Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9879
Peer-review started: May 12, 2022
First decision: July 14, 2022
Revised: July 27, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 127 Days and 5.9 Hours
Breast cancer poses a great threat to females worldwide. There are various therapies available to cure this common disease, such as surgery, chemotherapy, radiotherapy, and immunotherapy. Implantable venous access ports (IVAP, referred to as PORT) have been widely used for breast cancer chemotherapy. Venous malformations are possible conditions encountered during PORT im
We incidentally found that two patients had PLSVC while a PORT was implanted via the internal jugular vein. Due to chemotherapy for breast cancer, PORT was successfully implanted under the guidance of ultrasound into these 2 patients. Positive chest X-ray examination after the operation showed that the catheter ran beside the left mediastinum and the end was located in the seventh thoracic vertebra. The patients had no catheter-related complications and successfully completed the course of chemotherapy. Ultrasonography found that the ratio of PORT outer diameter to PLSVC inner diameter was less than 0.45, which was in line with the recommendations of relevant literature and operating guidelines. The purpose of this article is to introduce two rare cases and review the relevant literature.
Correct assessment of PLSVC status and ultrasound-guided PORT placement generally does not affect breast cancer patients chemotherapy.
Core Tip: We accidentally discovered two cases of implantable venous access ports (IVAP, referred to as PORT) placement through the persistent left superior vena cava (PLSVC). Both patients had no obvious clinical symptoms, and the operation was smooth and the postoperative recovery was good. According to relevant literature and operation guideline, the PORT should only be placed if the ratio of the PORT outer diameter to the PLSVC inner diameter is less than 0.45, otherwise catheter-related complications may occur. At the same time, it is recommended that all patients undergo ultrasonography before venous port placement to determine whether the coronary sinus is dilated.
- Citation: Zhou RN, Ma XB, Wang L, Kang HF. Accidental venous port placement via the persistent left superior vena cava: Two case reports. World J Clin Cases 2022; 10(27): 9879-9885
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9879.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9879
Implantable venous access ports (IVAP, referred to as PORT) have been widely used in intravenous therapy of breast cancer due to the advantages of fewer vascular-related complications, low incidence of local infection, and catheter displacement[1]. The use of PORTs usually involves the catheterization of the percutaneous internal jugular, the subclavian, the basilic and the femoral veins[2]. Among the cases of PORT implantation via the internal jugular vein in our department, two cases were opportunistically found, in which PORT was implanted through the persistent left superior vena cava (PLSVC). In embryology, PLSVC occurs because the left anterior main vein is not closed at 12 wk of pregnancy, resulting in the confluence of the left common jugular vein, and the left subclavian vein cannot return to the right superior vena cava but instead travels downward before the aortic arch and the left pulmonary hilum. This malformed vein- PLSVC, receives blood from the left superior intercostal vein and hemiazygos vein, and then passes through the pericardium into the coronary sinus (CS) or left atrium[3,4]. PLSVC is a rare venous malformation that has an influence on the implantation of PORT owing to its different course. This study reports two cases of opportunistic intravenous PORT implantation via the PLSVC and reviews the related literature on similar cases.
Case 1: A 40-year-old woman found double breast mass present for more than one month.
Case 2: A 54-year-old woman found right breast lump more than 3 mo.
Case 1: Her puncture biopsy confirmed right breast cancer with lymph node metastasis (T2N1M0) and left breast ductal carcinoma in situ. After the diagnosis was confirmed, subcutaneous adenectomy and axillary lymph node dissection were performed on the right breast, and breast prosthesis reconstruction on both breasts were performed under general anesthesia. Postoperative pathology revealed mucinous carcinoma of the right breast at the 10:00, 11:00, and 12:00 positions (size 2.5 cm, 0.7 cm, 1.2 cm, respectively), and 1/16 cancerous tissues were found in the right axillary lymph node. Immunohistochemistry suggested the following profile: Estrogen receptor (ER, 80%), progesterone receptor (PR, 90%), human epidermal growth factor receptor (HER2[-]), and Ki67 (30%). On the other hand, pathology of the left breast revealed papillomas with focal ductal epithelial atypical hyperplasia, formation of intraductal carcinoma, and reactive hyperplasia of the sentinel node (5), and immunohistochemistry showed a profile of ER (+), PR (+), and Ki67 (5%). The patient recovered well postoperatively and received adjuvant chemotherapy with a regimen consisting of doxorubicin and cyclophosphamide followed by paclitaxel (AC-T). The right breast mass of the patient had mucinous carcinoma with axillary lymph node metastasis; therefore, adjuvant radiotherapy was required after breast-conserving surgery.
Case 2: Her puncture biopsy showed right invasive breast cancer with axillary lymph gland metastasis. Immunohistochemistry suggested the following profile: ER (-), PR (-), androgen receptor (AR +) 2%, HER2 (2+), Ki67 (+) 50%, CK5/6 (-). Fluorescence in Situ hybridization detected HER2(-).
Case 1: The patient had no special history of past illness.
Case 2: Six months ago, the patient underwent lumbar vertebroplasty and steel screw implantation due to lumbar fracture.
Case 1: The patient had no personal and family history of venous malformation and cancer.
Case 2: The patient had no personal and history of cancer.
Case 1: 3 lumps in right breast at the 10:00, 11:00, and 12:00 positions (size 2.5 cm, 0.7 cm, 1.2 cm, respectively) could be palpated.
Case 2: In this patient's right breast, a 1.9 cm-sized mass was palpable at 11 o'clock, with ill-defined borders and poor mobility.
Case 1: Blood, urine, fecal routine, coagulation function, infectious disease detection and other laboratory tests are normal.
Case 2: There are no obvious abnormalities of her laboratory examination.
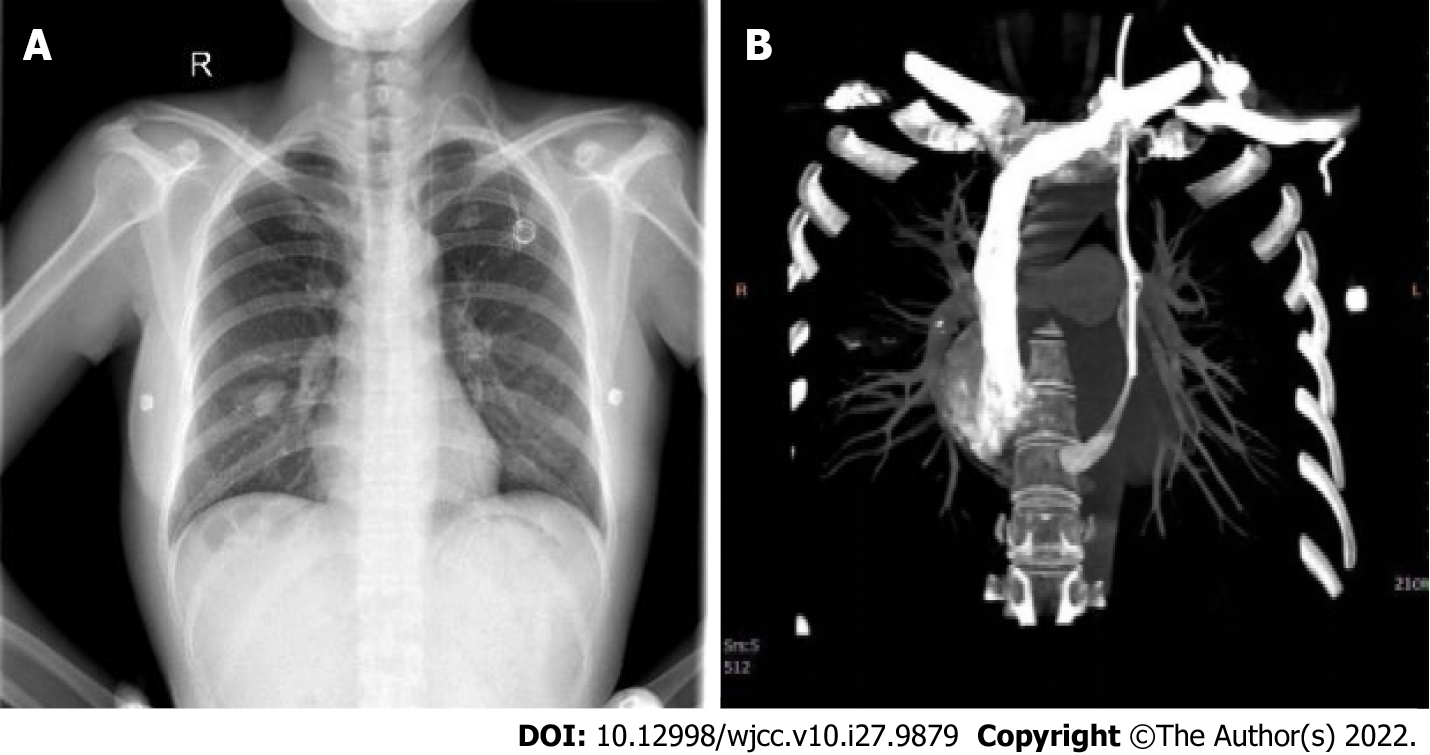
Case 1: Orthotopic chest radiography after the operation showed that the PORT catheter of the patient ran beside the left mediastinum (Figure 1A), which was different from that of healthy people who were implanted with PORT through the left internal jugular vein. Contrast-enhanced chest computed tomography (CT) confirmed that the patient had PLSVC, and the contrast medium flowed into the right atrium through the CS (Figure 1B). The PORT was implanted through the PLSVC, and the tip of the catheter was located at the nipple level.
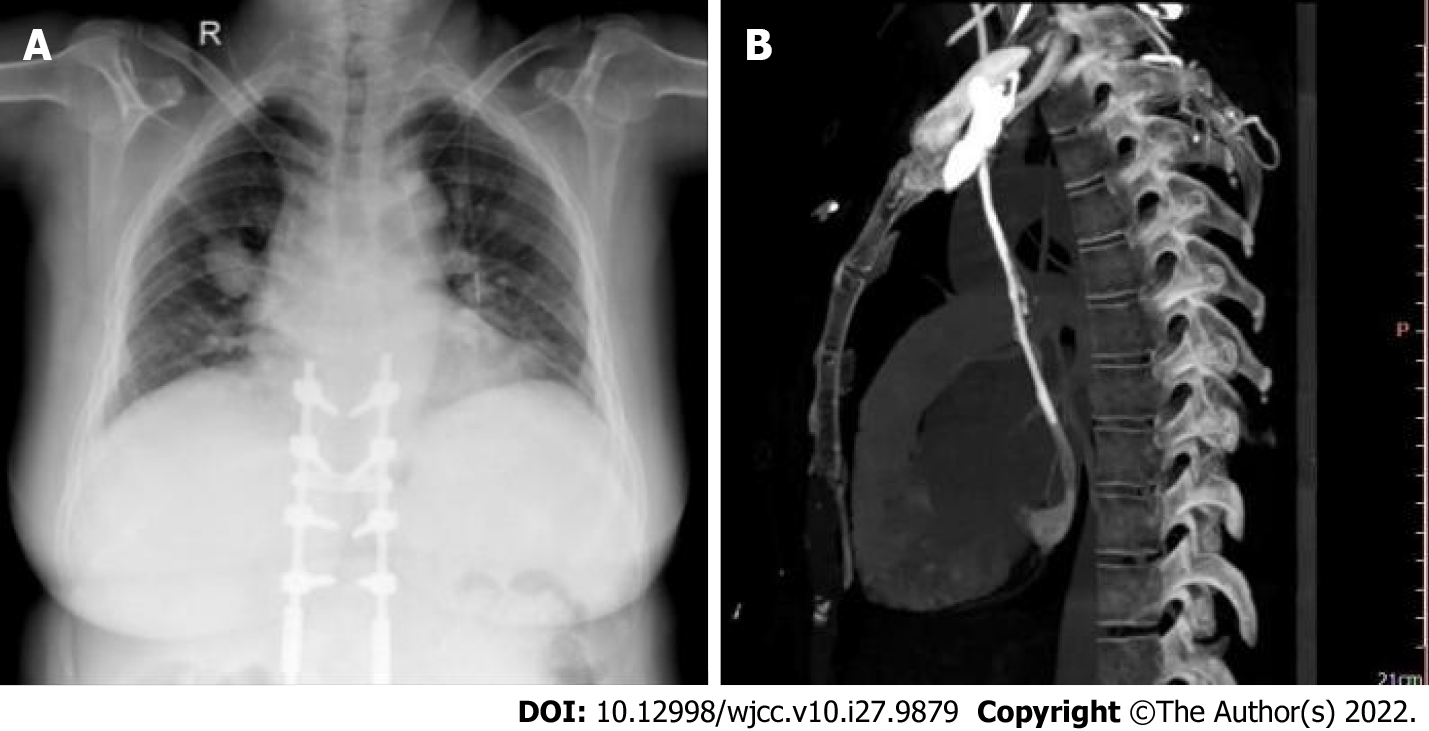
Case 2: A chest X-ray examination after operation showed that the catheter ran beside the left mediastinum, and the end was located in the seventh thoracic vertebra (Figure 2A), which resulted in an opportunistic discovery of PLSVC and PORT implantation via PLSVC in the patient. Similarly, contrast-enhanced CT of the chest was performed by median venography to determine the course of blood vessels, and PLSVC converged into the right atrium through the CS (Figure 2B).
The catheter-to-vein ratio was < 45% showed by ultrasound, so adjustments on the PORT were not required.
Intravenous port placement through the PLSVC.
The patient received adjuvant chemotherapy with a regimen consisting of doxorubicin and cyclophosphamide followed by paclitaxel (AC-T).
The PORT continued to function normally throughout adjuvant chemotherapy, and the catheter was removed at the end of treatment.
PORT is a fully implantable vascular channel device that can be punctured repeatedly. Intravenous therapy is an absolute indication for PORT implantation in patients with tumors. Compared with the past, peripherally inserted central catheters (PICC) can effectively reduce the incidence of peripheral phlebitis and vascular sclerosis and reduce the necrosis of surrounding tissue caused by drug extravasation[5,6]. Second, the use of PICC rarely restricts the daily activities of patients. Moreover, PICC can be easily used and maintained over extended periods, which can protect the patients' privacy and improve their quality of life[7].
After PORT was implanted through the left internal jugular vein in our patients, chest radiography showed abnormalities because the catheter was located next to the left mediastinum rather than the superior vena cava. CT angiography confirmed that the PORT catheter did not flow normally into the right superior vena cava through the left common jugular vein, but into the CS through the PLSVC and then into the right atrium. PLSVC was first discovered by Edwards and Dushane. It is a rare venous malformation, with an incidence rate of 0.3%–0.5% in healthy people and 3%–10% in patients with congenital heart disease[8]. The incidence of PLSVC is as high as 12% especially in cases of atrial septal defects, ventricular septal defects, coarctation of the aorta, transposition of major vessels, tetralogy of Fallot, and abnormal pulmonary venous connection[9]. However, no abnormality was found on color echocardiography in these two patients. PLSVC is caused by abnormal degeneration of the left anterior main vein, which starts in front of the left jugular vein and the left pulmonary artery, and in the lateral edge of the left atrium. It converges into the CS through the left atrioventricular sulcus, and finally enters the right atrium[10]. Based on the location of the flow into the heart, PLSVC is often divided into four types. Type I: PLSVC flows into the CS as the right superior vena cava is absent, accounting for 10%–20% of cases; type II: There is no communicating branch between the two superior vena cava, accounting for 50%–60% of cases; type III: Both sides of the superior vena cava exist and there are communicating branches between them, accounting for 25%–30% of cases; and type IV: PLSVC flows into the left atrium, accounting for 10%–20% of cases[11]. Type II has the highest incidence, but has no obvious effect on hemodynamics; therefore, patients generally have no symptoms and such cases are not easy to detect. In recent years, owing to the development of medical devices and interventional technology, an increasing number of PLSVCs have been found in cardiac pacemaker implantation, intravenous infusion port implantation, and PICC. However, there are few reports on the risk and experience of PORT implantation using PLSVC.
Since the PLSVC is an abnormal vascular flow, it affects the implantation of PORT. However, it is usually difficult to detect PLSVC before surgery, and only an abnormal catheter courses can be detected on postoperative imaging. PLSVC catheterization is prone to the following complications: (1) Obstruction of catheter entry; (2) Catheter misplacement into the right atrium; (3) Short catheter implantation and slow PLSVC blood flow velocity, which can easily lead to thrombosis; (4) Increased risk of infection caused by prolonged operation time; and (5) Changes in CS structure and venous flow velocity. In our report, the implantation and use of PORT were favored in both neoadjuvant and adjuvant chemotherapy for breast cancer. However, if it is difficult to enter the guidewire or catheter through the left internal jugular vein, PLSVC should be considered. Under the guidance of B-ultrasound or X-rays, PORT can be implanted normally. The need for catheter adjustment or re-implantation through other venous pathways requires an assessment of the PLSVC.
In these two patients, chest CT was performed through the ipsilateral median vein, and the contrast medium was administered into the right atrium through the PLSVC. At the same time, the ratio of the outer diameter of the PORT catheter to the internal diameter of the PLSVC vessel was less than 45%, suggesting that the venous velocity was normal; thus, PORT could be used, and the catheter path was not adjusted in both patients. The identification of the PLSVC before surgery to avoid opportunistic implantations of PORT through the PLSVC is conducive to the early selection of other venous pathways, which can be judged by the CS. The average caliber of the CS was 4.75 mm in diastole and 8.27 mm in systole[12]. Taking the end-systolic diameter of the CS orifice ≥ 16 mm as the critical value, the sensitivity, specificity, and accuracy of identifying PLSVC were 90.99%, 91.1%, and 91.1%, respectively[13]. When improving the relevant examination before PORT implantation, echocardiography should be recommended to evaluate CS, which has the advantages of being non-invasive, simple, and highly repeatable, and can be used as the first choice for the examination of PLSVC[14].
The position of the catheter should be confirmed by routine chest X-ray after the intravenous infusion port was implanted into the right subclavian vein and the ideal end of the catheter should be at the T5-7 level[15,16]. Postoperative CT scans of these two cases showed that the PORT was passing through the PLSVC, and the tube orifice was at the level of the nipple. In addition to routinely evaluating the position of the end of the catheter, an appropriate type of catheter should be selected according to the its vascular conditions. It is recommended that the ratio of the outer diameter of the catheter to the inner diameter of the indwelling vein be less than 45%[17]. According to the evaluation, the external diameter of the catheter is 6F; that is, the outer diameter of the catheter is about 1.91 mm, and the inner diameter of the indwelling vein of these 2 patients are 4.75 mm and 4.83 mm respectively, which meets the conditions of pipe placement. Both patients had no adjustments to the catheter position and completed adjuvant chemotherapy as planned, with no significant difference in chemotherapy side effects compared to other breast cancer patients. The reason may be that the two cases involve type II PLSVC, which do not affect hemodynamics and have no obvious effect on the absorption, distribution, metabolism, and excretion of chemotherapy drugs. Common intraoperative complications such as pneumothorax and/or hemothorax, air embolism, arterial injury, and pericardial tamponade[18] did not occur during the operation. Skin and soft tissue injuries caused by infection or noninfectious causes, phlebitis catheter-related infection, catheter-related thrombosis, and other common postoperative complications[19] also did not occur. After the treatment, the catheter was removed, and all indicators were normal during the regular review. The patients were followed up for more than a year, and their health and quality of life were significantly improved.
In summary, when opportunistic insertion of a PORT through the PLSVC occurs in clinically, it is necessary to analyze and perform echocardiography and contrast-enhanced chest CT for confirmation. It is important to assess whether the ratio of the inner diameter of the PLSVC to the outer diameter of the catheter meets the conditions for implantability, if there is a need to adjust the catheter and vascular path, and whether PORT can be used normally. At the same time, it is recommended to routinely perform a cardiac ultrasound to assess the condition of the CS when PORT is implanted through the left internal jugular vein. These practices may help improve the sensitivity of PLSVC in clinical work, improve the accuracy of diagnosis and treatment, and achieve precise and individualized treatment.
We thank the patients and their family who participated in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belosludtseva NV, Russia; Menendez-Menendez J, Spain S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Teichgräber UK, Pfitzmann R, Hofmann HA. Central venous port systems as an integral part of chemotherapy. Dtsch Arztebl Int. 2011;108:147-53; quiz 154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Giordano CR, Murtagh KR, Mills J, Deitte LA, Rice MJ, Tighe PJ. Locating the optimal internal jugular target site for central venous line placement. J Clin Anesth. 2016;33:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Zhang J, Yu WX. PICC catheterization in a patient with persistent left superior vena cava: a case report and literature review. Zhonguo Linchuang Yanjiu. 2019;32:1750-1752. |
| 4. | Voci P, Luzi G, Agati L. Diagnosis of persistent left superior vena cava by multiplane transesophageal echocardiography. Cardiologia. 1995;40:273-275. [PubMed] |
| 5. | Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, Eaton M, McLeay W, Price T, Ly M, Ullah S, Koczwara B, Kichenadasse G, Karapetis CS. Comparison of peripherally inserted central venous catheters (PICC) vs subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer. 2014;22:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, Berg S. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019;122:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Burbridge B, Lim H, Dwernychuk L, Le H, Asif T, Sami A, Ahmed S. Comparison of the Quality of Life of Patients with Breast or Colon Cancer with an Arm Vein Port (TIVAD) Versus a Peripherally Inserted Central Catheter (PICC). Curr Oncol. 2021;28:1495-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Sheikh AS, Mazhar S. Persistent left superior vena cava with absent right superior vena cava: review of the literature and clinical implications. Echocardiography. 2014;31:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Povoski SP, Khabiri H. Persistent left superior vena cava: review of the literature, clinical implications, and relevance of alterations in thoracic central venous anatomy as pertaining to the general principles of central venous access device placement and venography in cancer patients. World J Surg Oncol. 2011;9:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Horvath SA, Suraci N, D'Mello J, Santana O. Persistent left superior vena cava identified by transesophageal echocardiography. Rev Cardiovasc Med. 2019;20:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Tyrak KW, Holda J, Holda MK, Koziej M, Piatek K, Klimek-Piotrowska W. Persistent left superior vena cava. Cardiovasc J Afr. 2017;28:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | D'Cruz IA, Shala MB, Johns C. Echocardiography of the coronary sinus in adults. Clin Cardiol. 2000;23:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Zheng ZT, Zhang W, Li L. Clinical study on the causes of coronary sinus dilatation in adults. Zhonghua Chaosheng Yingxiangxue Zazhi. 2004;19-23. |
| 14. | Luo J, Zhu W, Zhou AY. Color Doppler diagnosis of persistent left superior vena cava and its significance. Journal of Nanchang University (Medical Edition). 2010;50:67-68. [DOI] [Full Text] |
| 15. | Cohn DE, Mutch DG, Rader JS, Farrell M, Awantang R, Herzog TJ. Factors predicting subcutaneous implanted central venous port function: the relationship between catheter tip location and port failure in patients with gynecologic malignancies. Gynecol Oncol. 2001;83:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Luciani A, Clement O, Halimi P, Goudot D, Portier F, Bassot V, Luciani JA, Avan P, Frija G, Bonfils P. Catheter-related upper extremity deep venous thrombosis in cancer patients: a prospective study based on Doppler US. Radiology. 2001;220:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Sharp R, Cummings M, Fielder A, Mikocka-Walus A, Grech C, Esterman A. The catheter to vein ratio and rates of symptomatic venous thromboembolism in patients with a peripherally inserted central catheter (PICC): a prospective cohort study. Int J Nurs Stud. 2015;52:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Guirindola B, Bothma P. Recognising air embolism as a complication of vascular access. Br J Nurs. 2015;24:S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Liu YJ, Qu X, Ge ZC. Expert consensus and Technical Guide for Clinical Application of Breast Cancer implantable intravenous Infusion Port (2017 Edition). Zhonguo Shiyong Waike Zazhi. 2017;37:1377-1382. |