DS. The hepatotoxicity of Polygonum multiflorum: The emerging role of the immune-mediated liver injury. Acta Pharmacol Sin. 2021;42:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Rattay B, Benndorf RA. Drug-Induced Idiosyncratic Agranulocytosis - Infrequent but Dangerous. Front Pharmacol. 2021;12:727717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 7. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8198] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 8. | Kalgutkar AS, Fate G, Didiuk MT, Bauman J. Toxicophores, reactive metabolites and drug safety: when is it a cause for concern? Expert Rev Clin Pharmacol. 2008;1:515-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, Devarbhavi H, Merz M, Lucena MI, Kaplowitz N, Aithal GP. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 469] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 10. | Byeon JH, Kil JH, Ahn YC, Son CG. Systematic review of published data on herb induced liver injury. J Ethnopharmacol. 2019;233:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Zhu Y, Niu M, Chen J, Zou ZS, Ma ZJ, Liu SH, Wang RL, He TT, Song HB, Wang ZX, Pu SB, Ma X, Wang LF, Bai ZF, Zhao YL, Li YG, Wang JB, Xiao XH; Specialized Committee for Drug-Induced Liver Diseases, Division of Drug-Induced Diseases, Chinese Pharmacological Society. Hepatobiliary and pancreatic: Comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients. J Gastroenterol Hepatol. 2016;31:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Lin Y, Xiao R, Xia BH, Zhang ZM, Li C, Wu P, Liao DF, Lin LM. Investigation of the idiosyncratic hepatotoxicity of Polygonum multiflorum Thunb. through metabolomics using GC-MS. BMC Complement Med Ther. 2021;21:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Lei X, Chen J, Ren J, Li Y, Zhai J, Mu W, Zhang L, Zheng W, Tian G, Shang H. Liver Damage Associated with Polygonum multiflorum Thunb.: A Systematic Review of Case Reports and Case Series. Evid Based Complement Alternat Med. 2015;2015:459749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Yu HS, Wang LL, He Y, Han LF, Ding H, Song XB, Gao XM, Yun NR, Li Z. Advances in the Study of the Potential Hepatotoxic Components and Mechanism of Polygonum multiflorum. Evid Based Complement Alternat Med. 2020;2020:6489648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Li C, Rao T, Chen X, Zou Z, Wei A, Tang J, Xiong P, Li P, Jing J, He T, Bai Z, Yin J, Tan Z, Yu P, Zhou H, Wang J, Xiao X, Ouyang D. HLA-B*35:01 Allele Is a Potential Biomarker for Predicting Polygonum multiflorum-Induced Liver Injury in Humans. Hepatology. 2019;70:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Björnsson ES, Vucic V, Stirnimann G, Robles-Díaz M. Role of Corticosteroids in Drug-Induced Liver Injury. A Systematic Review. Front Pharmacol. 2022;13:820724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Yao L, Zhang J, Jin J, Li H, Li L, Han X, Raza HK, Li X, Mao Y. An analysis of the efficacy and safety of compound glycyrrhizin injections in the treatment of drug-induced liver injury using a nationwide database. Int J Clin Pharm. 2022;44:731-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Curtis BR. Non-chemotherapy drug-induced neutropenia: key points to manage the challenges. Hematology Am Soc Hematol Educ Program. 2017;2017:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Pick AM, Nystrom KK. Nonchemotherapy drug-induced neutropenia and agranulocytosis: could medications be the culprit? J Pharm Pract. 2014;27:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Lorenzo-Villalba N, Alonso-Ortiz MB, Maouche Y, Zulfiqar AA, Andrès E. Idiosyncratic Drug-Induced Neutropenia and Agranulocytosis in Elderly Patients. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Yang J, Zhang J, Xu Q, Sheng GP, Weng WW, Dong MJ. Unusual Synchronous Methimazole-Induced Agranulocytosis and Severe Hepatotoxicity in Patient with Hyperthyroidism: A Case Report and Review of the Literature. Int J Endocrinol. 2015;2015:934726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Vilchez FJ, Torres I, Garcia-Valero A, López-Tinoco C, de Los Santos A, Aguilar-Diosdado M. Concomitant agranulocytosis and hepatotoxicity after treatment with carbimazole. Ann Pharmacother. 2006;40:2059-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Zarghami M, Hoseini SD, Kazemi A, Elyasi F. Concurrent Hepatotoxicity and Neutropenia Induced by Clozapine. Arch Iran Med. 2020;23:141-143. [PubMed] [DOI] [Full Text] |
| 24. | Bielen L, Kralj I, Ćurčić E, Vodanović M, Boban A, Božina N. Acute kidney injury, agranulocytosis, drug-induced liver injury, and posterior reversible encephalopathy syndrome caused by high-dose methotrexate-possible role of low activity ABC and SLC drug transporters. Eur J Clin Pharmacol. 2018;74:1191-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | He ZF, Chen L, Zhang JP, Wang QQ. Hepatotoxicity and hematologic complications induced by fusidic acid in a patient with hepatitis B cirrhosis: A case report. Medicine (Baltimore). 2019;98:e17852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Andrès E, Maloisel F, Zimmer J. The role of haematopoietic growth factors granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the management of drug-induced agranulocytosis. Br J Haematol. 2010;150:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Andrès E, Mourot-Cottet R. Non-chemotherapy drug-induced neutropenia - an update. Expert Opin Drug Saf. 2017;16:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |