Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9821
Peer-review started: April 22, 2022
First decision: June 8, 2022
Revised: June 17, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 146 Days and 23.8 Hours
Corrected transposition of the great arteries (cTGA) is a cardiac malformation in which the ventricular and arterial-ventricular positions in the heart are doubly reversed. In general, this defect puts a load on the systemic circulation and causes heart failure, resulting in a poor prognosis. This article reports a case of cTGA detected in a patient with post-caesarean pregnancy who had undergone elective caesarean section and was experiencing an episode of acute heart failure.
This was the case of a 36-year-old gravida 3 para 1 woman. No problems were noted in the puerperal course following the previous pregnancy. The current pregnancy was also uneventful. An elective caesarean section was performed and the patient was discharged from the hospital 7 d after the operation. On postoperative day 18, the patient became aware of breathing difficulty and presented at a nearby clinic, where she was referred to our institution after bilateral pleural effusions were detected. She was then diagnosed with acute heart failure after noting the presence of a prominent pedal oedema and SpO2 91% (supine position and room air); the patient was promptly hospitalised for close examination and treatment. Although chest computed tomography revealed the presence of cTGA, no other cardiac malformations were observed. Owing to improvements in both the pedal oedema and pleural effusions, the patient was discharged on day 9.
Close examination should be performed on the premise of congenital cardiac malformation when heart failure symptoms are noted during perinatal control.
Core Tip: Corrected transposition of the great arteries (cTGA) is a rare disorder that accounts for only less than 1% of all congenital heart diseases. Cases with no associated cardiac anomalies are even rarer, accounting for only 5% of all cTGA cases. We reported a case of cTGA that was detected on the occasion of acute heart failure following elective caesarean section carried out on a patient with post-caesarean pregnancy. Close examination should be performed on the premise of congenital cardiac malformation when symptoms of heart failure are noted during perinatal control.
- Citation: Ichii N, Kakinuma T, Fujikawa A, Takeda M, Ohta T, Kagimoto M, Kaneko A, Izumi R, Kakinuma K, Saito K, Maeyama A, Yanagida K, Takeshima N, Ohwada M. Diagnosed corrected transposition of great arteries after cesarean section: A case report. World J Clin Cases 2022; 10(27): 9821-9827
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9821.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9821
Corrected transposition of the great arteries (cTGA) is a rare disorder that accounts for only less than 1% of all congenital heart diseases[1]. Hemodynamically, systemic venous blood is ejected into the pulmonary artery via the right atrium and then the anatomical left ventricle, whereas pulmonary venous blood is ejected into the aorta via the left atrium and then the anatomical right ventricle. Cases with no associated cardiac anomalies are even rarer, only accounting for 5% of all cTGA cases. Without associated cardiac anomalies, the blood circulation is functionally corrected so that venous blood flows into the lungs and arterial blood into the rest of the body. However, the fact that the anatomical right ventricle acts as the functional left ventricle and the original mitral valve is replaced by the tricuspid valve has been reported to cause long-term functional disturbance[2]. In many cases, patients develop complications, such as ventricular septal defect, pulmonary artery stenosis/occlusion, and tricuspid valve abnormality[3].
Here, we report a case of cTGA that was detected on the occasion of acute heart failure following elective caesarean section carried out on a patient with post-caesarean pregnancy.
The patient was a 36-year-old woman on her third pregnancy. Preoperative tests (blood collection, chest X-ray and electrocardiography) performed at 36 wk of gestation detected no remarkable findings. On day 4 of week 37 of gestation, elective caesarean section was performed with an indication of post-caesarean pregnancy. The operation lasted 34 min, and the estimated blood loss during the operation was 1010 mL, including amniotic fluid. With no problems occurring during and after surgery, the patient was discharged 6 d after the operation. On day 18 postoperatively, the patient underwent emergency examination at our institution for breathing difficulty.
The patient became pregnant through natural conception and presented at the outpatient department of our institution at 7 wk of gestation. The subsequent progress in pregnancy was favorable.
The patient’s first pregnancy at 28 years of age ended in an abortion and her second pregnancy at 30 years of age ended with an elective caesarean section indicated for foetal malpresentation.
She had no other remarkable personal or family history. No problems were noted in the previous pregnancy and puerperal course.
On admission, she was 154.5 cm tall, weighed 50.0 kg (non-pregnant weight: 42.0 kg), had a body surface area of 1.46 m2, was fully conscious, had a blood pressure of 123/78 mmHg, a pulse rate of 99/min, and SpO2 of 91% (supine position, room air). On chest auscultation, systolic regurgitant murmurs at Levine IV were recorded at regular heartbeats that maximised at the cardiac apex. Bilateral pedal oedema was observed.
The brain natriuretic peptide (BNP) level was 642.5 pg/mL (normal range: ≤ 18.4). There were no other remarkable blood test results.
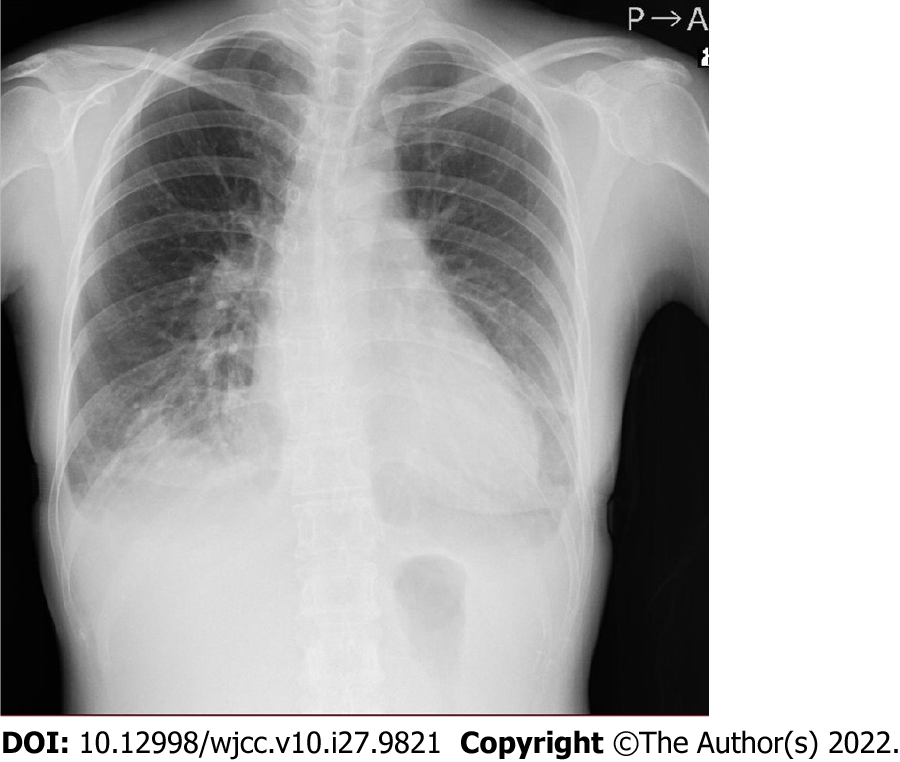
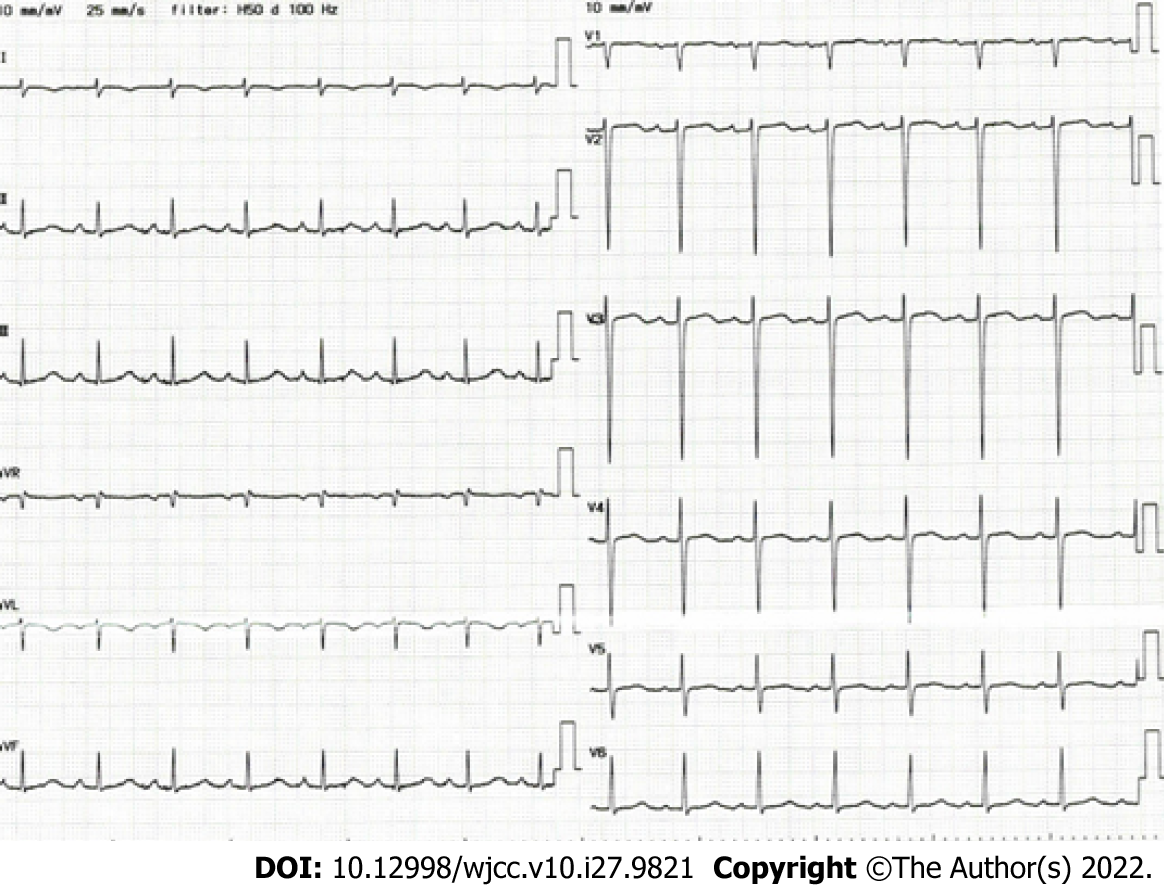
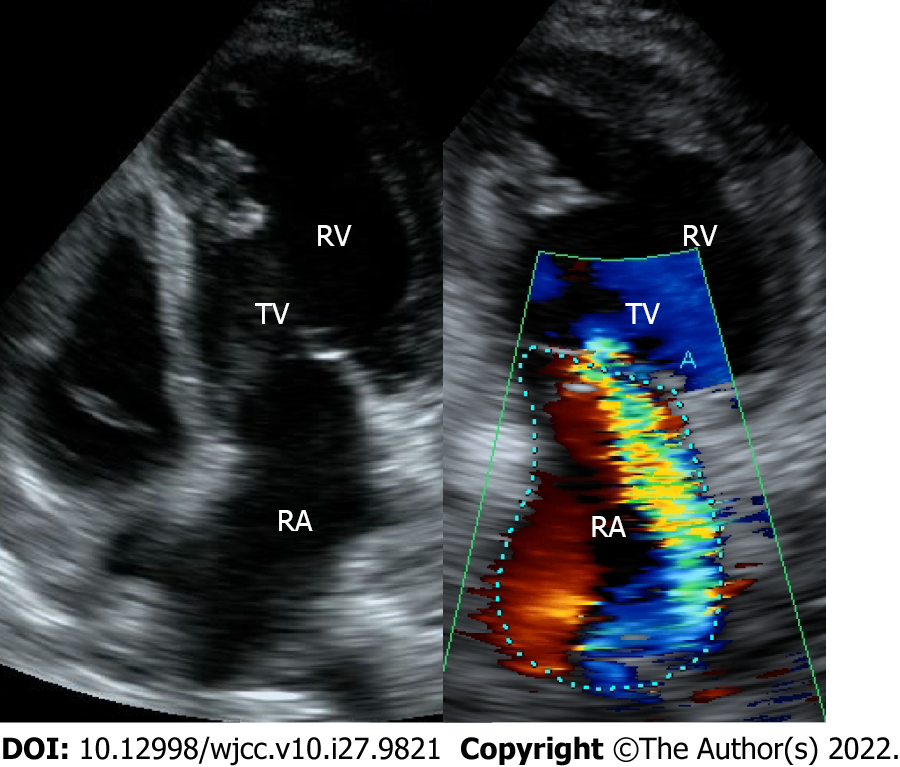
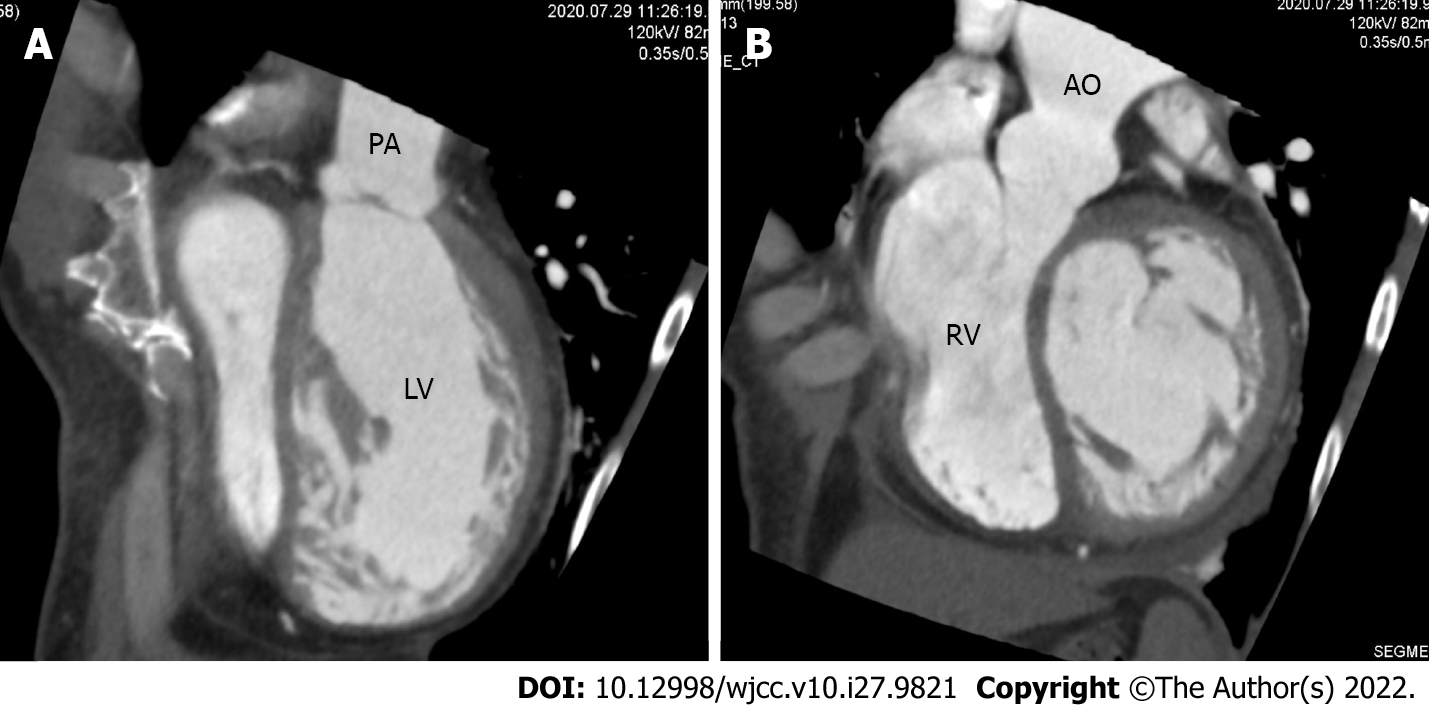
Chest X-ray revealed a cardiothoracic ratio of 55% and a bilateral pleural effusion (Figure 1). Electrocardiography revealed a pulse rate of 94/min, a sinus rhythm, no ST-segment abnormalities, and a slight right axis deviation (Figure 2). The patient’s ejection fraction (EF) was 44.6% (Simpson’s method) in the systemic circulation (right) ventricle, showing a diffuse decrease in wall motion. The systemic circulation ventricular diameter was 49.8 mm in the diastolic phase and 41.6 mm in the systolic phase, indicating a slight enlargement. Severe atrioventricular valve regurgitation was observed in the systemic circulation atrioventricular valve (tricuspid valve) (Figure 3). On contrast-enhanced chest computed tomography (CT), the aorta was found to have the shape of a rough adductor muscle starting from the anatomical right ventricle, and the pulmonary artery started from the anatomical left ventricle (Figure 4).
Ultrasonography and chest contrast CT examination revealed a cTGA.
The patient received furosemide and angiotensin converting enzyme inhibitors, which helped relieve her breathing difficulty and bilateral pedal oedema. By the fourth day of hospitalisation, these signs and symptoms were completely resolved. With the plain chest X-ray revealing a significant decrease in the amount of pleural effusion, the patient was discharged on the ninth day of hospitalisation.
As of 6 mo after delivery, the patient’s cardiac function improved with the echocardiography revealing an EF of 60.5% (Simpson’s method) in the systemic circulation (right) ventricle. In spite of slight regurgitation in the systemic circulation atrioventricular valve (tricuspid valve), the patient’s BNP level decreased to 9.7 pg/mL (normal range: ≤ 18.4). The patient is currently continuing with medical management.
cTGA is a rare disorder that accounts for less than 1% of all congenital heart diseases[2]. More than 80% of cTGA cases exhibit various clinical features, with associated cardiac anomalies such as atrial septal defect, ventricular septal defect, and pulmonary arterial stenosis[3]. Regarding the life prognosis of cTGA, Connelly et al[4] have reported a high mortality rate of 25% and that patients with this condition die at a relatively young age (38.5 years on average), which means that their mean life expectancy falls short of that of healthy people (mean survival age: Approximately 50 years; long-term survival age: Approximately 61 years)[4]. Likewise, Rutledge has reported a mortality rate of 16.5% among cTGA patients and a mean survival age of approximately 40 years. Either way, a generally poor life prognosis was indicated[5]. Graham et al[3] have also reported that the prevalence of right heart failure increases over years in cTGA patients, with 56% of cases with intracardiac lesions and 32% of cases without intracardiac lesions developing heart failure by the age of 45 years[3]. Symptoms and prognoses are contingent on associated cardiac malformations and functions of the anatomic right ventricle[6]. There are quite a few cases that are neither diagnosed nor receive adequate medical intervention until the patients become adults.
Meanwhile, the amount of circulating blood plasma greatly increases from the early to the middle stages of pregnancy, reaching 1.5-fold the non-pregnant volume on average. Moreover, the oxygen consumption nearly triples during delivery, with the amounts of circulating blood and cardiac output also increasing due to uterine contractions that are accompanied by labour pains. Immediately after delivery, the uterus contracts, and simultaneously, the pressure on the inferior vena cava applied by the late-pregnancy uterus is taken away, resulting in a rapid increase in the venous return flow[7]. As described above, the circulatory dynamics of the mother’s body changes significantly during pregnancy, delivery and the puerperium. Even in a normal heart, the force of cardiac contraction reportedly decreases temporarily from late pregnancy to the puerperal period[8].
Furthermore, Hilfiker-Kleiner et al[9] noted the high occurrence rates of cardiomyopathy and heart failure on the occasion of pregnancy and delivery among female mice in which the STAT3 protein was knocked out specifically in the cardiac muscle and reported research results on the etiological mechanism of peripartum cardiomyopathy[9]. It has been revealed that the increased expression of cathepsin D (a protease in the cardiac muscle) leads to the cleavage of 23 kDa prolactin in the blood, resulting in an increase in 16 kDa prolactin. This suggests that an increase in the blood concentration of cleaved prolactin during the perinatal period suppresses angiogenesis around the cardiac muscle. This, in turn, applies oxidative stress on myocardial cells due to a low oxygen load, eventually causing heart failure.
Therefore, the patient of the present case progressed without any particular problems following the previous delivery because she had no associated intracardiac anomalies. However, in this pregnancy, pregnancy-derived factors over the years, such as poor response to circulatory loads coupled with endocrine abnormalities, may have induced tricuspid insufficiency leading to heart failure.
Recent advances in medical technology have contributed to increasing diagnoses of adult congenital heart diseases (ACHDs), posing a major inevitable challenge of controlling and managing pregnancy and delivery among female patients with ACHD. When patients with cTGA complications become pregnant, pregnancy risks vary depending on the degree of complications, including the severity of tricuspid regurgitation, right ventricular functions, VSD, and atrioventricular block. According to a report of 60 pregnancies in 22 cTGA patients, 83% ended in delivery, of which there was one case of premature birth (before 30 complete weeks of gestation), and no new-borns had congenital heart diseases. Except for one case of heart failure due to atrioventricular valve regurgitation on the systemic circulation side, only multiparous patients (with a history of 12 previous pregnancies) developed heart failure and endocardial inflammation, and there was no case of death[10]. However, the exact mechanism by which pregnancy and delivery affect the long-term prognosis remains unknown. Given that the right side of the heart is known to be more susceptible to circulatory loads during pregnancy and delivery, special care is needed in controlling and managing pregnancy and delivery in cases with significant atrioventricular valve regurgitation and decline in systemic right ventricular functions. As obstetric and gynaecological complications in ACHD patients, high-risk ones include premature birth, post-delivery haemorrhage, and pregnancy-related hypertension. As complications in foetuses and new-borns, premature birth and intra-uterine maldevelopment are feared[11]. However, given the diversity of ACHDs, no concrete guidelines have been established on the pregnancy and delivery of ACHD patients. Accordingly, medical practitioners are forced to deal with pregnancy and delivery control and management on a case-by-case basis. Nevertheless, facilitating accurate diagnoses will help implement more effective therapeutic interventions. Therefore, it is essential to perform close examination on the premise of congenital cardiac malformation when cardiac dysfunction and heart failure symptoms are observed during a perinatal stage, in which hormonal and circulatory dynamics change prominently. For the control and management of pregnancy and delivery in ACHD patients, a comprehensible framework needs to be formulated, involving not only obstetricians and gynaecologists but also specialists in the fields of neonatal care, circulatory medicine, and cardiac surgery.
In this article, we reported a case of cTGA that was detected on the occasion of acute heart failure following an elective caesarean section carried out on a patient with post-caesarean pregnancy. During the perinatal stage associated with significant hormonal and circulatory dynamics changes, performing a close physical examination on the premise of congenital cardiac malformation is essential.
Performing a close physical examination on the premise of congenital cardiac malformation is crucial for heart failure symptoms during the perinatal stage associated with significant hormonal and circulatory dynamics changes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naem AA, Syria; Zeng Y, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Sharland G, Tingay R, Jones A, Simpson J. Atrioventricular and ventriculoarterial discordance (congenitally corrected transposition of the great arteries): echocardiographic features, associations, and outcome in 34 fetuses. Heart. 2005;91:1453-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 2. | Beauchesne LM, Warnes CA, Connolly HM, Ammash NM, Tajik AJ, Danielson GK. Outcome of the unoperated adult who presents with congenitally corrected transposition of the great arteries. J Am Coll Cardiol. 2002;40:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 3. | Graham TP Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, Gersony WM, Gessner IH, Hurwitz RA, Kaemmerer H, Kugler JD, Murphy DJ, Noonan JA, Morris C, Perloff JK, Sanders SP, Sutherland JL. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 424] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 4. | Connelly MS, Liu PP, Williams WG, Webb GD, Robertson P, McLaughlin PR. Congenitally corrected transposition of the great arteries in the adult: functional status and complications. J Am Coll Cardiol. 1996;27:1238-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 178] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 5. | Rutledge JM, Nihill MR, Fraser CD, Smith OE, McMahon CJ, Bezold LI. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr Cardiol. 2002;23:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 6. | Lundstrom U, Bull C, Wyse RK, Somerville J. The natural and "unnatural" history of congenitally corrected transposition. Am J Cardiol. 1990;65:1222-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 7. | Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060-H1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 163] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 8. | Geva T, Mauer MB, Striker L, Kirshon B, Pivarnik JM. Effects of physiologic load of pregnancy on left ventricular contractility and remodeling. Am Heart J. 1997;133:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 9. | Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 577] [Article Influence: 32.1] [Reference Citation Analysis (1)] |
| 10. | Connolly HM, Grogan M, Warnes CA. Pregnancy among women with congenitally corrected transposition of great arteries. J Am Coll Cardiol. 1999;33:1692-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 11. | Kamiya CA, Iwamiya T, Neki R, Katsuragi S, Kawasaki K, Miyoshi T, Sasaki Y, Osato K, Murohara T, Ikeda T. Outcome of pregnancy and effects on the right heart in women with repaired tetralogy of fallot. Circ J. 2012;76:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (1)] |