Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9743
Peer-review started: November 9, 2021
First decision: December 27, 2021
Revised: January 24, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: September 26, 2022
Processing time: 310 Days and 19.5 Hours
The prognosis of intrahepatic cholangiocarcinoma (ICC) with lymph node metastasis is poor. The feasibility of surgery is not certain, which is a contraindication according to the National Comprehensive Cancer Network guidelines. The role of immunotherapy as a neoadjuvant therapy for ICC is not clear. We herein describe a case of ICC with lymph node metastasis that was successfully treated with neoadjuvant therapy.
A 60-year-old man with a liver tumor was admitted to our hospital. Enhanced computed tomography and magnetic resonance imaging revealed a space-occupying lesion in the right lobe of the liver. Multiple subfoci were found around the tumor, and the right posterior branch of the portal vein was invaded. Liver biopsy indicated poorly differentiated cholangiocytes. According to the American Joint Committee on Cancer disease stage classification, ICC with hilar lymph node metastasis (stage IIIB) and para-aortic lymph node metastasis was suspected. A report showed that two patients with stage IIIB ICC achieved a complete response (CR) 13 mo and 16 mo after chemotherapy with a PD-1 monoclonal antibody. After multidisciplinary consultation, the patient was given neoadjuvant therapy, surgical resection and lymph node dissection, and postoperative adjuvant therapy. After three rounds of PD-1 immunotherapy (camrelizumab) and two rounds of gemcitabine combined with cisplatin regimen chemotherapy, the tumor size was reduced. Therefore, a partial response was achieved. Exploratory laparotomy found that the lymph nodes of Group 16 were negative, and the tumor could be surgically removed. Therefore, the patient underwent right hemihepatectomy plus lymph node dissection. The patient received six rounds of chemotherapy and five rounds of PD-1 treatment postoperatively. After 8 mo of follow-up, no recurrence was found, and a CR was achieved.
Neoadjuvant therapy combined with surgical resection is useful for advanced-stage ICC. This is the first report of successful treatment of stage IIIB ICC using neoadjuvant therapy with a PD-1 inhibitor.
Core Tip: At present, the objective remission rate of intrahepatic cholangiocarcinoma (ICC) in the treatment of advanced hepatocellular carcinoma (HCC) patients is approximately 20%. The development of more sensitive and efficient predictive methods will improve the benefits of ICC therapy in potential patients with advanced HCC and benefit patients who are suitable for ICC therapy. This will hopefully open up a new prospect of immunotherapy for liver tumors. Immunotherapy may be a potential treatment option for ICC.
- Citation: Zhu SG, Li HB, Dai TX, Li H, Wang GY. Successful treatment of stage IIIB intrahepatic cholangiocarcinoma using neoadjuvant therapy with the PD-1 inhibitor camrelizumab: A case report. World J Clin Cases 2022; 10(27): 9743-9749
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9743.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9743
Intrahepatic cholangiocarcinoma (ICC) is an adenocarcinoma originating from the secondary bile duct and its branch epithelium. ICC accounts for approximately 10%-15% of primary liver cancers[1]. ICC is the primary malignant tumor of the liver after hepatocellular carcinoma (HCC)[2]. The incidence rate has increased in recent years.The exact cause of ICC is not clear. The well-known risk factors for ICC include congenital choledochal cysts, chronic cholangitis, chronic inflammatory bowel disease, primary sclerosing cholangitis, parasitic infection, chemical carcinogens (such as thorium dioxide and nitrosamine), genetic factors, biliary cirrhosis, cholelithiasis, alcoholic liver disease, and nonspecific cirrhosis. Hepatitis viruses are also closely related to ICC[3]. The general morphology of ICC is divided into three types: mass type, peritubular infiltration type, and intratubular growth type. The most common type is the mass type, which accounts for 60% to 80% of ICCs. The periductal infiltration type accounts for 15% to 35%. This type may have diffuse infiltration along the biliary system and portal vein system, which results in bile duct stenosis and peripheral bile duct dilatation. The intraductal growth type accounts for 8% to 29%. This type mostly shows papillary, polypoid or granular growth that spreads along the superficial bile duct[4]. We herein report the case of a patient with advanced ICC who was successfully treated with neoadjuvant therapy combined with surgical resection.
Right upper abdominal pain for 1 wk.
A 60-year-old male patient developed right upper abdominal pain with no obvious cause 1 wk ago. The pain was dull and persistent, and the patient had no radiating pain, fatigue, poor appetite, cold, or fever. He visited a local hospital, where upper abdominal computed tomography (CT) examination suggested liver space occupying lesions. Since the onset of the disease, the patient’s spirit, sleep, and diet were a little poor, and his urine and feces were normal. He has lost 7.5 kg in weight in the past 2 mo.
The patient had a history of hepatitis B for 30 years and was currently receiving oral entecavir antiviral therapy.
The patient had no smoking or drinking history, and her family members were healthy.
The abdomen was soft, without tenderness or rebound pain. The liver can be touched 2 cm below the right costal margin, and the spleen can be touched 3 cm below the left costal margin. There was percussion pain in the liver area.
The patient’s CA19-9 level was 1844 U/mL, and his hepatitis B virus deoxyribonucleic acid level was 6.0e2 IU/mL. Carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) and liver function were normal.
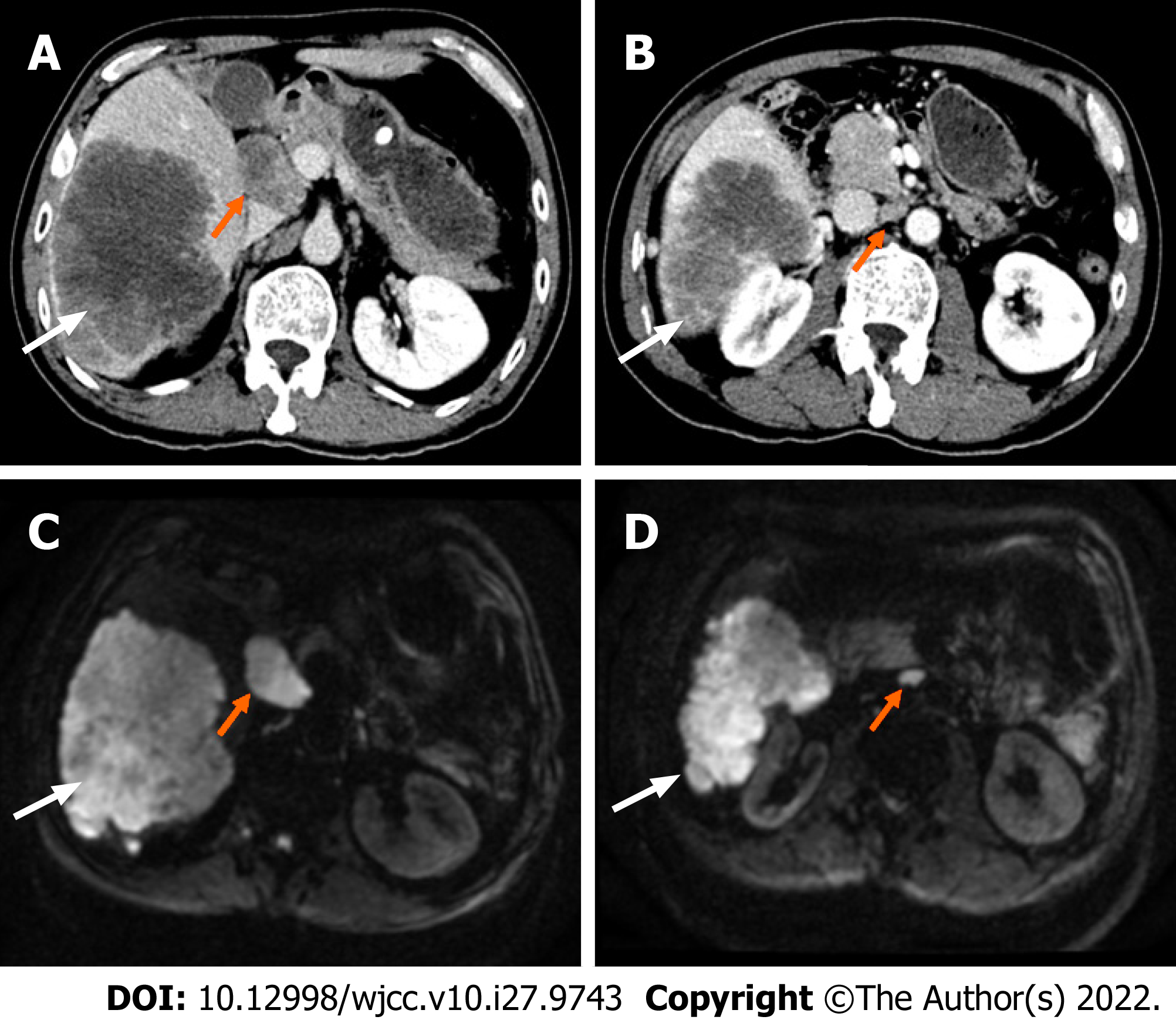
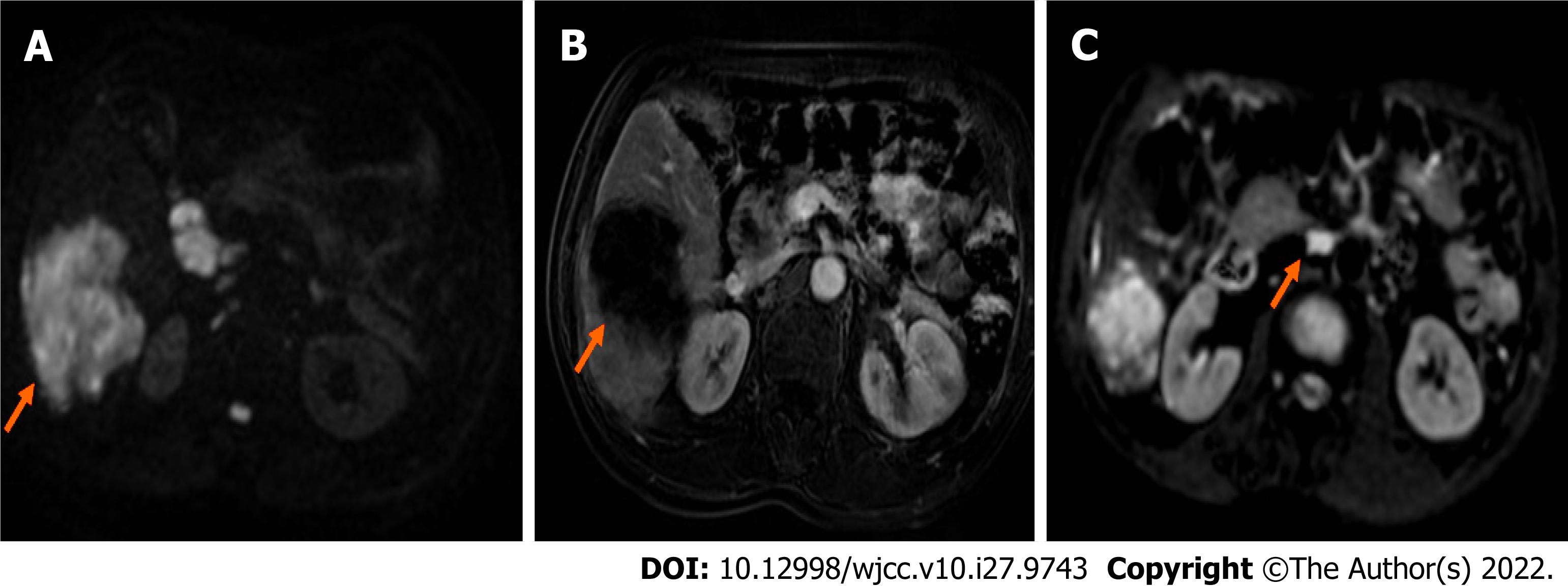
Computed tomography and magnetic resonance imaging (Figure 1) showed that the tumors were located in the right lobe, and the longest diameter was about 20 cm; hilar lymph node metastasis was found.
ICC (stage IIIB) with hilar and retroperitoneal lymph node metastases was initially diagnosed.
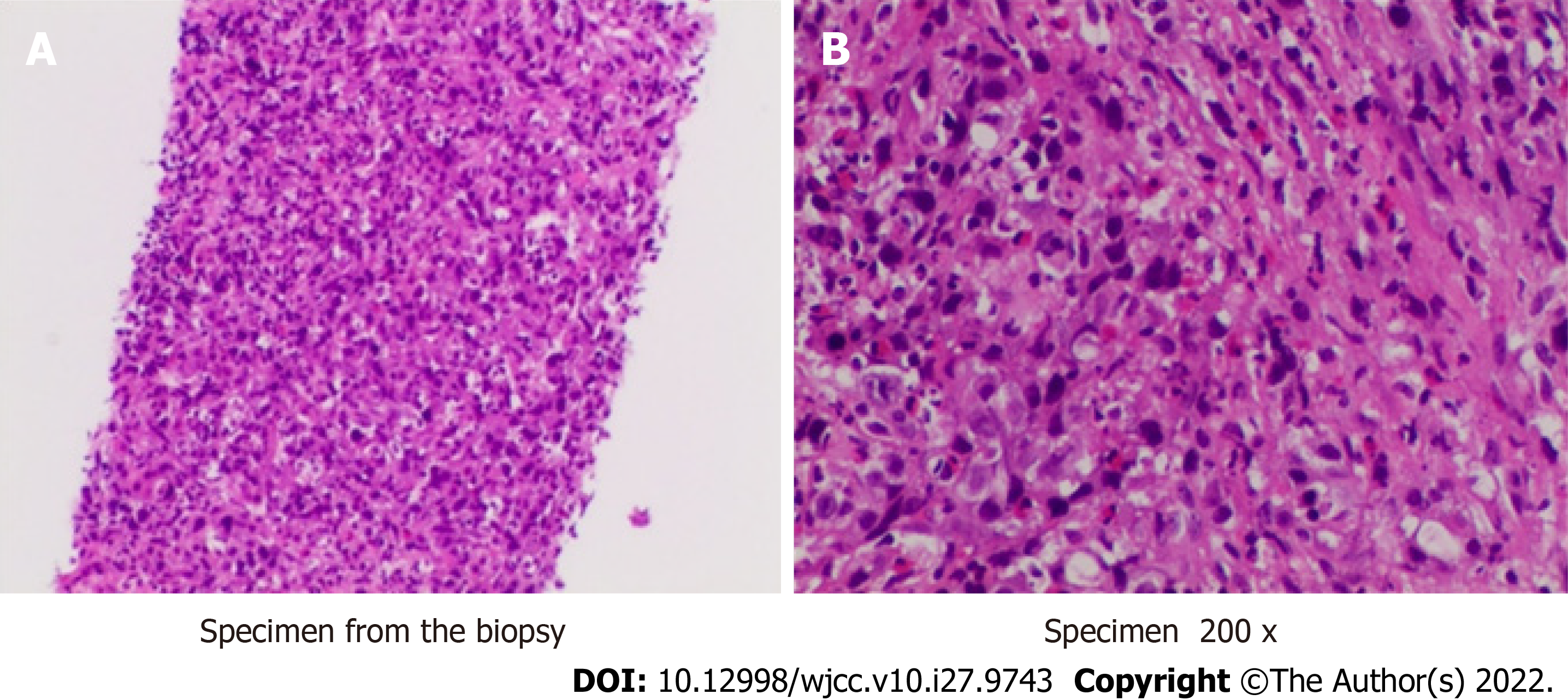
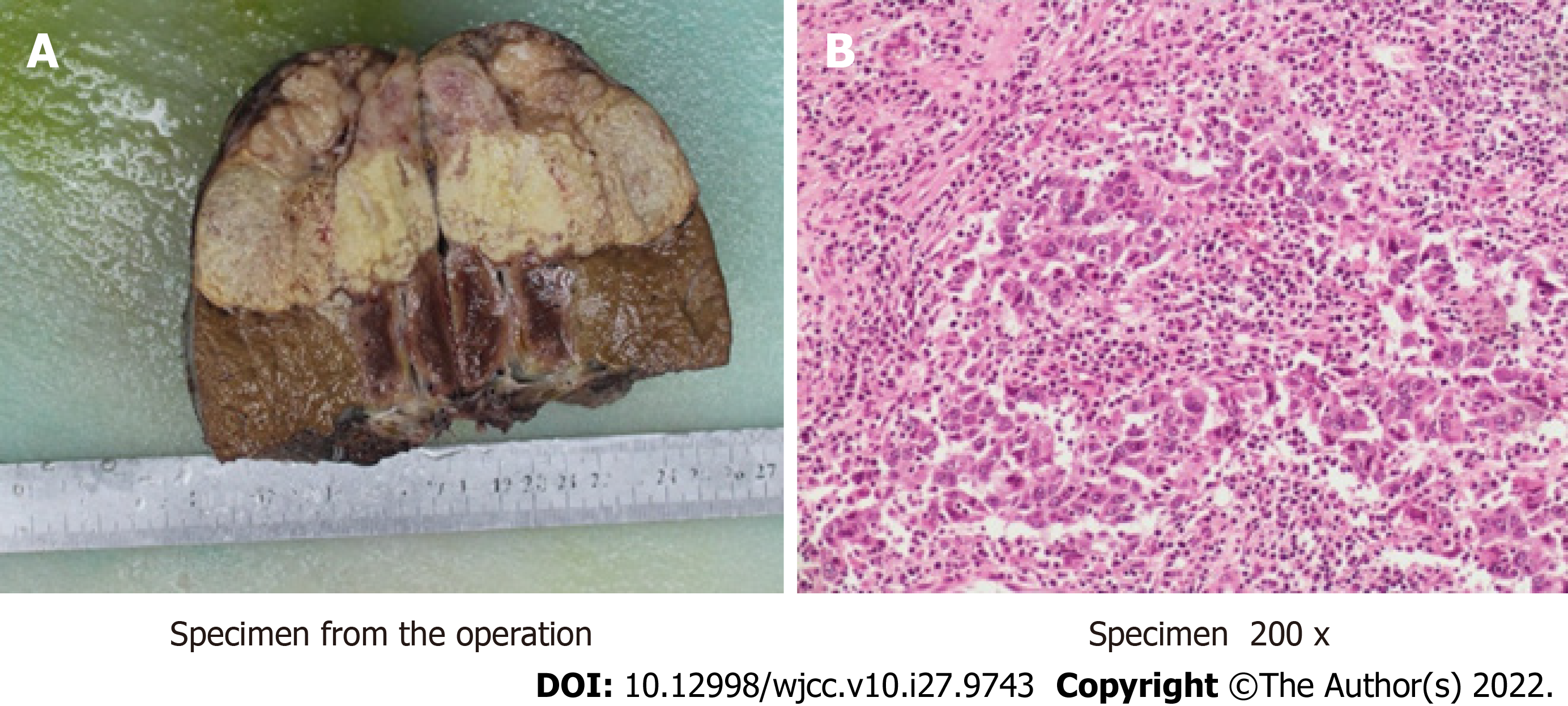
We performed liver biopsy, and the pathological results (Figure 2) showed poorly differentiated cholangiocarcinoma. After multidisciplinary consultation, we determined that the patient should be given neoadjuvant therapy, surgical resection (Figure 3) and lymph node dissection, and postoperative adjuvant therapy.
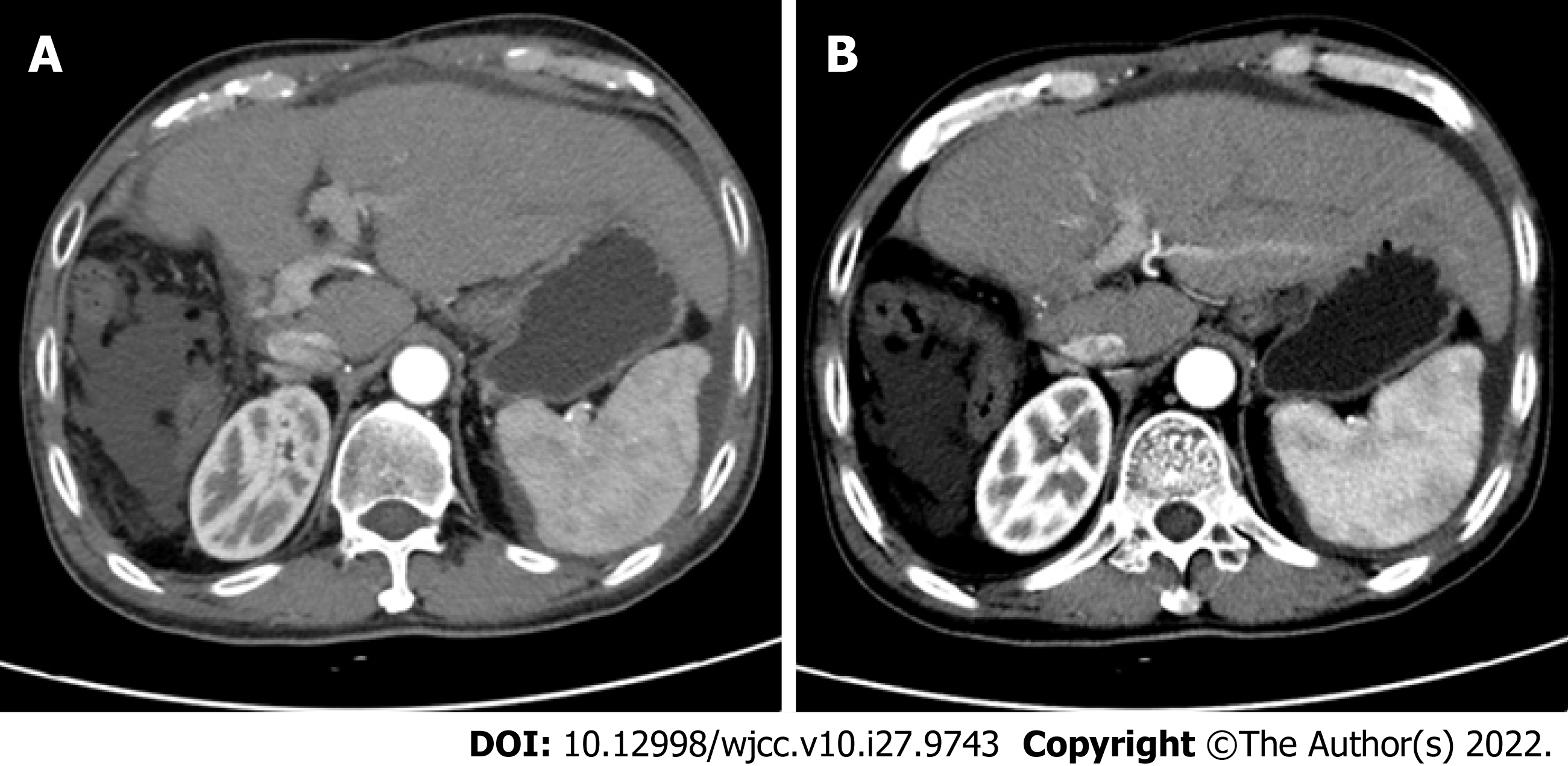
CT imaging (Figure 4) revealed no recurrence after 8 mo of follow-up, and CA199 decreased from 1844 U/mL to 4.76 U/mL. An oncology assessment suggested a partial response.
ICC has high malignancy and a poor prognosis, and there are few long-term survivors. The prognosis of patients without surgery is very poor, and the 3-year survival rate is only 40%-50% after surgery[5]. Compared to other liver malignancies, ICC is associated with a shorter survival time and lower resection and cure rates[6]. The gross classification of ICC is related to tumor prognosis. The peritubular infiltration type has the worst prognosis, followed by the mass type. The intratubular growth type has the best prognosis. Tumor markers lack sensitivity and specificity for the diagnosis of ICC. CA19-9 is valuable in the diagnosis of tumors, evaluation of tumor resection, and prediction of prognosis. However, CEA, AFP, and CA-125 are less valuable in the diagnosis of ICC[7]. The definite diagnosis depends on the combination of imaging and pathological examination[8]. ICC is more likely to recur than HCC[9]. The survival of patients with ICC is affected by the number of tumors, the extent of liver resection, the degree of tumor differentiation, and the type of tumor cells. According to the National Comprehensive Cancer Network (NCCN) guidelines, preoperative biopsy is not necessary, especially in surgically resectable cases. When imaging results indicate a suspicious nodule, it should be treated as malignant, and diagnostic laparoscopic exploration is recommended to exclude unresectable disseminated lesions[10]. Exploration and evaluation should include examinations for multiple intrahepatic cancers, lymph node metastasis, and distant metastasis. Lymph node metastasis and distant metastasis outside the portal hepatis should be considered as surgical contraindications. Hilar lymph node dissection should be performed routinely to provide staging and prognosis information. According to the NCCN guidelines, radical surgery (plus lymph node dissection) is the only curative treatment[11]. Surgical indications and neoadjuvant therapy remain the focus of clinical judgments[12]. One study reported that two patients with stage IIIB ICC achieved a CR 13 mo and 16 mo after chemotherapy with PD-1 monoclonal antibody[13]. For the present case of ICC with hilar lymph node metastasis, abdominal aortic lymph node metastasis was suspected and it was classified as American Joint Committee on Cancer stage IIIB. Multidisciplinary discussion determined that the patient should receive neoadjuvant therapy, surgical resection and lymph node dissection, and postoperative adjuvant therapy. Neoadjuvant therapy included chemotherapy and immunotherapy. After immunotherapy with a PD-1 inhibitor and chemotherapy with the gemcitabine combined with cisplatin regimen, a re-evaluation using magnetic resonance imaging (Figure 5) showed that the tumor had shrunk and was necrotic. An oncology evaluation determined a partial response. According to the NCCN guidelines, the patient continued to receive immunotherapy and chemotherapy after tumor resection, and no recurrences were found. Lymph node dissection is another focus of clinical debate. The existing research shows that extended dissection does not improve the prognosis. However, the NCCN and European Society of Medical Oncology guidelines recommend routine hilar lymph node dissection. The NCCN guidelines consider positive extrahepatic lymph node metastasis as a surgical contraindication and recommend neoadjuvant therapy followed by surgery at a lower stage. Therefore, the independent risk factors for the prognosis of the patient included a less than 1-cm distance between the cutting edge and the tumor, a tumor diameter greater than 5 cm, multiple tumors, microvascular invasion, and positive pathological lymph node metastasis[12,14]. A previous study confirmed that lymph node dissection did not improve the prognosis of patients with ICC[15]. Postoperative pathology confirmed that positive lymph node metastasis is an independent risk factor affecting the prognosis of patients with ICC. This relationship confirms the clinical value of lymph node dissection and shows that lymph node dissection remains associated with many problems[15]. Lymph node dissection is routine, and the 2019 Chinese Society of Clinical Oncology biliary system tumor diagnosis and treatment expert consensus recom
Immunotherapy is a current research hotspot. PD-1 inhibitors are effective for solid tumors, but only melanoma and lung cancer are clinical indications for immunotherapy. The objective remission rate in advanced ICC patients is approximately 20%, but more phase III clinical trials are needed for verification. The development of more sensitive and efficient predictive methods will improve the benefits of ICC therapy and benefit patients who are suitable for such therapy. These improvements will hopefully open a new prospect of immunotherapy for liver tumors[19]. Immunotherapy may be a treatment option for ICC, but it must be confirmed by a study including a larger sample of cases[20]. Its role in preoperative neoadjuvant therapy for ICC is a hot topic in clinical trials[21]. Overall, the value of neoadjuvant therapy, the time of surgery after neoadjuvant therapy, the necessity of lymph node dissection, the means of adjuvant therapy, and the treatment plan after recurrence remain hot topics of current research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mocan T, Romania; Tantau AI, Romania S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Nuzzo G, Giuliante F, Ardito F, Giovannini I. Intrahepatic cholangiocarcinoma. Ann Surg. 2009;249:541-2; author reply 542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 967] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 4. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 5. | Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 6. | Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 7. | Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: Molecular markers for diagnosis and prognosis. Surg Oncol. 2017;26:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 833] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 9. | Chaisaingmongkol J, Budhu A, Dang H, Rabibhadana S, Pupacdi B, Kwon SM, Forgues M, Pomyen Y, Bhudhisawasdi V, Lertprasertsuke N, Chotirosniramit A, Pairojkul C, Auewarakul CU, Sricharunrat T, Phornphutkul K, Sangrajrang S, Cam M, He P, Hewitt SM, Ylaya K, Wu X, Andersen JB, Thorgeirsson SS, Waterfall JJ, Zhu YJ, Walling J, Stevenson HS, Edelman D, Meltzer PS, Loffredo CA, Hama N, Shibata T, Wiltrout RH, Harris CC, Mahidol C, Ruchirawat M, Wang XW; TIGER-LC Consortium. Common Molecular Subtypes Among Asian Hepatocellular Carcinoma and Cholangiocarcinoma. Cancer Cell. 2017;32:57-70.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 10. | Cheng Z, Lei Z, Shen F. Coming of a precision era of the staging systems for intrahepatic cholangiocarcinoma? Cancer Lett. 2019;460:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Idrees JJ, Merath K, Gani F, Bagante F, Mehta R, Beal E, Cloyd JM, Pawlik TM. Trends in centralization of surgical care and compliance with National Cancer Center Network guidelines for resected cholangiocarcinoma. HPB (Oxford). 2019;21:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Li T, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, Wang L, Tang ZY, Fan J. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int. 2014;34:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Sui M, Li Y, Wang H, Luo Y, Wan T, Wang X, Hu B, Cheng Y, Lv X, Xin X, Xu Q, Wang G, Lu S. Two cases of intrahepatic cholangiocellular carcinoma with high insertion-deletion ratios that achieved a complete response following chemotherapy combined with PD-1 blockade. J Immunother Cancer. 2019;7:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Zhou R, Lu D, Li W, Tan W, Zhu S, Chen X, Min J, Shang C, Chen Y. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? HPB (Oxford). 2019;21:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Ji GW, Zhu FP, Zhang YD, Liu XS, Wu FY, Wang K, Xia YX, Jiang WJ, Li XC, Wang XH. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol. 2019;29:3725-3735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 17. | Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7:943-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 18. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 19. | Jing CY, Fu YP, Yi Y, Zhang MX, Zheng SS, Huang JL, Gan W, Xu X, Lin JJ, Zhang J, Qiu SJ, Zhang BH. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer. 2019;7:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Lowery MA, Burris HA 3rd, Janku F, Shroff RT, Cleary JM, Azad NS, Goyal L, Maher EA, Gore L, Hollebecque A, Beeram M, Trent JC, Jiang L, Fan B, Aguado-Fraile E, Choe S, Wu B, Gliser C, Agresta SV, Pandya SS, Zhu AX, Abou-Alfa GK. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 21. | Lu JC, Zeng HY, Sun QM, Meng QN, Huang XY, Zhang PF, Yang X, Peng R, Gao C, Wei CY, Shen YH, Cai JB, Dong RZ, Shi YH, Sun HC, Shi YG, Zhou J, Fan J, Ke AW, Yang LX, Shi GM. Distinct PD-L1/PD1 Profiles and Clinical Implications in Intrahepatic Cholangiocarcinoma Patients with Different Risk Factors. Theranostics. 2019;9:4678-4687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |