Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9760
Peer-review started: May 4, 2022
First decision: June 16, 2022
Revised: June 28, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 26, 2022
Processing time: 135 Days and 7.5 Hours
Aortic intramural hematoma (IMH) associated with aortic branch tear and intra
During logging operations, a 66-year-old man experienced blunt force trauma after being injured by a fallen tree. He arrived at our trauma center with a left flank pain complaint. Computed tomography (CT) revealed a pseudoaneurysm arising from the proximal renal artery (localized within the aortic media) and Stanford type A IMH. A covered stent was deployed along the left main renal artery, bridging the pseudoaneurysm and covering the parent artery, successfully excluding the pseudoaneurysm as confirmed using aortography. However, although the degree of the pseudoaneurysm decreased, follow-up CT revealed remnant pseudoaneurysm, likely caused by an endoleak. Subsequently, a covered stent was additionally installed through the previously deployed covered stent. Successful exclusion of the pseudoaneurysm was confirmed using final aortography. In the 7-mo follow-up CT scan, the IMH and pseudoaneurysm completely disappeared with no evidence of stent-related complications.
Endovascular treatment such as stent-graft placement can be an effective and safe treatment for traumatic renal artery injury.
Core Tip: We report the case of an adult male who presented with left flank pain after experiencing blunt trauma from being injured by a fallen tree. Investigations revealed a pseudoaneurysm arising from the proximal renal artery and Stanford type A IMH. The patient received endovascular treatment, and the successful exclusion of the pseudoaneurysm was confirmed using final aortography. A postoperative computed tomography scan showed the complete disappearance of the intramural hematoma and pseudoaneurysm with no stent-related complications. Consequently, we propose that endovascular treatment such as stent-graft placement can be an effective and safe treatment for traumatic renal artery injury.
- Citation: Kim Y, Lee JY, Lee JS, Ye JB, Kim SH, Sul YH, Yoon SY, Choi JH, Choi H. Endovascular treatment of traumatic renal artery pseudoaneurysm with a Stanford type A intramural haematoma: A case report. World J Clin Cases 2022; 10(27): 9760-9767
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9760.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9760
Aortic intramural hematoma (IMH) is classically defined as a hematoma in the aortic wall, secondary to rupture of the vasa vasorum, and it is distinguished from aortic dissection (AD) by the absence on imaging of any evident intimal tear[1,2]. However, improved thin-section multidetector computed tomography (CT) images and surgical reports have confirmed small intimomedial tears in a variable percentage of patients diagnosed with IMH[1-4]. Recently, acute aortic syndrome (AAS) was defined as AD, IMH, and penetrating aortic ulcer (PAU)[5]. AAS is characterized by disruption of the media layer of the aorta with bleeding within IMH, along the aortic wall resulting in dissection, or transmurally through the wall in the case of ruptured PAU or trauma[5]. A total of 94% of the reported IMHs had a nontraumatic cause[2,4-6]. The natural history of IMH is highly variable; it may resolve or progress to aneurysm, dissection, or rupture. The morbidity and mortality caused by aortic IMH are similar to that of AD[5,7]. Uncomplicated IMH can be managed conservatively with antihypertensive medication[5]. Conversely, prompt surgical or endovascular intervention is required when IMH cases have significant predictors or symptoms of complications. The type of endovascular intervention depends on the Stanford classification, maximum aortic diameter, maximum IMH thickness, and intramural focal contrast enhancement[1,2,5,7]. To the best of our knowledge, aortic IMH caused by aortic branch artery tear with intramurally located pseudoaneurysm after blunt trauma has not been reported. Here, we report a case of progressive type A aortic IMH associated with a pseudoaneurysm arising from the injured proximal renal artery after blunt trauma, successfully managed using endovascular treatment.
A 66-year-old man arrived at our trauma center with a left flank pain complaint.
The patient’s symptoms started 1 h after being injured by a fallen tree during logging operations.
The patient’s previous medical history was not significant.
The patient’s family history was not significant.
The patient was aerodynamically stable with a blood pressure of 19.3/11.3 kPa, heart rate of 72 beats per min, respiratory rate of 24 breaths per min, and body temperature of 36.0. There was no evidence of gross hematuria.
Laboratory findings were normal, including hemoglobin level, electrolytes, liver function, kidney function, and coagulation profile.
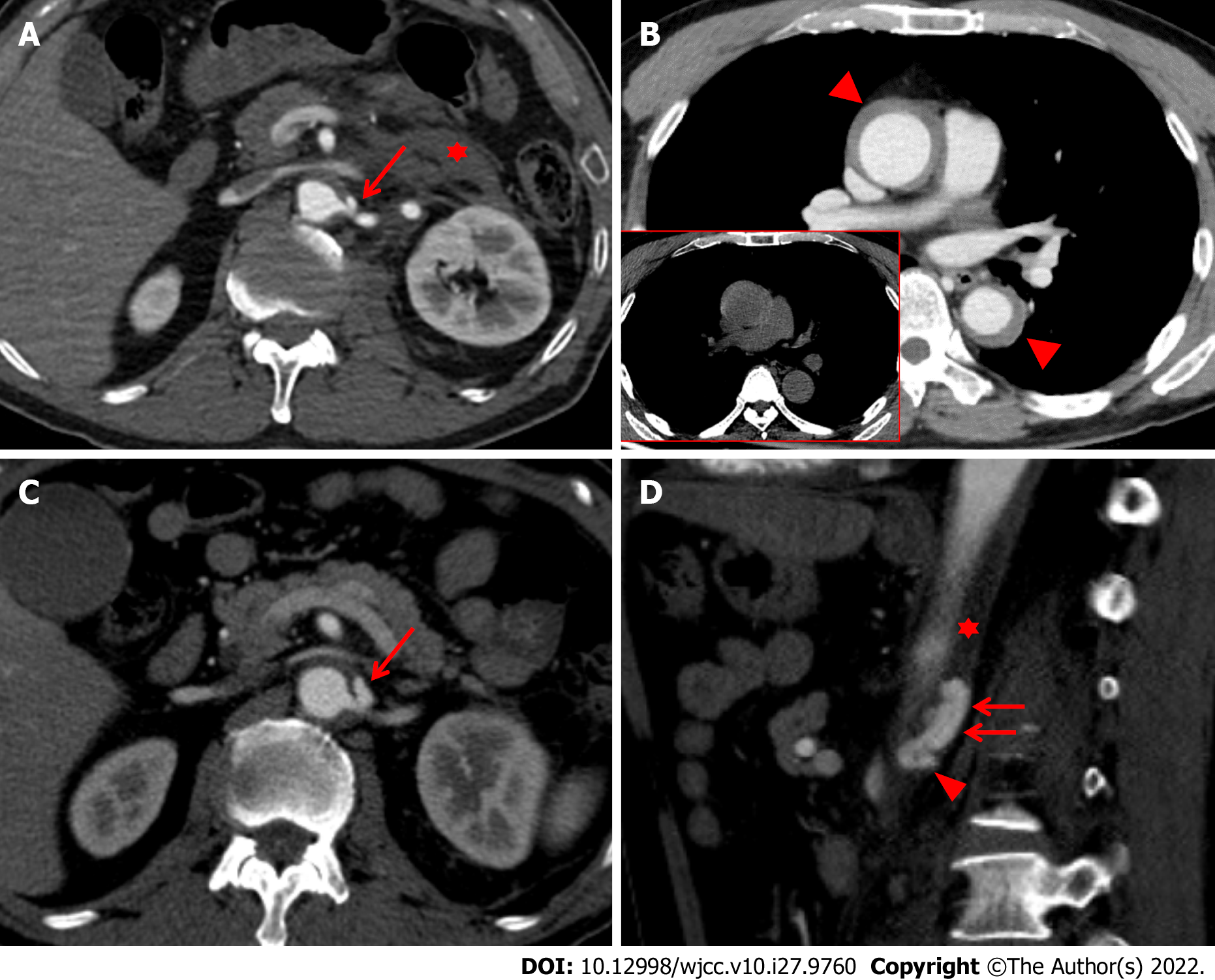
Stanford type A IMH was detected on a chest CT scan (Figure 1A), and an abdominal CT scan revealed a focal out-pouching nodular lesion, suggesting the presence of a pseudoaneurysm arising from the proximal left renal artery with retroperitoneal hematoma (Figure 1B). We decided on conservative management by controlling systolic blood pressure to < 16.0 kPa due to the stable vital signs and small size of the pseudoaneurysm (< 0.5 cm) on the initial CT scan. However, a follow-up CT scan (axial image) taken 15 d after the initial CT scan revealed an increase in the size and extent of the pseudoaneurysm (Figure 1C), which was localized within the aortic media, resulting in an intramural blood collection (Figure 1D). In addition, the maximum diameter of the type A aortic IMH also increased on the follow-up chest CT scan taken 15 d after the initial scan.
The final diagnosis of the presented case is progressive type A IMH associated with renal artery injury after blunt trauma.
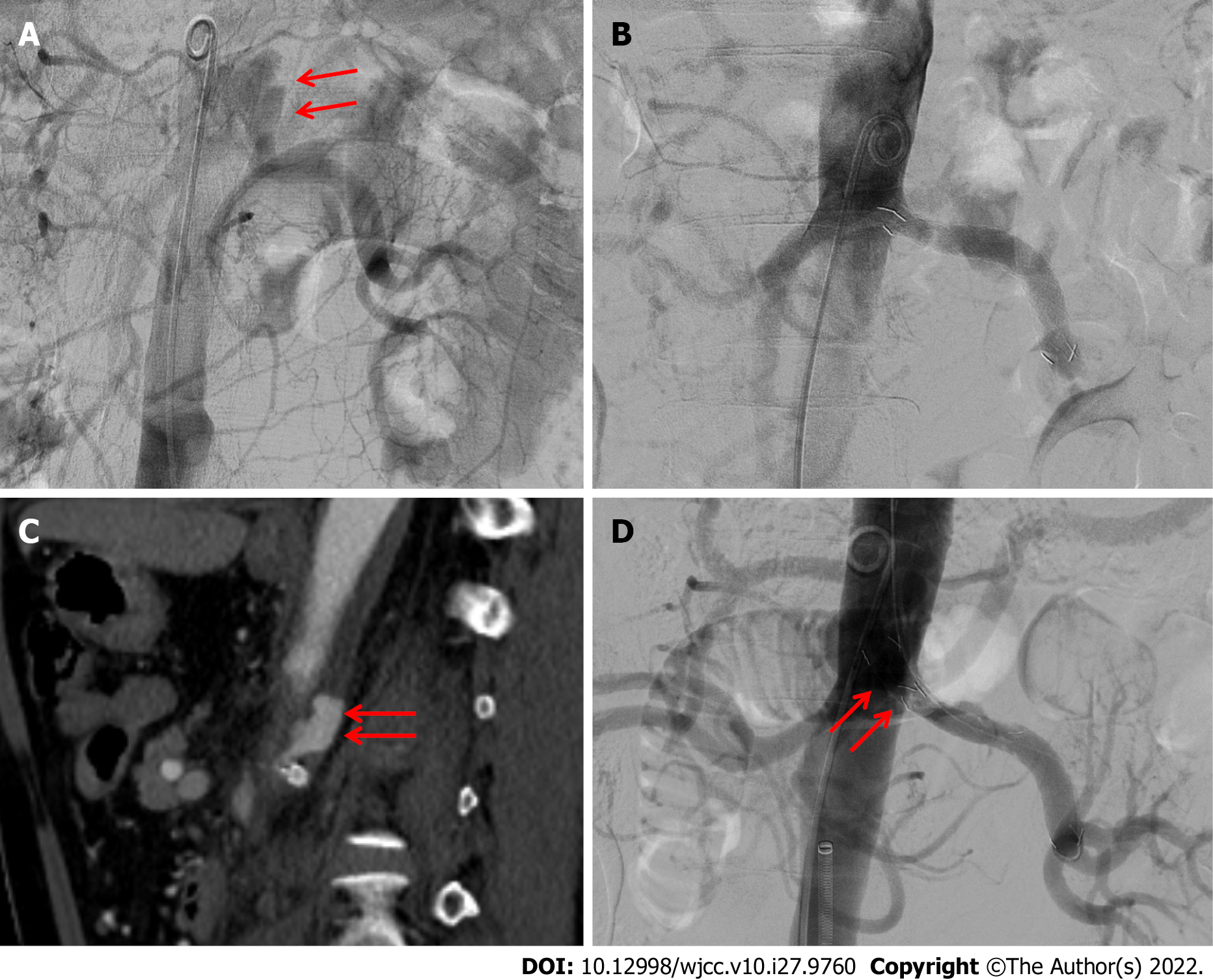
Considering the aggravated pseudoaneurysm and progressive IMH, an emergent endovascular procedure was performed immediately. The pseudoaneurysm arising from the left proximal renal artery was detected using abdominal aortography (Figure 2A), corresponding to the CT images. We opted for complete exclusion of the pseudoaneurysm with a covered stent to manage the pseudoaneurysm while preserving the blood flow to the kidney. Therefore, an 8 mm × 55 mm covered stent (Hercules: S&G Biotech Inc., Sungnam, Korea) was deployed along the left main renal artery, bridging the pseudoaneurysm and covering the parent artery, successfully excluding the pseudoaneurysm as confirmed using the final aortography (Figure 2B).
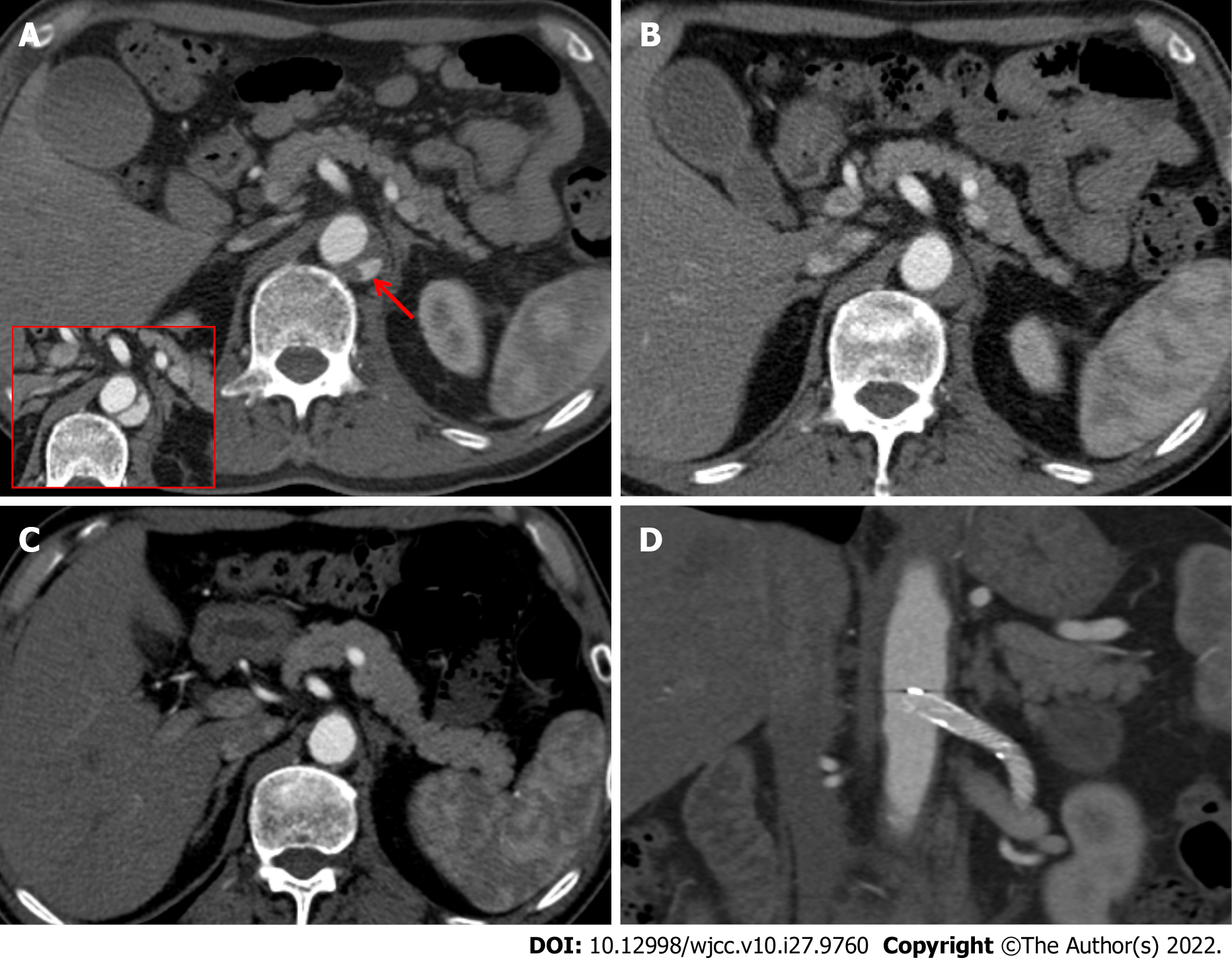
A repeated CT scan performed 1 d after the first endovascular procedure revealed a decrease in the size of the pseudoaneurysm arising from the renal artery. However, a large intramural blood collection initially noted on the sagittal image remained (Figure 2C), likely caused by an endoleak. Therefore, we immediately performed a re-endovascular procedure. Although the degree of the pseudoaneurysm decreased compared with the previous CT scan, evidence of the pseudoaneurysm was still observed using aortography. Subsequently, an additional 8 mm × 45 mm covered stent was installed through the previously deployed covered stent in the left main renal artery. The proximal portion of the newly covered stent was placed into the abdominal aortic lumen to prevent or resolve the endoleak. As a result of the complete coverage of the orifice of the left renal artery, the pseudoaneurysm was successfully excluded, as confirmed using the final aortography (Figure 2D). Eight days after the second en
There are few reports of small intimomedial tears in IMH cases identified prospectively during imaging or surgery[3,4,8]. As mentioned in the introduction, IMH is classically considered secondary to the rupture of the vasa vasorum. However, with improved technology in the last two decades, more intimomedial tears are being identified in patients with IMH. Therefore, a better distinction may be that AD contains two intimomedial tears: an entry tear from the lumen into the media and a re-entry tear back into the aortic lumen. Conversely, IMH with an intimomedial tear often only has an entry tear[1,7]. In the present case, an intimal injury of the renal artery from the aorta occurred by blunt trauma, leading to the formation of a small orifice in the intimal-medial flap. The focal out-pouching saccular aneurysm arose from the proximal portion of the left renal artery, a pseudoaneurysm, associated with an aortic IMH.
The natural history of IMH is variable. It may resolve, enlarge, or progress to an aneurysm or dissection[9]. Multiple imaging prognostic factors indicating a higher risk of complications or progression have been identified[1,7]. When imaging an IMH, the following items should be considered: Maximum aortic diameter, maximum hematoma thickness, lack of focal contrast enhancement (i.e., intramural blood collection or ulcer-like projection), and Stanford classification[1,2,5,9]. An intramural blood collection is a similar small localized area of contrast enhancement with the IMH and is more likely to occur in the descending aorta[9]. An intramural blood collection may appear as a string of contrast agents pooling on the coronal and sagittal images[1,2]. Continued improvements in the thin-section CT may allow for increased visibility of the connection to the branch artery and the aortic lumen[1,2,8]. Although studies of intramural blood collection are limited and the prognostic significance is uncertain, patients with a large intramural blood collection and those with a visible connection to a branch artery are at a higher risk of incomplete hematoma resorption. The blood collection may increase over time, necessitating endovascular treatment. In the present case, the multidetector CT differentiated the pseudoaneurysm as being confined within the IMH and not having a wall of its own. The pseudoaneurysm originated from the renal artery, extended along type A IMH, and gradually increased in size over time, as seen in the follow-up CT scans. This situation requires emergent surgical or endovascular treatment of the injured proximal renal artery.
Main renal artery injury after blunt trauma is rare, with an estimated incidence of only 0.05%[10-12]. A wide spectrum of treatment plans has been proposed for traumatic renal arterial injury, including observation, endovascular treatment, and surgical procedures. The preferred management is decided based on the hemodynamic stability or the need to control an acute and heavy hemorrhage. In the present case, the clinicians decided first to manage the pseudoaneurysm from the renal artery conservatively, considering the relatively small size of the pseudoaneurysm and the stable hemodynamics of the patient. However, a follow-up CT scan showed an increase in the size and extent of the pseudoaneurysm arising from the proximal renal artery, necessitating immediate endovascular intervention. The endovascular options may play an important role in diagnosing and treating renal artery injury with several advantages such as rapid recovery, a short length of hospital stays, and early resumption of physical activities[11]. Rundback et al[13] proposed an angiographic classification system by which renal arterial injuries could be divided into three main types for treatment. Type I lesions, as described in the present case, are saccular and arise from either the main renal artery trunk or proximally from a large segment artery. These lesions can be successfully treated with stent grafts, excluding the pseudoaneurysm arising from the main renal artery, while preserving the distal perfusion of the normal parenchyma to prevent kidney dysfunction. Several studies have reported endovascular stent-graft placement with promising results[10-12]. However, the endovascular stent-graft option also has some limitations depending on the available devices (such as stents), and we experienced them in the present case. After the stent-graft deployment along the main renal artery on the first attempt, the aortography seemed to show the proximal and distal parts of the pseudoaneurysm completely. However, a large intramural blood collection was still identified on the follow-up CT scan 1 d later.
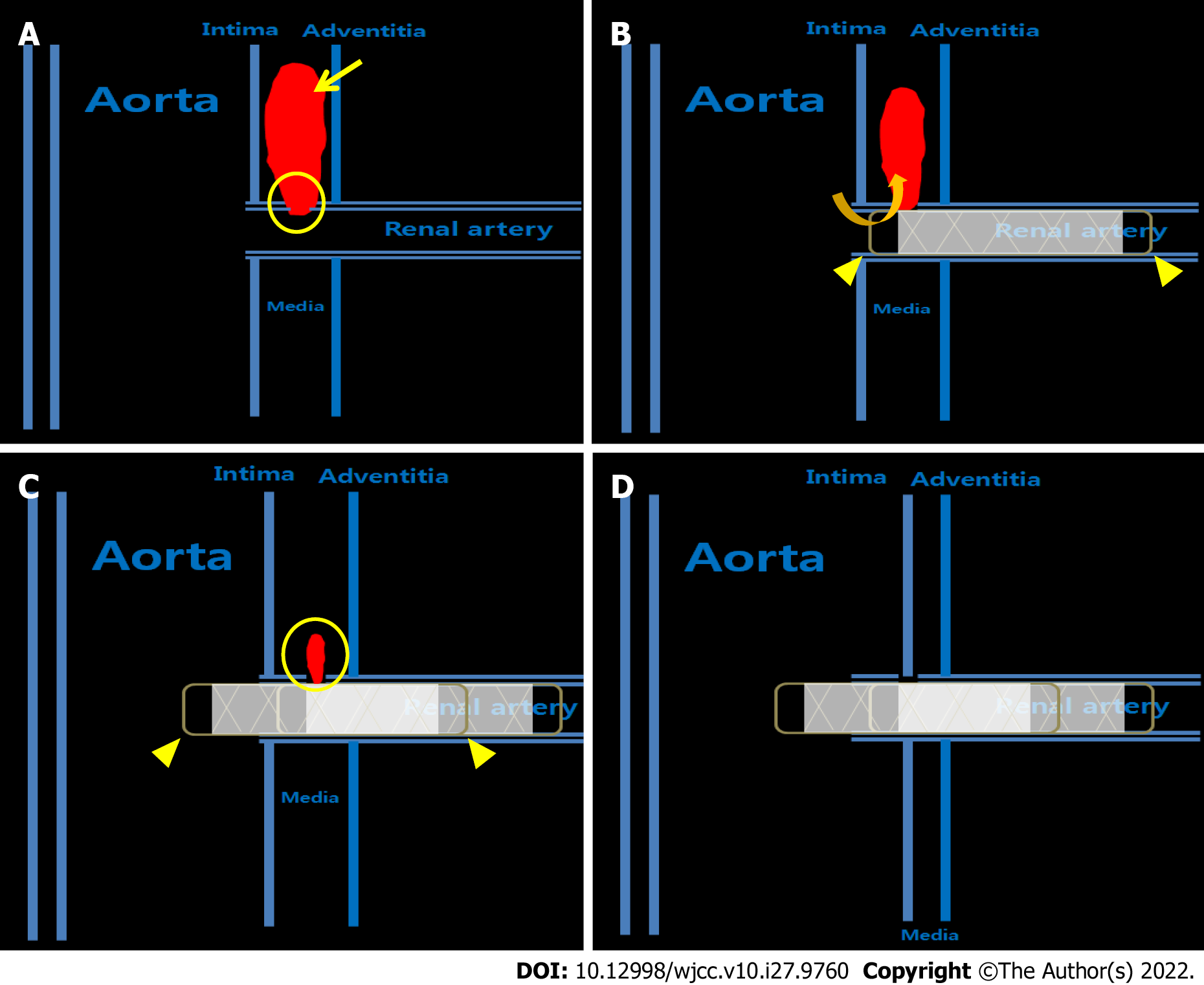
Eventually, the pseudoaneurysm was managed after installing an additional stent graft with the proximal part placed in the middle of the aorta lumen to cover the injured area of the renal artery sufficiently (Figure 4). At the time of the urgent procedures, the only type of covered stent available in Korea was the expanded polytetrafluoroethylene-covered stent graft (Hercules: S&G Biotech Inc., Sungnam, Korea), which has an uncovered area of 2.5 mm at each end. Therefore, the injured area of the proximal renal artery may not have been fully covered by the stent graft in the first procedure, resulting in the type 1 endoleak. Considering that the pseudoaneurysm had small communication through an intimomedial tear of only 1-2 mm in diameter and that the tear origin of the renal artery was located too close to the aorta, the stent graft should have been safely placed as far as possible into the aorta beyond the intimomedial flap to prevent the endoleak, rather than adequately covering it.
Understanding IMH pathogenesis, imaging findings, and increasing evidence suggesting that many IMHs are associated with intimomedial tears will help clinicians identify patients with IMHs and select the appropriate treatment, thus improving outcomes. Endovascular treatment, including stent-graft placement, can be an effective and safe treatment strategy for IMHs associated with intimomedial tears. A careful and repetitive approach, based on awareness of the various possible causes of endoleaks, could increase the success rate of endovascular treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gálvez Salazar P, Ecuador; Lovi NK, Ghana; Tan B, China; Yang L, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Chao CP, Walker TG, Kalva SP. Natural history and CT appearances of aortic intramural hematoma. Radiographics. 2009;29:791-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Gutschow SE, Walker CM, Martínez-Jiménez S, Rosado-de-Christenson ML, Stowell J, Kunin JR. Emerging Concepts in Intramural Hematoma Imaging. Radiographics. 2016;36:660-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Seitun S, Rossi UG, Cademartiri F, Maffei E, Cronin P, Ferro C, Williams DM. MDCT findings of aortic branch artery pseudoaneurysms associated with type B intramural haematoma. Radiol Med. 2012;117:789-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Williams DM, Cronin P, Dasika N, Upchurch GR Jr, Patel HJ, Deeb MG, Abrams G. Aortic branch artery pseudoaneurysms accompanying aortic dissection. Part I. Pseudoaneurysm anatomy. J Vasc Interv Radiol. 2006;17:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012;33:26-35b. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Park KH, Lim C, Choi JH, Sung K, Kim K, Lee YT, Park PW. Prevalence of aortic intimal defect in surgically treated acute type A intramural hematoma. Ann Thorac Surg. 2008;86:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Sawaki S, Hirate Y, Ashida S, Takanohashi A, Yagami K, Usui M. Clinical outcomes of medical treatment of acute type A intramural hematoma. Asian Cardiovasc Thorac Ann. 2010;18:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Bosma MS, Quint LE, Williams DM, Patel HJ, Jiang Q, Myles JD. Ulcerlike projections developing in noncommunicating aortic dissections: CT findings and natural history. AJR Am J Roentgenol. 2009;193:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Kaji S, Nishigami K, Akasaka T, Hozumi T, Takagi T, Kawamoto T, Okura H, Shono H, Horibata Y, Honda T, Yoshida K. Prediction of progression or regression of type A aortic intramural hematoma by computed tomography. Circulation. 1999;100:II281-II286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Lim KH, Ryeom HK, Park J. Endovascular treatment of renal arterial perforation after blunt trauma: Case report. Int J Surg Case Rep. 2018;42:208-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Loffroy R, Chevallier O, Gehin S, Midulla M, Berthod PE, Galland C, Briche P, Duperron C, Majbri N, Mousson C, Falvo N. Endovascular management of arterial injuries after blunt or iatrogenic renal trauma. Quant Imaging Med Surg. 2017;7:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Lopera JE, Suri R, Kroma G, Gadani S, Dolmatch B. Traumatic occlusion and dissection of the main renal artery: endovascular treatment. J Vasc Interv Radiol. 2011;22:1570-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Rundback JH, Rizvi A, Rozenblit GN, Poplausky M, Maddineni S, Crea G, Agrawal U, Olson C, Matalon TA. Percutaneous stent-graft management of renal artery aneurysms. J Vasc Interv Radiol. 2000;11:1189-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |