Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9865
Peer-review started: May 11, 2022
First decision: May 31, 2022
Revised: June 19, 2022
Accepted: August 17, 2022
Article in press: August 17, 2022
Published online: September 26, 2022
Processing time: 127 Days and 20.9 Hours
During skull base surgery, intraoperative internal carotid artery (ICA) injury is a catastrophic complication that can lead to fatal blood loss or secondary cerebral ischemia. Appropriate management of ICA injury plays a crucial role in the prognosis of patients. Neurosurgeons have reported multiple techniques and management strategies; however, the literature on managing this complication from the anesthesiologist’s perspective is limited, especially in the aspect of circulation management and airway management when patients need transit for further endovascular treatment.
We describe 4 cases of ICA injury during neurosurgery; there were 3 cases of pathologically proven pituitary adenoma and 1 case of cavernous sinus en
ICA injury imposes a high risk of massive hemorrhage and subsequent infarction. Immediate treatment is critical and requires interdisciplinary collaboration among neurosurgeons, anesthesiologists, and interventional neuroradiologists. Effective hemostatic methods, stable hemodynamics sufficient to ensure perfusion of vital organs, airway safety during transit, rapid localization and implementation of appropriate measures to occlude the damaged vessel are strong guarantees of patient safety.
Core Tip: Intraoperative internal carotid artery (ICA) injury is an uncommon but life-threatening event that usually requires transfer to a hybrid or catheter operating room for urgent endovascular treatment; however, the literature on the management of this complication from the anesthesiologist’s perspective is limited. This case series documents four cases of ICA injury during skull base neurosurgery. Effective hemostatic procedures, hemodynamic stabilization and maintenance of mechanical ventilation during and after transfer, rapid localization and implementation of necessary measures to occlude the injured vessel are solid guarantees of patient safety.
- Citation: Wang J, Peng YM. Emergency treatment and anesthesia management of internal carotid artery injury during neurosurgery: Four case reports. World J Clin Cases 2022; 10(27): 9865-9872
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9865.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9865
Internal carotid artery (ICA) injury is a rare but catastrophic complication. As anterior, medial, and lateral skull base tumors often close to the ICA, there is an inherent risk of injuring the ICA[1]. Due to publication bias, the reported incidence of ICA injury varies widely. The incidence of ICA injury in traditional craniotomy for the resection of skull base masses is approximately 3%-8%[2] and varies from 0.4%-9% in endoscopic neurosurgery[3-5]. Nasal packing, muscle patches, direct vessel closure, and endovascular techniques have been described as useful strategies for managing ICA bleeds[6]. In this paper, we described the anesthetic management of 4 cases of ICA injury during neurosurgery at Tiantan Hospital, especially in the aspect of circulation management and airway management when patients need transit for further endovascular treatment.
Case 1: A 51-year-old female presented with paroxysmal headache for 7 mo (Table 1).
| Case | Age (yr) | Sex | BMI (kg/m2) | Primary disease | Comorbidities | Maximum diameter of tumor (mm) | Tumor recurrence (yes/no) | Operative approach | Location of ICA injury |
| 1 | 51 | Female | 25.28 | Pituitary adenoma | None | 20 | Yes | ETTS | C5 segment of right internal carotid artery |
| 2 | 57 | Female | 21.50 | Pituitary adenoma | Hyperthyroidism, cured | 46 | No | ETTS | C4 segment of left internal carotid artery |
| 3 | 32 | Male | 26.12 | Pituitary adenoma | None | 30 | No | MTTS | C5 segment of right internal carotid artery |
| 4 | 53 | Female | 25.63 | Right cavernous sinus endothelial meningioma | Allergic asthma | 35 | No | Right frontotemporal approach | C5 segment of right internal carotid artery |
Case 2: A 57-year-old female presented with an intermittent headache for over a month.
Case 3: A 32-year-old male presented with facial changes for three months.
Case 4: A 53-year-old female presented with intermittent headache and dizziness for 1 year and exacerbation for 3 mo.
Case 1: The patient underwent pituitary adenoma surgery 7 years prior and denied any other symptoms.
Case 2: There were no other symptoms.
Case 3: There were no other symptoms.
Case 4: There were no other symptoms.
Case 1: The patient had no other medical history.
Case 2: The patient had a history of hyperthyroidism that had been cured.
Case 3: The patient had no other medical history.
Case 4: The patient had a history of allergic asthma.
Case 1: The patient had no history of smoking or drinking, and no family history.
Case 2: The patient had no history of smoking or drinking, and no family history.
Case 3: The patient had a history of smoking. The patient’s parents are alive and healthy.
Case 4: The patient had no history of smoking or drinking. The patient’s father had died of a stroke. The patient’s mother is alive and healthy.
Case 1: The visual acuity of both eyes decreased, and the left upper visual field partially defected; the rest nervous system examination was negative.
Case 2: The nervous system examination was negative.
Case 3: Physical examination revealed hypertrophy of the lips and acromegaly; the rest nervous system examination was negative.
Case 4: The nervous system examination was negative.
Case 1: The growth hormone was mildly elevated at 8.3 ng/mL (reference range: 0-8.0 ng/mL).
Case 2: The patient’s blood test results were all normal.
Case 3: The patient exhibited multiple pituitary hormone abnormal (growth hormone level, > 40.0 ng/mL, reference range: 0-8.0 ng/mL; prolactin level, 143 ng/mL, reference range: 1.9-25 ng/mL).
Case 4: The patient’s blood test results were all normal.
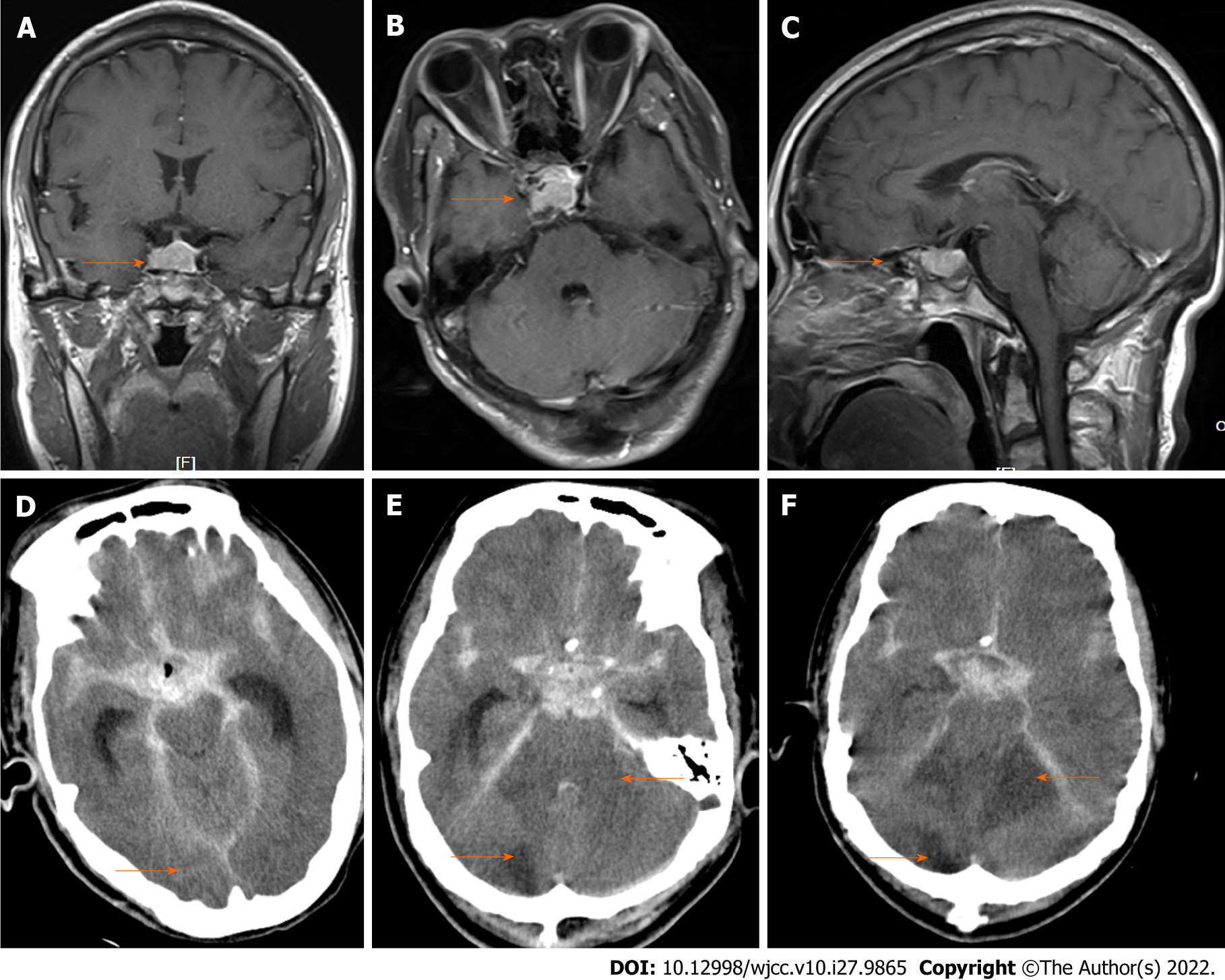
Magnetic resonance imaging (Figures 1A-C) performed before surgery showed that the ICA was enveloped by a sellar tumor. Time-varying computed tomography (CT) (Figures 1D-F) performed after surgery showed intracerebral hemorrhage and progressive cerebral infarction (orange arrow).
Case 1: The patient was diagnosed with pituitary adenoma.
Case 2: The patient was diagnosed with pituitary adenoma.
Case 3: The patient was diagnosed with pituitary adenoma.
Case 4: The patient was diagnosed with right cavernous sinus endothelial meningioma.
Case 1: Endoscopic transsphenoidal pituitary adenoma surgery was performed under general anesthesia. After curettage of most of the tumor, a large amount of bloody fluid gushed out from the sellar region, at which time sponges were immediately placed to temporarily stop the bleeding. The patient was immediately transferred to a hybrid operating room with controlled ventilation. During the transfer, vital signs were monitored, and the depth of anesthesia was maintained with a combination of propofol and remifentanil infusions. Injury of the right ICA was confirmed by digital subtraction angiography, and the injury was sealed with a covered stent. The overall volume of blood loss was 2400 mL. Fluid resuscitation, vasoactive drugs (epinephrine), and blood products were used to maintain circulation during the operation. After surgery, the patient was returned to the intensive care unit (ICU) under tracheal intubation and mechanical ventilation (Table 2).
| Case | ASA class | Type of anesthesia | Vasoactive drugs | Total infusion (mL) | RBC (mL) | FFP (mL) | Blood loss (mL) | Urine output (mL) | Tumor removal | Endovascular treatment | Prolonged intubation (d) | Length of stay (d) | Outcome | Hospital costs (RMB) |
| 1 | IV | TIVA | Adrenaline | 6000 | 520 | 800 | 2400 | 750 | Near total | Covered stent | 55 | 62 | Died | 402167.49 |
| 2 | III | TIVA | Dopamine | 6000 | 1560 | 1200 | 3000 | 3500 | Near total | Covered stent | 0 | 12 | Recovered | 221113.41 |
| 3 | III | CIIA | Norepinephrine and adrenaline | 4000 | 260 | 400 | 900 | 800 | Near total | Covered stent | 1 | 17 | Recovered | 209218.11 |
| 4 | III | CIIA | Norepinephrine and adrenaline | 6600 | 1000 | 400 | 2200 | 4100 | Total | Right ICA embolism | 1 | 22 | Recovered | 165178.78 |
Case 2: Endoscopic transnasal resection of the sphenoidal tumor was performed under general anesthesia. When the operation was continued to remove the tumor in the left cavernous sinus, severe bleeding occurred. After compression with a hemostatic sponge, the patient was immediately transferred to a catheter operating room with controlled ventilation. During the transfer, vital signs were monitored, and the depth of anesthesia was maintained with a combination of propofol and remifentanil infusions. The C4 segment of the left ICA was confirmed to be injured and was treated with a covered stent. The total volume of blood loss was 3000 mL. Fluid resuscitation, vasoactive drugs (dopamine), and blood products were used to maintain circulation during the operation. After surgery, the patient was extubated and returned to the ward.
Case 3: Transsphenoidal microsurgery was performed under general anesthesia. When the bone window was enlarged, the ICA was penetrated by bone debris. Immediately after the injury, the area of hemorrhage was packed, and the patient was transferred to the hybrid operating room. The C5 segment of the right ICA was confirmed to be injured and was treated with a covered stent. During the transfer, vital signs were monitored, and the depth of anesthesia was maintained with a combination of midazolam and rocuronium. The total volume of blood loss was 1000 mL. Fluid resuscitation, vasoactive drugs (norepinephrine and epinephrine), and blood products were used to maintain circulation during the operation. After surgery, the patient was returned to the ICU under tracheal intubation and spontaneous breathing.
Case 4: Craniotomy with the right frontotemporal approach was performed under general anesthesia. The tumor tightly encircled the right ICA and invaded the arterial wall. During the separation of the residual tumor, injury to the ICA occurred. The area of hemorrhage was immediately packed, and the patient was transferred to the hybrid operating room. The C5 segment of the right ICA was confirmed to be injured and was treated with coil embolization. During the transfer, vital signs were monitored, and the depth of anesthesia was maintained with a combination of propofol and remifentanil infusions. The total volume of blood loss was 2200 mL. Fluid resuscitation, vasoactive drugs (norepinephrine and epinephrine), and blood products were used to maintain circulation during the operation. After surgery, the patient was returned to the ICU under tracheal intubation with spontaneous breathing.
Case 1: The patient remained in a coma with a Glasgow Coma Scale score of 3 without spontaneous breathing until postoperative day 11. Subsequently, brainstem failure, multiple cerebral infarcts, respiratory and circulatory failure, severe electrolyte disturbances, and deep venous thrombosis occurred gradually. The time-varying CT images are shown in Figure 1. The patient died 62 d after the surgery (Table 2).
Case 2: The patient did not develop any neurological sequelae and was discharged after 12 d of hospitalization.
Case 3: The patient did not develop any neurological sequelae and was discharged after 17 d of hospitalization.
Case 4: The patient experienced transient left upper extremity weakness and was discharged after 22 d of hospitalization.
Although ICA injury is rare during skull base neurosurgery, it receives considerable attention due to its potentially catastrophic consequences, which include massive hemorrhage and even circulation collapse and secondary ischemia. The reported incidence of ICA injury varies from 0.34%-2.6% with trans
Due to different approaches and angles of craniotomy, intraoperative ICA injury is more likely to occur in the cavernous segment during endoscopic approaches and in the postcavernous segment during anterior and middle skull base surgery. The petrous segment is at risk during both open and endoscopic lateral skull base surgery[1]. As Gardner et al[12] mentioned, primary prevention is the best management strategy. Individual preoperative risk evaluation is mandatory if intraoperative manipulation of the ICA is anticipated[13]. A comprehensive preoperative evaluation includes an assessment of the tumor size, the relationship between the tumor and the ICA (encapsulation, invasion, or displacement of the ICA), and the patient’s risk tolerance. It should be noted that previous surgery, previous radiotherapy, sphenoid wall defects, encapsulation of the ICA by the tumor, and more ectatic arteries due to acromegaly are risk factors for ICA injury[1,7,14,15]. Regarding the preparation for anesthesia, placing two peripheral lines for rapid perfusion and keeping two units of blood products in the operating room for patients with a high risk of ICA injury are necessary[16]. In addition to routine monitoring, it has been recommended to place an arterial catheter in the dorsal pedis artery or radial artery after anesthesia induction.
In the event of intraoperative ICA injury, neurosurgeons, anesthesiologists, and interventional neuroradiologists should collaborate as a team to manage this emergency situation. Successful primary repair of an injured ICA by encasing the injured vessel with a synthetic or autologous material, patching or wrapping with muscle grafts, or other methods of hemostasis is of the utmost importance. In this paper, all 4 patients who experienced ICA injury during skull base neurosurgery under general anesthesia were immediately transferred to a hybrid or catheter operating room for endovascular treatment after effective hemostasis. Patient safety must be ensured during the transfer process. Multivital sign monitoring, controlled breathing and stable blood pressure to maintain target organ perfusion during transport are critical to patient safety. Previous studies recommended that blood pressures should be kept normal to high to ensure adequate cerebral perfusion during the management of an ICA injury[12,16,17].
Since the patients have been endotracheally intubated, it is relatively easy to manage the airway and control breathing to maintain oxygenation during transfer, and if conditions permit, transport ventilators can be used, which may be safer. Continuous intravenous infusion of propofol and remifentanil in combination with a muscle relaxant (rocuronium or cisatracurium) could be used to maintain a certain depth of anesthesia to prevent coughing and any body movement during transfer to avoid exacerbation of the ICA injury and deterioration of the current situation. However, patients with ICA injury usually experience hypovolemic shock. Our primary goal was to prevent circulation collapse. Patients with massive bleeding and profound hemodynamic instability could be treated with resuscitation measures, including limited transfusion of crystalloids, whole blood, or balanced blood components and vasoactive agents as needed. In this paper, 3 of 4 patients lost more than 2000 mL of blood. All patients received an infusion of blood products and vasoactive drugs, such as dopamine, epinephrine, and norepinephrine, or a combination of both to maintain hemodynamic stability.
Subsequent arteriography was performed under general anesthesia. Both intravenous and volatile agents can be used to maintain anesthesia, although short-acting agents are preferred. The tracheal tube was successfully removed in 1 patient, and removal was delayed in 3 patients. One patient died of brainstem failure, multiple cerebral infarcts, and respiratory and circulatory failure (Figure 1) despite active postoperative hemostasis, vascular preservation, and intracranial pressure reduction. One patient experienced transient neurological impairment but had recovered by the time of discharge, whereas the other 2 patients exhibited no neurological sequelae.
Anesthesia management of ICA injury during skull base surgery requires optimal surgical conditions while maintaining hemodynamic stability to ensure vital organ perfusion and airway safety during and after transfer. Preoperative risk assessment and intraoperative multidisciplinary collaboration are the cornerstones of perioperative safety.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Al-Ani RM, Iraq; Oley MH, Indonesia S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Van Der Veken J, Simons M, Mulcahy MJ, Wurster C, Harding M, Van Velthoven V. The surgical management of intraoperative intracranial internal carotid artery injury in open skull base surgery-a systematic review. Neurosurg Rev. 2022;45:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Inamasu J, Guiot BH. Iatrogenic carotid artery injury in neurosurgery. Neurosurg Rev. 2005;28:239-47; discussion 248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Hosemann W, Schroeder HW. Comprehensive review on rhino-neurosurgery. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2015;14:Doc01. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | AlQahtani A, London NR Jr, Castelnuovo P, Locatelli D, Stamm A, Cohen-Gadol AA, Elbosraty H, Casiano R, Morcos J, Pasquini E, Frank G, Mazzatenta D, Barkhoudarian G, Griffiths C, Kelly D, Georgalas C, Janakiram N, Nicolai P, Prevedello DM, Carrau RL. Assessment of Factors Associated With Internal Carotid Injury in Expanded Endoscopic Endonasal Skull Base Surgery. JAMA Otolaryngol Head Neck Surg. 2020;146:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Valentine R, Padhye V, Wormald PJ. Management of arterial injury during endoscopic sinus and skull base surgery. Curr Opin Otolaryngol Head Neck Surg. 2016;24:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Hamour AF, Laliberte F, Padhye V, Monteiro E, Agid R, Lee JM, Witterick IJ, Vescan AD. Development of a management protocol for internal carotid artery injury during endoscopic surgery: a modified Delphi method and single-center multidisciplinary working group. J Otolaryngol Head Neck Surg. 2022;51:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Sylvester PT, Moran CJ, Derdeyn CP, Cross DT, Dacey RG, Zipfel GJ, Kim AH, Uppaluri R, Haughey BH, Tempelhoff R, Rich KM, Schneider J, Chole RA, Chicoine MR. Endovascular management of internal carotid artery injuries secondary to endonasal surgery: case series and review of the literature. J Neurosurg. 2016;125:1256-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Valentine R, Wormald PJ. Carotid artery injury after endonasal surgery. Otolaryngol Clin North Am. 2011;44:1059-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Bafaquh M, Khairy S, Alyamany M, Alobaid A, Alzhrani G, Alkhaibary A, Aldhafeeri WF, Alaman AA, Aljohani HN, Elahi BN, Alghabban FA, Orz Y, Alturki AY. Classification of internal carotid artery injuries during endoscopic endonasal approaches to the skull base. Surg Neurol Int. 2020;11:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chin OY, Ghosh R, Fang CH, Baredes S, Liu JK, Eloy JA. Internal carotid artery injury in endoscopic endonasal surgery: A systematic review. Laryngoscope. 2016;126:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Gardner PA, Tormenti MJ, Pant H, Fernandez-Miranda JC, Snyderman CH, Horowitz MB. Carotid artery injury during endoscopic endonasal skull base surgery: incidence and outcomes. Neurosurgery. 2013;73:ons261-9; discussion ons269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Gardner PA, Snyderman CH, Fernandez-Miranda JC, Jankowitz BT. Management of Major Vascular Injury During Endoscopic Endonasal Skull Base Surgery. Otolaryngol Clin North Am. 2016;49:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Xiao L, Xie S, Tang B, Wu X, Ding H, Bao Y, Hong T. A novel technique to manage internal carotid artery injury in endoscopic endonasal skull base surgery in the premise of proximal and distal controls. Neurosurg Rev. 2021;44:3437-3445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Morrison T, Jukes A, Wong J. Rupture from cavernous internal carotid artery pseudoaneurysm 11 years after transsphenoidal surgery. J Clin Neurosci. 2020;79:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Zada G, Cavallo LM, Esposito F, Fernandez-Jimenez JC, Tasiou A, De Angelis M, Cafiero T, Cappabianca P, Laws ER. Transsphenoidal surgery in patients with acromegaly: operative strategies for overcoming technically challenging anatomical variations. Neurosurg Focus. 2010;29:E8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Kassir ZM, Gardner PA, Wang EW, Zenonos GA, Snyderman CH. Identifying Best Practices for Managing Internal Carotid Artery Injury During Endoscopic Endonasal Surgery by Consensus of Expert Opinion. Am J Rhinol Allergy. 2021;35:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | AlQahtani A, Castelnuovo P, Nicolai P, Prevedello DM, Locatelli D, Carrau RL. Injury of the Internal Carotid Artery During Endoscopic Skull Base Surgery: Prevention and Management Protocol. Otolaryngol Clin North Am. 2016;49:237-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |