Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9805
Peer-review started: April 12, 2022
First decision: June 10, 2022
Revised: June 16, 2022
Accepted: August 12, 2022
Article in press: August 12, 2022
Published online: September 26, 2022
Processing time: 157 Days and 2.5 Hours
Gastric metastasis from renal cell carcinoma (RCC) is an extremely rare clinical entity. Due to an easily neglected RCC history, nonspecific symptoms and under-recognized endoscopic presentation may lead to a potential diagnostic pitfall in daily clinical practice.
We present a case of metastatic gastric tumors arising from RCC 5 years after radical nephrectomy. Simultaneous, multifocal metastases to the gallbladder, pancreas and soft tissue were observed. One year previously, a solitary submucosal discoid tumor with a central depression was detected in the gastric fundus in a 65-year-old man. Endoscopic ultrasonography (EUS) showed a 1.12 x 0.38 cm lesion originating from the deeper mucosal layers with partially discontinuous submucosa. One year later, the endoscopic findings of the lesion showed various changes. A large lesion of the protruding type (2.5 cm × 2 cm) was found in the fundus at the same location. EUS showed a heterogeneous mass that involved the mucosa and submucosal layer. In addition, two small similar submucosal lesions 0.4-0.6 cm in size were detected. These lesions had a central depression, surface mucosal congestion and thickened vessels. The two adjacent lesions in the fundus were resected by endoscopic submucosal dissection. Based on the postoperative pathological analysis, the patient was diagnosed with gastric metastasis from RCC.
Gastric metastasis from RCC should be considered in patients with a history of RCC irrespective of the time interval involved.
Core Tip: Gastric metastasis from renal cell carcinoma (RCC) is an extremely rare clinical entity that may be missed or misdiagnosed due to lack of knowledge. We present a case of metastatic gastric tumors arising from RCC 5 years after radical nephrectomy. Simultaneous, multifocal metastases to the gallbladder, pancreas and soft tissue were detected. This case highlights the dynamic changes in white light endoscopy and endoscopic ultrasonography, demonstrating the tumors’ growth pattern during the 1-year follow-up. Our diagnostic excision approach using endoscopic submucosal dissection has been rarely reported in this entity.
- Citation: Chen WG, Shan GD, Zhu HT, Chen LH, Xu GQ. Gastric metastasis presenting as submucosa tumors from renal cell carcinoma: A case report. World J Clin Cases 2022; 10(27): 9805-9813
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9805.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9805
Renal cell cancer (RCC) originating from the epithelium of the proximal tubules is the third most frequently observed urological tumor and accounts for 2%-3% of malignant neoplasms in adults[1]. Clear cell RCC is the most common histopathological type of RCC. RCC has an abundant blood supply and an aggressive metastatic nature. Approximately 25%-30% of patients are initially diagnosed with local infiltration or metastasis and 20%-40% develop distant metastases or local recurrence after nephrectomy[2]. Lung, lymph nodes, bone, soft tissue and liver are the main metastatic RCC sites reported[3]. RCC can also metastasize to unusual sites such as the gastrointestinal (GI) tract. Gastric metastatic tumors are detected in only 0.2%-0.7% of cases based on clinical and autopsy findings, and gastric metastasis from RCC is extremely rare[4]. Due to an easily neglected RCC history, nonspecific symptoms and uncommon under-recognized endoscopic presentation may lead to a potential diagnostic pitfall in daily clinical practice.
In this study, we present a case of metastatic gastric tumors arising from RCC 5 years after radical nephrectomy with the absence of GI symptoms in the early stages. Simultaneous, multifocal metastases to the gallbladder, pancreas and soft tissue were detected. This case highlights the dynamic changes in white light endoscopy and endoscopic ultrasonography (EUS), which demonstrated the tumors’ growth pattern, none of which have been described previously. In addition, our diagnostic excision approach using endoscopic submucosal dissection (ESD) has been rarely reported in this entity. We report this rare case to raise awareness of this uncommon type of tumor.
A gastric submucosal tumor was detected in a 65-year-old man 1 year ago.
One year previously, the patient attended a local hospital for a physical check-up, and underwent gastroscopy. He had no evidence of GI bleeding, abdominal pain, nausea and vomiting or weight loss. At gastroscopy, a solitary discoid-shaped submucosal tumor in the gastric fundus was detected. The preliminary diagnosis by EUS was possible ectopic pancreas, and close follow-up was suggested by the doctor in the local hospital. One year later, there was a gradual onset of abdominal discomfort in the subxiphoid, which was more noticeable after eating; therefore, the patient visited our hospital, and underwent EUS follow-up examination. Surprisingly, the endoscopic findings of the lesion showed multiple changes. A large protruding lesion was found in the same location. In addition, two small similar submucosal lesions were detected. EUS showed that the large lesion involved the mucosa and submucosal layer. The EUS diagnosis was not ectopic pancreas. Accordingly, the patient was hospitalized and endoscopic management was suggested.
The patient had undergone nephrectomy for RCC 5 years previously. The targeted agent sunitinib was administered to prevent recurrence for 3 years and treatment was discontinued 2 years ago.
There was no significant family history.
No obvious abnormalities were observed.
Laboratory data showed no abnormalities except mildly elevated serum creatinine (133 mmol/L).
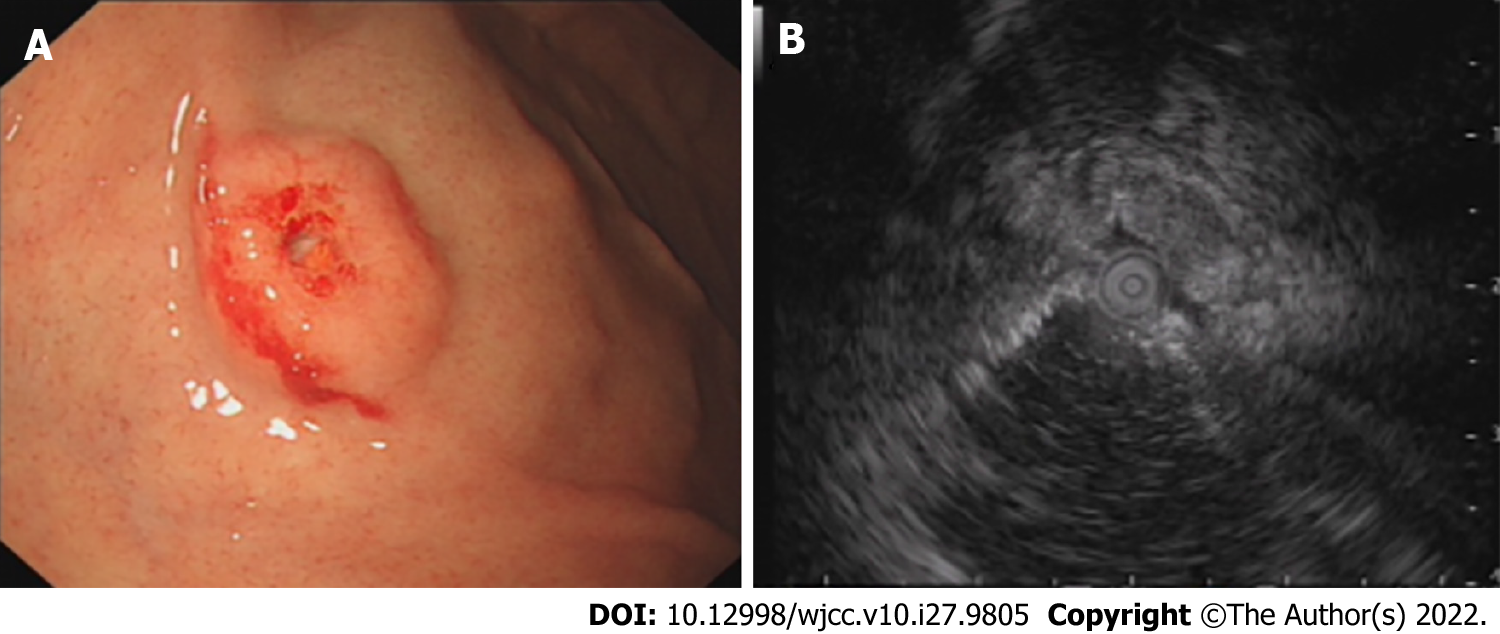
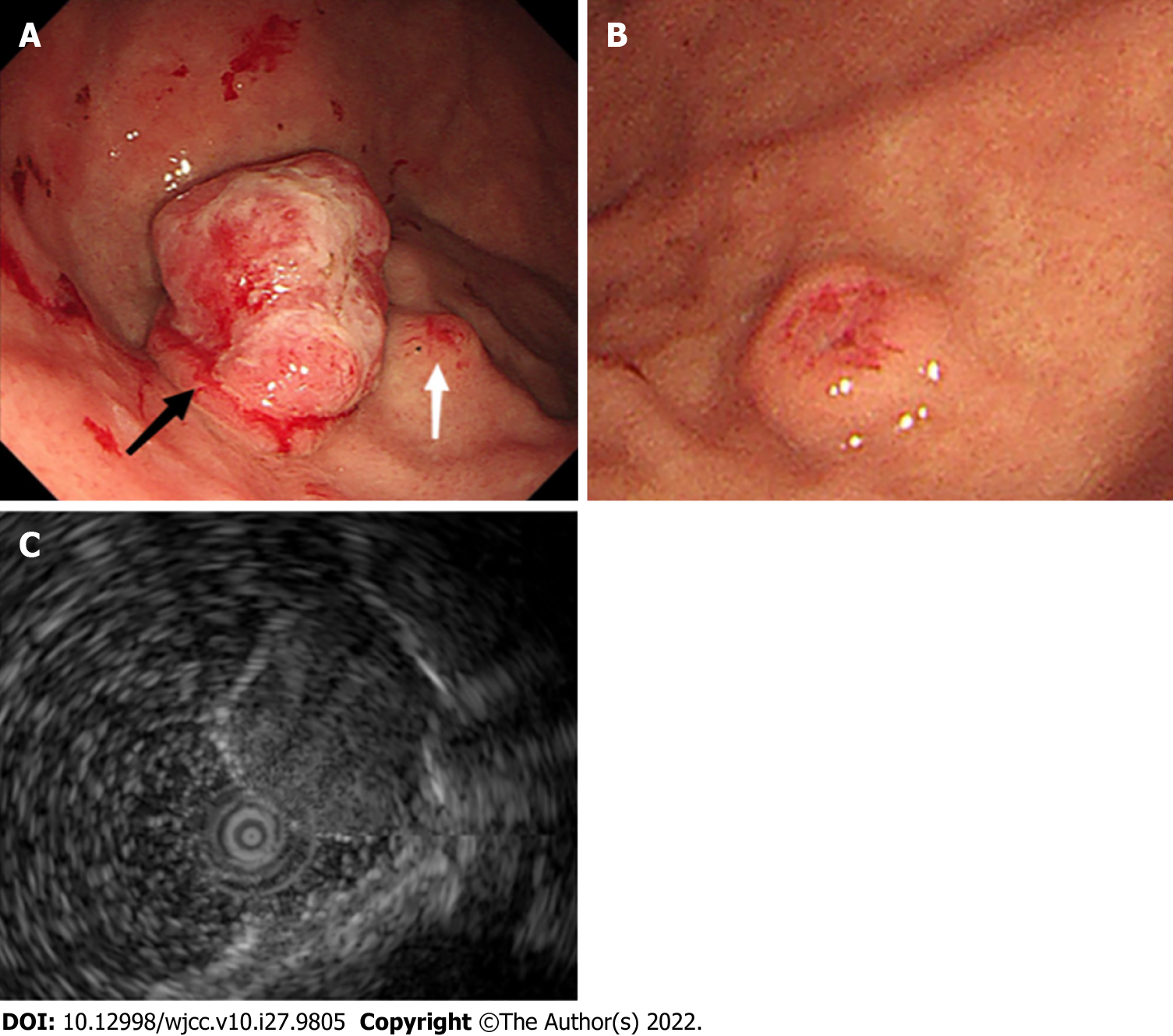
Gastroscopy at the local hospital detected a solitary submucosal tumor in the gastric fundus with central depression and surface mucosal congestion 1 year ago. The lesion was slightly elevated and discoid, and no ulcer or other mass was found in the stomach. EUS showed that the lesion originated from the deeper mucosal layers with partially discontinuous submucosa, with medium and hypoechoic changes and was 1.12 cm × 0.38 cm in size (Figure 1). One year later, a large protruding lesion was found in the fundus in the same location, congestion and erosion were presented on the mucosal surface. The basal layer lesion remained discoid in shape. Two similar small submucosal lesions 0.4-0.6 cm in size were also found; one in the middle section of the stomach body, and the other in the fundus adjacent to the large lesion. Central depression, surface mucosal congestion and thickened vessels were observed in the two small lesions. EUS showed a heterogeneous mass that involved the mucosa and submucosal layer with hypoechoic changes. The lesion had increased in size (2.5 cm × 2 cm) (Figure 2). No significant lymphadenopathy was observed in the periphery.
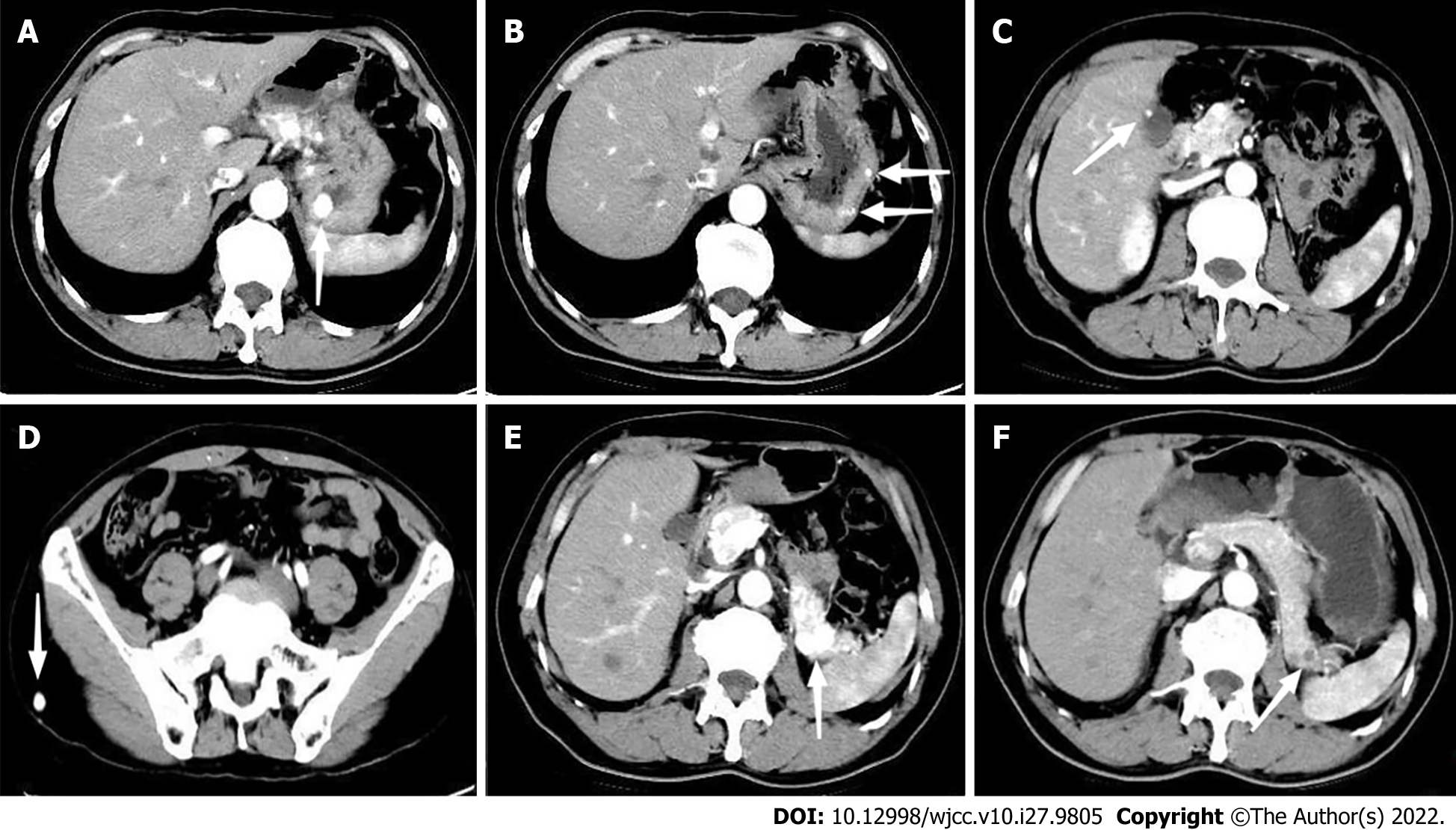
In addition to the 3 lesions observed during gastroscopy, another 2 small lesions were found in the stomach body by Computed Tomography (CT). Apart from multiple lesions in the stomach, CT revealed hypervascular lesions with abnormal enhancement in the arterial and venous phases in the gallbladder, pancreas, and subcutaneous soft tissue of the right buttock (Figure 3). The left kidney was absent, and no mass was detected in the vicinity of the kidney. Thus, multiple metastases were the preliminary diagnosis. Abdominal CT showed no abnormalities 1 year previously at the local hospital. No additional lesions were visible on further evaluation by chest and head CT, Doppler echocardiography, and ultrasound examination of the thyroid gland and adrenal gland.
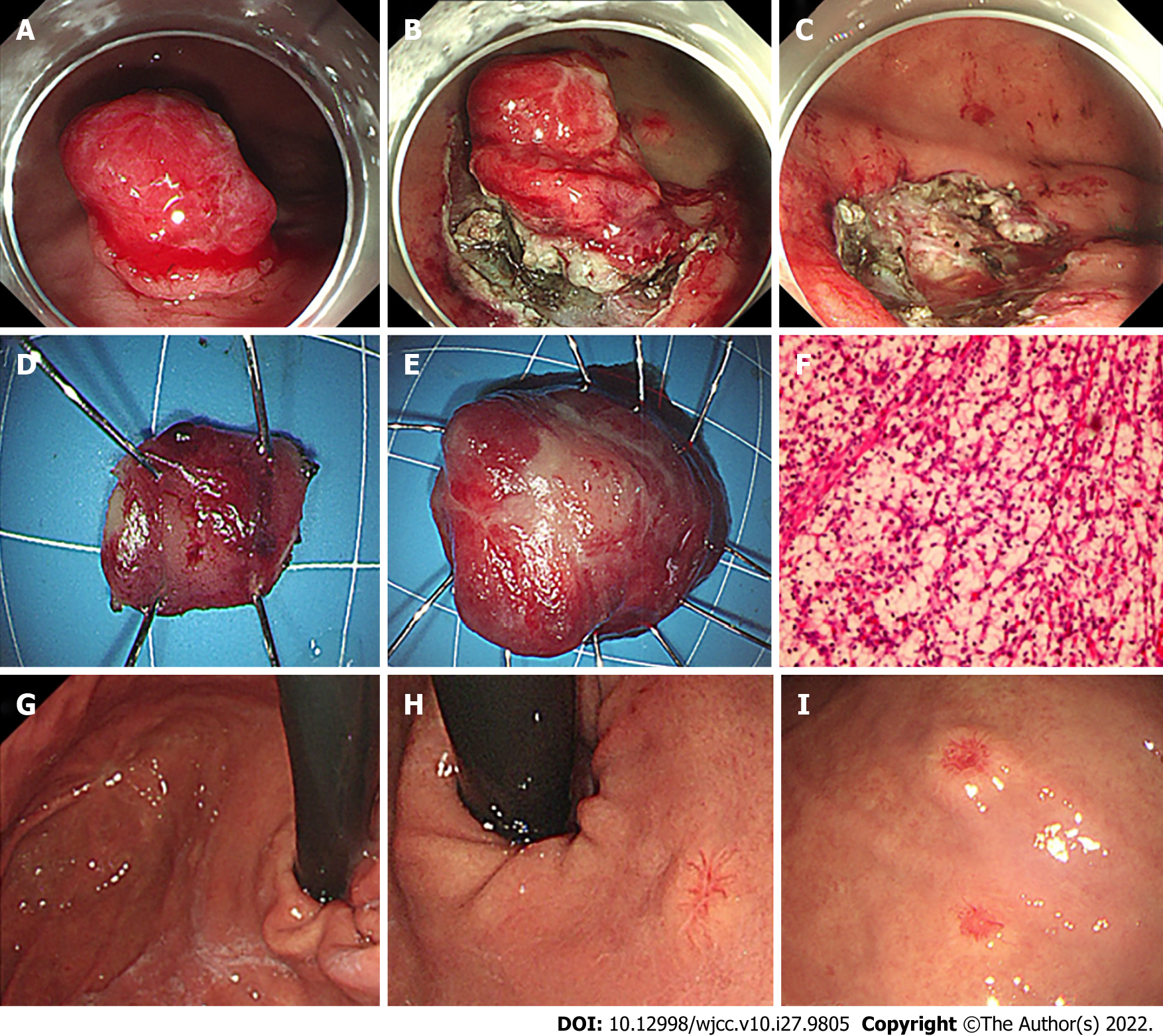
Consequently the rapidly infiltrating growth lesion in fundus associated with the symptom of abdominal discomfort, feasibility of endoscopic resection judgment through EUS, the lesions were resected by ESD for the purposes of symptomatic remission and acquiring sufficient histological proof (Figure 4). Because the large and small lesions in the fundus were adjacent, we removed them together. Based on postoperative pathological and immunohistochemical analyses of the specimens, gastric metastasis from clear cell RCC was diagnosed. From the two gastroscopy examinations at a 1-year interval, we detected three different types of gastric metastasis from RCC and dynamic changes in the lesions. Due to their similar imaging characteristics, the multifocal lesions in the gallbladder, pancreas and soft tissue were also thought to be metastases.
The patient was unable to undergo radical surgery and was considered to be resistant to sunitinib; therefore, another targeted agent, second-line therapy with axitinib, was suggested.
The patient did not develop postoperative morbidity, and he was discharged 5 d after ESD. At 8-mo follow-up, no targeted agent-related side effects were observed. Following targeted therapy and resection of the fundus mass, the patient had complete regression of abdominal discomfort. A follow-up CT scan showed no new lesions. The diameters of the lesions in the pancreas were significantly smaller, enhancement in the arterial phase was significantly reduced, and appeared hypodense (Figure 3). The lesions in the stomach body and gallbladder were slightly smaller, metastasis in the subcutaneous soft tissue of the right buttock showed no change. At 1-year follow-up, gastroscopy showed scars in the fundus with no relapse, the lesion in the middle section of the stomach body demonstrated no obvious change, and 2 new small lesions were found in the middle section of the stomach body and the upper section close to the cardia (Figure 4).
RCC is the most common type of kidney malignancy, accounting for approximately 90% of cases, which is known to develop metachronous metastatic recurrence even several years after nephrectomy. The highest incidence of RCC is found in patients aged 50-70 years, with a male to female ratio of 2:1[5]. Clinically, clear cell RCC is the most common histological type and has a poor prognosis compared to other types. Common metastatic sites include the lungs, lymph nodes, bone, soft tissue, liver, brain and skin. There are an increasing number of case reports of uncommon metastases from RCC, such as the pancreas, thyroid gland, adrenal gland and GI tract[6]. In our hospital, extremely rare sites of metastasis have included the abdominal cavity, parotid gland, small intestine, and pleura. Gastric metastasis from RCC is extremely rare and seems to be a late event, which is an indicator of aggressive behavior. Gastric metastasis is commonly found with metastasis to other areas. The occurrence of gastric metastasis from RCC may take a significantly longer period of time and is easily underestimated; the longest docu
The mechanism responsible for gastric metastasis from RCC is not fully known. Typically, RCC can spread through the lymphatic, hematogenous, transcoelomic or by direct invasion routes[9]. The rich vascular proliferation noted in RCC is thought to be the reason behind increased hematogenous dissemination leading to distant metastasis, and tumor cells may become trapped in vessels in areas of the stomach wall with a rich blood supply, such as the submucosa or subserosa. The clinical presentation of metastatic gastric tumors is usually asymptomatic or nonspecific symptoms. In our case, the patient had no symptoms and gastric metastases were initially detected during a routine physical examination. As the lesion grew, gradual onset of abdominal discomfort in the subxiphoid appeared. GI bleeding and anemia are the most common presentations of symptomatic gastric metastasis when the tumor invades the gastric mucosa. Other presenting symptoms include dysphasia, epigastric pain, and vomiting[10]. This rare entity should be considered in patients with the above GI symptoms and a history of RCC.
Usually, laboratory findings are within the normal range, and only a few cases have shown minor associated elevations in carcinoembryonic antigen. The imaging findings of gastric metastasis from RCC are similar to those of primary RCC, which are all hypervascular tumors. Abdominal contrast-enhanced CT shows a heterogeneous, well-defined hypervascular mass with marked early enhancement in the arterial phase, with reduced enhancement during the venous phase[11]. Metastasis to other organs may show the same contrast-enhanced pattern. Thus, CT scans play an important role in the diagnosis of metastases. In our case, abdominal CT revealed multiple hypervascular metastatic foci with abnormal enhancement in the stomach, pancreas, gallbladder, and subcutaneous soft tissue of the right buttock. Concomitantly, abdominal CT detected small lesions that were not evident during gastroscopy. In our case, two other small lesions were found in the stomach body by CT with negative endoscopic findings. Positron emission tomography (PET)/CT are recognized as efficient imaging modalities for the management of oncology patients, and are considered to have high specificity and a complementary role in the diagnosis of RCC[12]. PET/CT was reported to be superior to conventional imaging methods for detecting local recurrence and distant metastasis of RCC, and appeared to be an effective surveillance tool[13].
Endoscopically, gastric metastasis from RCC may present as submucosal tumor-like (with or without central depression) ulcerative lesions which can be called “volcano-like lesions”, polypoid lesions, or minor erosion[14]. Generally, submucosal-like tumor is the most frequent endoscopic type. It is reported that lesions are most commonly located in the gastric body, followed by the fundus and antrum, and metastasis is more likely to be single rather than multiple[15]. Recently, more small gastric metastases from RCC have been documented, with the smallest being approximately 0.6 cm, which is similar to our two small lesions, underlining the importance of careful gastroscopy for identification of early metastasis[16]. In our patient, the EUS image mainly showed a heterogeneous hypoechoic change with definite borders that involved the deeper layers of the mucosa and submucosal layer. Due to tumor growth, the tumor may affect the whole mucosal layer. Endoscopic biopsy is meaningless and may reveal only nonspecific changes unless the tumor has infiltrated the mucosa. EUS-guided fine needle aspiration or deep excavation biopsy may be helpful in obtaining tissue from these lesions. After 1-year of follow-up, our case showed three types of endoscopic characteristics in different periods, from which we could derive the tumor’s growth pattern. Firstly, the metastatic foci presented as small and flat submucosal-like tumors, with central depression, surface mucosal congestion and thickened vessels. As time progressed, the diameter of the tumor became enlarged, discoid-shaped, and located in the deeper layers of the mucosa and submucosal layer. As the tumor grew further, it infiltrated from the deeper layers of the mucosa and submucosal layer to the superficial mucosal layer and presented as a large protruding lesion. The primary discoid-shape remained in the basal layer of the protruding lesion, so it is assumed that this submucosal tumor infiltrated the mucosal layer, and then grew to the stomach cavity through the prior central depressed localization.
Histopathological examination is the gold standard for the diagnosis of gastric metastasis from RCC. Histologically, metastatic RCC is predominantly the clear cell type[17]. The presence of clear cell morphology in any unknown gastric lesion should prompt pathologists to consider the possibility of metastatic RCC. Metastatic tumors can be distinguished from gastric carcinoma based on the absence of cellular atypia in the gastric glandular structures that may appear compressed by the metastatic tumor[18]. Immunohistochemical markers are helpful in establishing the correct diagnosis. Several previously published studies have shown RCC-associated markers including CD10, CD15, vimentin, EMA, PAX-2 and E-cadherin, but negative for CK7, CK20 and cKIT[19]. In our case, immunohistochemistry was positive for CK(pan), PAX-8, vimentin, CD10, CAIX and Ki-67 and negative for CK7, CD117, TFE3, Melan A, CgA, Syn, S-100, CDX2 and CK20, which confirmed the diagnosis of metastatic RCC.
To date, no standard therapeutic guideline for patients with gastric metastasis arising from RCC has been established. A variety of management options have been described for this entity on a case-by-case basis, including endoscopic resection, surgery, chemotherapy, immunotherapy, and embolization of the metastasis. More studies, similar to that of Dabestani et al[20], have concluded that complete resection of metastases benefits patients’ overall and cancer-specific survival. In addition, treatment of patients with solitary gastric metastasis shows a good outcome compared with treatment of multiple metastases[21]. When it is feasible, resection of the solitary gastric metastasis should be performed. Endoscopic treatment, either with endoscopic mucosal resection (EMR) or ESD, may be performed for solitary gastric metastasis confined to the mucosa and submucosa. Accordingly, for tumors with a characteristic growth pattern with submucosal invasion, ESD is more effective in the early stages, and endoscopic full-thickness resection may be needed in selected cases. In our study, complete en bloc resection was performed using ESD for the two lesions. EMR may only be attempted in partial pedunculated gastric metastasis. Surgical resection of metastatic gastric tumors (either total or subtotal gastrectomy) is indicated only after careful consideration, and remains the best therapeutic option for a resectable solitary gastric metastasis. For symptomatic patients with hemorrhage, tumor perforation, other related complications and those without other coexisting organ metastasis, surgical treatment may improve the quality of life and prolong survival time[22]. If the tumor is unresectable, systemic treatment is required. Compared with chemotherapy or hormonal therapy, targeted agents for metastatic tumors in the stomach are the preferred option. Molecularly targeted therapy, including sorafenib, sunitinib, temsirolimus and bevacizumab, is effective and extends the survival period in patients with advanced metastatic RCC[23]. In our case, second-line therapy with axitinib showed clinical efficacy in this patient who was resistant to sunitinib.
Prediction of the prognosis of gastric metastasis from RCC is limited due to its rare occurrence. Generally, when patients with RCC are diagnosed with gastric metastasis, they usually have metastatic lesions in other organs. Therefore, the outcome of this rare tumor is unfavorable, and the 5-year survival rates of patients with solitary metastatic RCC ranges from 23% to 35%[12]. In cases with resectable oligometastasis from RCC, the 5-year survival rate is higher (72.6%)[24]. Patients with a single metastasis fare better than those with multiple metastases. In the report by Namikawa, factors for poor prognosis included protruding gastric lesion, multiple metastases, and gastric metastasis detected within 6.3 years after therapeutic intervention for renal cancer[4]. Regular follow-up is crucial in patients who undergo nephrectomy for RCC. In patients with GI symptoms or those who are endoscopy positive for an unknown gastric lesion, recurrence should initially be excluded. In retrospect, the lesson learned from our case was that the solitary submucosal tumor was detected and misdiagnosed as ectopic pancreas in the local hospital. Thus, it was more accurate to obtain cyto-histological evidence by a deep excavation biopsy or EUS-guided biopsy, or shorten the follow-up time to 3-6 mo.
This report highlights the rare clinical entity of gastric metastasis from RCC which should be considered in patients with a history of RCC irrespective of the long interval after RCC treatment. Additionally, our case presented three types of endoscopic characteristics and we deduced the tumors’ growth pattern. Due to the unfavorable prognosis, watchful follow-up for patients with a history of RCC is necessary, especially in patients with GI symptoms. Further investigations with more samples to describe this uncommon entity are needed in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Batyrbekov K, Kazakhstan; Yelamanchi R, India S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Curti BD. Renal cell carcinoma. JAMA. 2004;292:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1221] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 3. | Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176:2397-400; discussion 2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Namikawa T, Munekage M, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Metastatic gastric tumors arising from renal cell carcinoma: Clinical characteristics and outcomes of this uncommon disease. Oncol Lett. 2012;4:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Mubarak MF, Daglilar ES, Smilari T, Ray A. Metastatic Renal Cell Carcinoma Presenting as a Bleeding Gastric Polyp. Ochsner J. 2018;18:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P, Menon M, Montorsi F, Karakiewicz PI. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 480] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 7. | Namikawa T, Iwabu J, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Solitary gastric metastasis from a renal cell carcinoma, presenting 23 years after radical nephrectomy. Endoscopy. 2012;44 Suppl 2 UCTN:E177-E178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Gilg MM, Gröchenig HP, Schlemmer A, Eherer A, Högenauer C, Langner C. Secondary tumors of the GI tract: origin, histology, and endoscopic findings. Gastrointest Endosc. 2018;88:151-158.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surg Today. 2014;44:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Abu Ghanimeh M, Qasrawi A, Abughanimeh O, Albadarin S, Helzberg JH. Gastric Metastasis from Renal Cell Carcinoma, Clear Cell Type, Presenting with Gastrointestinal Bleeding. Case Rep Gastrointest Med. 2017;2017:5879374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Yoshida R, Yoshizako T, Ando S, Shibagaki K, Ishikawa N, Kitagaki H. Dynamic CT findings of a polypoid gastric metastasis of clear renal cell carcinoma: a case report with literature review. Radiol Case Rep. 2020;15:237-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Sugasawa H, Ichikura T, Ono S, Tsujimoto H, Hiraki S, Sakamoto N, Yaguchi Y, Shimazaki H, Yamamoto J, Hase K. Isolated gastric metastasis from renal cell carcinoma 19 years after radical nephrectomy. Int J Clin Oncol. 2010;15:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Park JW, Jo MK, Lee HM. Significance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography for the postoperative surveillance of advanced renal cell carcinoma. BJU Int. 2009;103:615-619. [PubMed] [DOI] [Full Text] |
| 14. | Arakawa N, Irisawa A, Shibukawa G, Sato A, Abe Y, Yamabe A, Takasakia Y, Yoshida Y, Maki T, Igarashi R, Yamamoto S, Ikeda T, Hojo H. Simultaneous Gastric Metastasis From Renal Cell Carcinoma: A Case Report and Literature Review. Clin Med Insights Case Rep. 2018;11:1179547618775095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kim MY, Jung HY, Choi KD, Song HJ, Lee JH, Kim DH, Choi KS, Kim SA, Lee GH, Kim JH. Solitary synchronous metastatic gastric cancer arising from t1b renal cell carcinoma: a case report and systematic review. Gut Liver. 2012;6:388-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Xu J, Latif S, Wei S. Metastatic renal cell carcinoma presenting as gastric polyps: A case report and review of the literature. Int J Surg Case Rep. 2012;3:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Rita H, Isabel A, Iolanda C, Alexander H, Pedro C, Liliana C, Lucília M, Sofia S, Leopoldo M. Treatment of gastric metastases from renal cell carcinoma with endoscopic therapy. Clin J Gastroenterol. 2014;7:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Riviello C, Tanini I, Cipriani G, Pantaleo P, Nozzoli C, Poma A, Riccardo V, Valeri A. Unusual gastric and pancreatic metastatic renal cell carcinoma presentation 10 years after surgery and immunotherapy: A case report and a review of literature. World J Gastroenterol. 2006;12:5234-5236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 19. | Yamamoto D, Hamada Y, Okazaki S, Kawakami K, Kanzaki S, Yamamoto C, Yamamoto M. Metastatic gastric tumor from renal cell carcinoma. Gastric Cancer. 2009;12:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Dabestani S, Marconi L, Hofmann F, Stewart F, Lam TB, Canfield SE, Staehler M, Powles T, Ljungberg B, Bex A. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15:e549-e561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 21. | Namikawa T, Munekage E, Ogawa M, Oki T, Munekage M, Maeda H, Kitagawa H, Sugimoto T, Kobayashi M, Hanazaki K. Clinical presentation and treatment of gastric metastasis from other malignancies of solid organs. Biomed Rep. 2017;7:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Sakurai K, Muguruma K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Kubo N, Tanaka H, Yashiro M, Ohira M, Hirakawa K. Gastric metastasis from renal cell carcinoma with gastrointestinal bleeding: a case report and review of the literature. Int Surg. 2014;99:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Marion J Pollheimer, Thomas A Hinterleitner, Verena S Pollheimer, Andrea Schlemmer, Cord Langner. Renal cell carcinoma metastatic to the stomach: single-center experience and literature review. BJU Int. 2008;102(3):315-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Tanis PJ, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. 2009;96:579-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |