Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9828
Peer-review started: April 20, 2022
First decision: June 19, 2022
Revised: June 30, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: September 26, 2022
Processing time: 148 Days and 22 Hours
Esophageal carcinosarcoma (ECS) is a rare biphasic tumor and a type of eso
A 63-year-old man was admitted to the hospital with dysphagia. During the endoscopic examination, an elevated lesion was found with an erosive and hyperemic surface covered with white pseudomembranous inflammation. Endoscopic ultrasonography (EUS), biopsies, and enhanced thoracic computed tomography were performed, suggesting that it was a benign lesion and located within the submucosal layer. This lesion was diagnosed as a fibrovascular polyp with a Paris classification of 0-Ip. The patient was then referred to ESD treatment. However, the post-ESD pathological and immunohistochemical study showed that this lesion was ECS with a vertical positive margin (T1b stage), indicating that we made a misdiagnosis and achieved a noncurative resection. Due to the potential tumor residue, additional open surgery was performed at the patient's request. In the postoperative pathological study, no tumor remnants or metastases were discovered. The patient was followed for 1 year and had no recurrence.
ECS can be misdiagnosed at the initial endoscopy. EUS can help to identify the tumor stage. Patients with T1b stage ECS cannot be routinely referred to ESD treatment due to the high risk of metastasis and recurrence rate.
Core Tip: Esophageal carcinosarcoma (ECS) is a rare type of esophageal malignancy. ECS commonly presents as a pedunculated characteristic (0-Ip), which is often misdiagnosed due to the lack of specific features. Endoscopic ultrasonography can help to evaluate whether ECS invasion is within the submucosal layer (T1 or T2 stage) but cannot further distinguish whether it is T1a or T1b stage. Due to the high risk of metastasis and recurrence based on the literature review, endoscopic submucosal dissection treatment cannot be routinely recommended for ECS patients with T1b stage disease.
- Citation: Ma XB, Ma HY, Jia XF, Wen FF, Liu CX. Misdiagnosis of an elevated lesion in the esophagus: A case report. World J Clin Cases 2022; 10(27): 9828-9833
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9828.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9828
Patients routinely undergo endoscopic evaluation for dysphagia, during which protruding or elevated lesions are frequently found. Some of the lesions are presented as pedunculated lesions, including esophageal adenoma, inflammatory polyps, fibrovascular polyps, carcinosarcoma[1-3], etc. However, they lack specific features and have similar endoscopic ultrasonography (EUS) characteristics. Therefore, it is difficult to conclusively diagnose the lesion without the support of postsurgical pa
Herein, we report a rare case of esophageal carcinosarcoma (ECS), which was assessed as a benign tumor and treated by endoscopic submucosal dissection (ESD). Nevertheless, post-ESD pathology indicated that it was preoperatively misdiagnosed. Therefore, we systematically evaluated the endoscopic and clinicopathological characteristics of ECS and analyzed the feasibility of endoscopic treatment.
Dysphagia for 3 mo.
A 63-year-old man was admitted to the hospital with dysphagia for 3 mo. The patient can only swallow semi-solid food for 2 wk, with intermittent swallowing pain.
The patient was in good health in the past.
The patient had no personal and family history.
The patient was in good condition. The physical examination was completely normal.
Routine laboratory tests were all within the normal range.
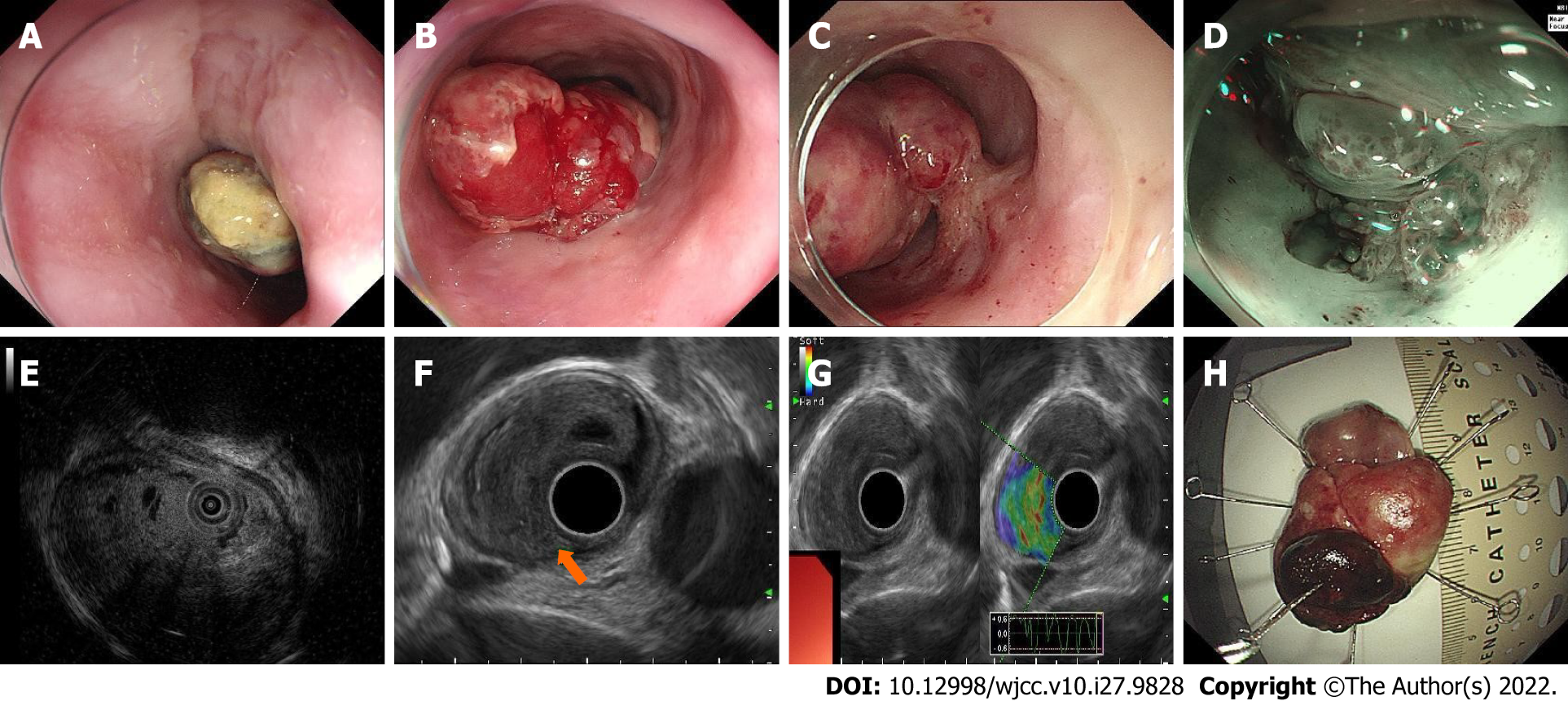
During the endoscopic examination, an elevated lesion with an erosive and hyperemic surface covered with white pseudomembranous inflammation was found. It had a short peduncle connected to the mid-esophagus wall and was 30 mm × 40 mm in size. EUS revealed a lesion derived from the submucosal layer with an intact inherent muscle layer, and this lesion was hypoechoic and consisted of internal multicystic components. Ultrasonic elastography revealed that the lesion was blue–green, indicating a tough texture (Figure 1). Multiple biopsies showed necrosis and active fibroblast proliferation. An enhanced thoracic computed tomography scan showed a protuberant lesion in the middle of the esophagus, suggesting a benign tumor. A multidisciplinary consultation was performed, and we preliminarily diagnosed this lesion as a fibrovascular polyp.
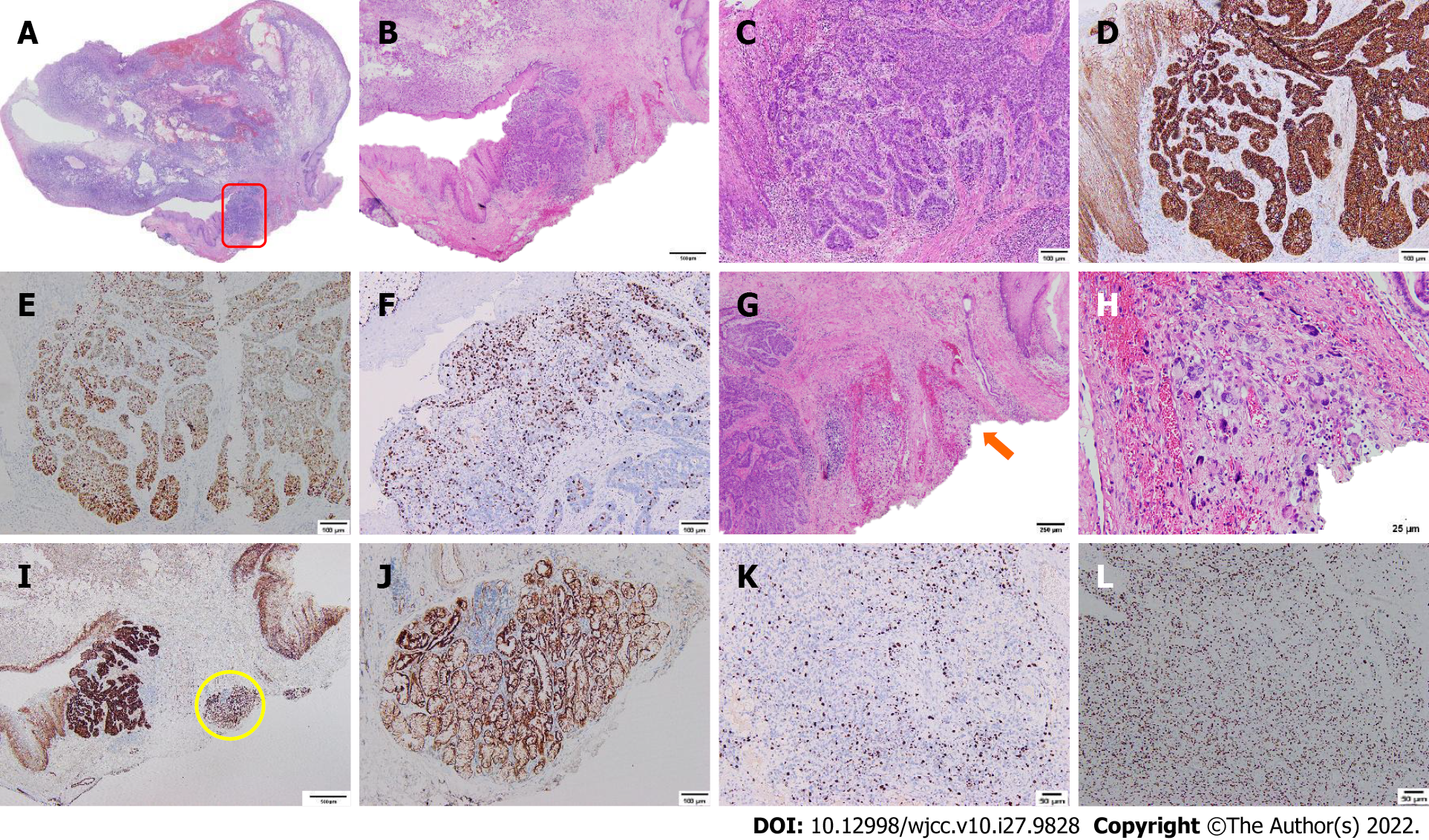
The post-ESD pathological study showed that this lesion was composed of a malignant fibroblast apoptosis component and a basal-like squamous cell carcinoma (BSC) component, indicating ECS. The polypoid mass was predominantly composed of malignant fibrous histiocytoma with a vertical positive margin, horizontal negative margin, and no evidence of vascular or lymphatic invasion. Immunohistochemical (IHC) staining showed CK (-), vimentin (+), CD68 (+), β-catenin (+), p53 (+), S-100 (-), CD34 (-), SMA (-), desmin (-), Twist1 (+), ZEB1 (+), Snai2 (-), PDGFR alpha (-), and a Ki-67 index of 20%. BSC was observed in the neck of the tumor, and its vertical and horizontal margins were negative. IHC staining showed CK (+), E-cadherin (+), p53 (+), vimentin (-), S-100 (-), CD34 (-), CD68 (-), SMA (-), desmin (-), and a Ki-67 index of 30% (Figure 2).
The following ESD treatment was successful with no obvious adhesion in the submucosal layer after the patient’s informed consent was obtained.
Due to the potential tumor residue, additional open surgery was performed at the patient's request. No tumor remnants or metastases were discovered in the postoperative pathological study. The patient was followed for 1 year and had no recurrence.
ECS is a rare biphasic tumor that accounts for 0.2%-2.8% of all esophageal malignancies. It is characterized by the presence of both malignant epithelial and mesenchymal components[4]. ECS usually presents as a large intraluminal polypoid mass on the upper and middle esophagus, with a median diameter of 55-75 mm. The endoscopic features of this lesion may include a hyperemia surface, erosion, ulceration, brittleness, and easy bleeding, which lack specificity for endoscopic diagnosis[5].
The diagnosis of ECS mainly relies on pathological studies[4]. However, untargeted endoscopic biopsies of this lesion usually reveal components of sarcoma, which makes it easily misdiagnosed. Efforts can be made to potentially improve the biopsy accuracy by targeting the root or peduncle as the epithelial cancer component always exceeds the mass in the range[6].
EUS evaluation of the lesion plays a role in the assessment before treatment. Although lacing specificity in diagnosis, EUS can provide information on invasion depth. According to a report from Taiwan[7], five of six ECS patients were correctly assessed on the invasion depth by EUS. However, all lesions were in the deep invasion (T2 stage). Our preoperative EUS showed that the origin of the ECS was derived from the T1 stage, which was proven by postoperative pathology. However, we also noticed that EUS could not further distinguish whether it was T1a or T1b stage. The reason could be that the echo of sarcoma that invaded the submucosa was similar to the original interstitial composition and therefore could not be distinguished by EUS.
Data on lymph node metastases of ECS at T1 stage are limited[7,8]. In 2006, Sanada et al[9] reviewed 57 cases of ECS reported in Japan between 1995 and 2004, among which one was a T1a stage case and 17 were T1b stage cases[9]. Seven (41%) of the T1b stage cases were found to have lymph node metastasis compared with none of the T1a stage cases. In 2021, Chen et al[10] reported that none of the ten ECS patients at T1 stage were found to have lymph node metastasis, with no report of T1 subtypes[10]. Since lymph node metastasis is related to prognosis, a detailed assessment is required before treatment.
Data on the prognosis of ESD treatment for ECS in the T1 stage are also limited. One Korean case reported by Cha et al[11] in 2014 is very similar to ours[11]. The lesion located within the submucosal layer without evidence of metastasis was treated by ESD. The post-ESD pathological study reported ECS with a vertical positive margin (T1b stage). In contrast, the patient from this Korean study refused to receive additional surgery, and a recurrence was found during an endoscope examination 21 mo later. Two Chinese cases were also treated by ESD, and one case was followed by additional surgery. Unfortunately, neither of them had long-term follow-ups[12,13]. Therefore, robust data on the prognosis of ESD for ECS are needed.
We report a rare case of ECS with BSC, which can be misdiagnosed due to the lack of specific characteristics. Targeted biopsies on the root or peduncle after observation by narrow-band imaging or iodine staining may potentially improve the diagnostic accuracy. EUS can help to evaluate the layer of the origin (T1 or T2 stage) but cannot further distinguish whether it is at the T1a or T1b stage. ESD treatment should not be routinely recommended to ECS patients with T1b stage disease due to the risk of metastasis and high recurrence rate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mohamed SY, Egypt; Nakamura K, Japan; Suresh Kumar VC, United States; Villa E, United States S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Tsai SJ, Lin CC, Chang CW, Hung CY, Shieh TY, Wang HY, Shih SC, Chen MJ. Benign esophageal lesions: endoscopic and pathologic features. World J Gastroenterol. 2015;21:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 2. | Tomita H, Miyakawa K, Wada S, Okamoto S, Morimoto T, Kishimoto K, Nakajima Y. The imaging features of protruding esophageal lesions. Jpn J Radiol. 2016;34:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ha C, Regan J, Cetindag IB, Ali A, Mellinger JD. Benign esophageal tumors. Surg Clin North Am. 2015;95:491-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Madan AK, Long AE, Weldon CB, Jaffe BM. Esophageal carcinosarcoma. J Gastrointest Surg. 2001;5:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Cavallin F, Scarpa M, Alfieri R, Cagol M, Ruol A, Rugge M, Ancona E, Castoro C. Esophageal carcinosarcoma: management and prognosis at a single Italian series. Anticancer Res. 2014;34:7455-7459. [PubMed] |
| 6. | Ishida H, Fujishima F, Onodera Y, Konno-Kumagai T, Maruyama S, Okamoto H, Sato C, Heishi T, Sakurai T, Taniyama Y, Kamei T, Sasano H. Esophageal Carcinosarcoma with Basaloid Squamous Cell Carcinoma: A Case Report and Review of the Literature. Tohoku J Exp Med. 2019;249:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kuo CJ, Lin TN, Lin CJ, Wu RC, Chang HK, Chu YY, Lien JM, Su MY, Chiu CT. Clinical manifestation of esophageal carcinosarcoma: a Taiwan experience. Dis Esophagus. 2010;23:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Schizas D, Mastoraki A, Bagias G, Ioannidi M, Kanavidis P, Moris D, Tsilimigras DI, Spartalis E, Arkadopoulos N, Liakakos T. Carcinosarcomas of the esophagus: systematic review of a rare nosologic entity. J BUON. 2018;23:1432-1438. [PubMed] |
| 9. | Sanada Y, Hihara J, Yoshida K, Yamaguchi Y. Esophageal carcinosarcoma with intramural metastasis. Dis Esophagus. 2006;19:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Chen S, Shi Y, Lu Z, Wang M, Cong L, Yang B, Chen X, Cai J, Yang X. Esophageal Carcinosarcoma: Analysis of Clinical Features and Prognosis of 24 Cases and a Literature Review. Cancer Control. 2021;28:10732748211004886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Cha RR, Jung WT, Oh HW, Kim HJ, Ha CY, Kim TH, Ko GH. A case of metachronous development of esophageal squamous cell carcinoma in the patient with esophageal carcinosarcoma. Korean J Gastroenterol. 2014;64:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Jiang ZD, Zhang YS, Wang ZB, Gao B, Wang L, Zhang ZY, Yang XB. Endoscopic submucosal dissection for esophageal sarcomatoid carcinoma: a case report and literature review. Weichangbingxue. 2016;21:767-768. [DOI] [Full Text] |
| 13. | Zhu Z, Zhu HH, Liu D, Yin J, Wang L, Chen L. Endoscopic submucosal dissection removed one case of esophageal sarcoma-like carcinoma. Weichangbingxue. 2017;23:109-110. [DOI] [Full Text] |